Melandrii Herba Extract Attenuates H2O2-Induced Neurotoxicity in Human Neuroblastoma SH-SY5Y Cells and Scopolamine-Induced Memory Impairment in Mice
Abstract
:1. Introduction
2. Results
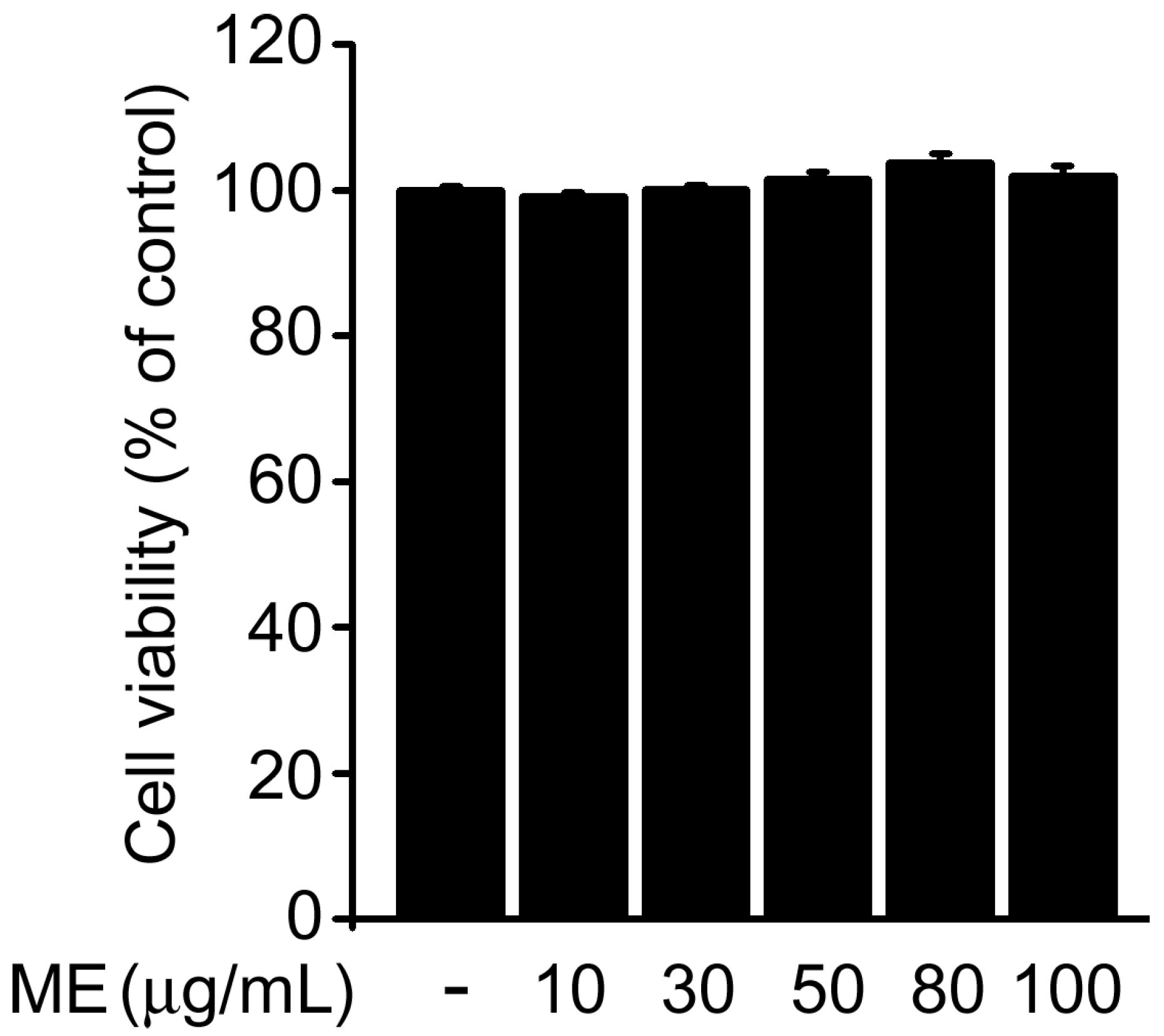
2.1. Melandrii Herba Extract Alleviated H2O2-Induced Damage in SH-SY5Y Cells
2.2. Melandrii Herba Extract Inhibited H2O2-Induced Bax Upregulation and Bcl-2 Downregulation in SH-SY5Y Cells
2.3. Melandrii Herba Extract Prohibited H2O2-Stimulated MAPK Pathways
2.4. Melandrii Herba Extract Attenuated Scopolamine-Induced Cognitive Impairment
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatment
4.2. Preparation of Melandrii Herba Extract (ME)
4.3. Western Blotting
4.4. Experimental Animals
4.5. Spatial Memory Test
4.6. Passive Avoidance Test
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Choi, J.; Sullards, M.C.; Olzmann, J.A.; Rees, H.D.; Weintraub, S.T.; Bostwick, D.E.; Gearing, M.; Levey, A.I.; Chin, L.S.; Li, L. Oxidative damage of DJ-1 is linked to sporadic Parkinson and Alzheimer diseases. J. Biol. Chem. 2006, 281, 10816–10824. [Google Scholar] [CrossRef] [PubMed]
- Caviness, J.N.; Lue, L.; Adler, C.H.; Walker, D.G. Parkinson’s disease dementia and potential therapeutic strategies. CNS Neurosci. Ther. 2011, 17, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Stoy, N.; Mackay, G.M.; Forrest, C.M.; Christofides, J.; Egerton, M.; Stone, T.W.; Darlington, L.G. Tryptophan metabolism and oxidative stress in patients with Huntington’s disease. J. Neurochem. 2005, 93, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Bellingham, M.C. A review of the neural mechanisms of action and clinical efficiency of riluzole in treating amyotrophic lateral sclerosis: What have we learned in the last decade? CNS Neurosci. Ther. 2011, 17, 4–31. [Google Scholar] [CrossRef] [PubMed]
- Gorman, A.M.; McGowan, A.; O’Neill, C.; Cotter, T. Oxidative stress and apoptosis in neurodegeneration. J. Neurol. Sci. 1996, 139 (Suppl.), 45–52. [Google Scholar] [CrossRef]
- Halliwell, B.; Aruoma, O.I. DNA damage by oxygen-derived species. Its mechanism and measurement in mammalian systems. FEBS Lett. 1991, 281, 9–19. [Google Scholar] [CrossRef]
- Barbouti, A.; Doulias, P.T.; Nousis, L.; Tenopoulou, M.; Galaris, D. DNA damage and apoptosis in hydrogen peroxide-exposed jurkat cells: Bolus addition versus continuous generation of H2O2. Free Radic. Biol. Med. 2002, 33, 691–702. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Satoh, T.; Enokido, Y.; Nishio, C.; Ikeuchi, T.; Hatanaka, H. Generation of reactive oxygen species, release of l-glutamate and activation of caspases are required for oxygen-induced apoptosis of embryonic hippocampal neurons in culture. Brain Res. 1999, 824, 71–80. [Google Scholar] [CrossRef]
- Simonian, N.A.; Coyle, J.T. Oxidative stress in neurodegenerative diseases. Annu. Rev. Pharmacol. Toxicol. 1996, 36, 83–106. [Google Scholar] [CrossRef] [PubMed]
- Behl, C.; Davis, J.B.; Lesley, R.; Schubert, D. Hydrogen peroxide mediates amyloid beta protein toxicity. Cell 1994, 77, 817–827. [Google Scholar] [CrossRef]
- Hyslop, P.A.; Zhang, Z.; Pearson, D.V.; Phebus, L.A. Measurement of striatal H2O2 by microdialysis following global forebrain ischemia and reperfusion in the rat: Correlation with the cytotoxic potential of H2O2 in vitro. Brain Res. 1995, 671, 181–186. [Google Scholar] [CrossRef]
- Kelsey, N.A.; Wilkins, H.M.; Linseman, D.A. Nutraceutical antioxidants as novel neuroprotective agents. Molecules 2010, 15, 7792–7814. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, E.K.; Kelsey, N.A.; Doyle, J.; Breed, E.; Bouchard, R.J.; Loucks, F.A.; Harbison, R.A.; Linseman, D.A. Green tea epigallocatechin 3-gallate accumulates in mitochondria and displays a selective antiapoptotic effect against inducers of mitochondrial oxidative stress in neurons. Antioxid. Redox Signal 2009, 11, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Cho, H.S.; Park, E.; Kim, S.; Lee, S.Y.; Kim, C.S.; Kim, D.K.; Kim, S.J.; Chun, H.S. Rosmarinic acid protects human dopaminergic neuronal cells against hydrogen peroxide-induced apoptosis. Toxicology 2008, 250, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Budzynska, B.; Boguszewska-Czubara, A.; Kruk-Slomka, M.; Skalicka-Wozniak, K.; Michalak, A.; Musik, I.; Biala, G. Effects of imperatorin on scopolamine-induced cognitive impairment and oxidative stress in mice. Psychopharmacol. Berl. 2015, 232, 931–942. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.W.; Chang, S.Y.; Jeng, K.C. Protective effect of a sesamin derivative, 3-bis(3-methoxybenzyl)butane-1,4-diol on abeta-stressed pc12 cells. Arch. Pharm. Res. 2015, 38, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Huang, Z.; Chen, X.; Rong, Y.; Zhang, S.; Jiao, Y.; Huang, Q.; Huang, R. Protective effect of Millettia pulchra polysaccharide on cognitive impairment induced by D-galactose in mice. Carbohydr. Polym. 2014, 101, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Miyazaki, I.; Sogawa, N.; Miyoshi, K.; Asanuma, M. Neuroprotective effects of metallothionein against rotenone-induced myenteric neurodegeneration in parkinsonian mice. Neurotox. Res. 2014, 26, 285–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perry, L.M.; Metzger, J. Medicinal Plants of East and Southeast Asia: Attributed Properties and Uses; MIT Press: Cambridge, MA, USA, 1980; p. 620. [Google Scholar]
- Lee, M.Y.; Shin, I.S.; Kyoung, H.; Seo, C.S.; Son, J.K.; Shin, H.K. Alpha-spinasterol from Melandrium firmum attenuates benign prostatic hyperplasia in a rat model. Mol. Med. Rep. 2014, 9, 2362–2366. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.S.; Hwang, N.K.; Kim, D.H.; Moon, T.C.; Son, J.K.; Chang, H.W. Chemical constituents of Melandrium firmum rohrbach and their anti-inflammatory activity. Arch. Pharm. Res. 2008, 31, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Woo, E.H.; Woo, W.S.; Chmurny, G.N.; Hilton, B.D. Melandrioside A: A saponin from Melandrium firmum. J. Nat. Prod. 1992, 55, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Chang, I.S.; Han, Y.B.; Woo, W.S.; Kang, S.S.; Lotter, H.; Wagner, H. Sapogenins from Melandrium firmum. Planta Med. 1989, 55, 544–547. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.M.; Xu, F.H.; Fady, C.; Jacoby, F.J.; Duffey, D.C.; Tu, Y.; Lichtenstein, A. Apoptotic vs. Nonapoptotic cytotoxicity induced by hydrogen peroxide. Free Radic. Biol. Med. 1997, 22, 73–83. [Google Scholar] [CrossRef]
- Jeon, B.S.; Kholodilov, N.G.; Oo, T.F.; Kim, S.Y.; Tomaselli, K.J.; Srinivasan, A.; Stefanis, L.; Burke, R.E. Activation of caspase-3 in developmental models of programmed cell death in neurons of the substantia nigra. J. Neurochem. 1999, 73, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Hunot, S.; Michel, P.P.; Muriel, M.P.; Vyas, S.; Faucheux, B.A.; Mouatt-Prigent, A.; Turmel, H.; Srinivasan, A.; Ruberg, M.; et al. Caspase-3: A vulnerability factor and final effector in apoptotic death of dopaminergic neurons in Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2000, 97, 2875–2880. [Google Scholar] [CrossRef] [PubMed]
- Matsura, T.; Kai, M.; Fujii, Y.; Ito, H.; Yamada, K. Hydrogen peroxide-induced apoptosis in HL-60 cells requires caspase-3 activation. Free Radic. Res. 1999, 30, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Korsmeyer, S.J. Bcl-2 gene family and the regulation of programmed cell death. Cancer Res. 1999, 59, 1693s–1700s. [Google Scholar] [CrossRef]
- Ferrer, I.; Blanco, R.; Carmona, M.; Puig, B.; Barrachina, M.; Gomez, C.; Ambrosio, S. Active, phosphorylation-dependent mitogen-activated protein kinase (MAPK/ERK), stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK), and p38 kinase expression in Parkinson’s disease and dementia with lewy bodies. J. Neural Transm. Vienna 2001, 108, 1383–1396. [Google Scholar] [CrossRef] [PubMed]
- Nakano, H.; Nakajima, A.; Sakon-Komazawa, S.; Piao, J.H.; Xue, X.; Okumura, K. Reactive oxygen species mediate crosstalk between NF-kappaB and JNK. Cell Death Differ. 2006, 13, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Pandareesh, M.D.; Mythri, R.B.; Srinivas Bharath, M.M. Bioavailability of dietary polyphenols: Factors contributing to their clinical application in cns diseases. Neurochem. Int. 2015, 89, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, H.; Sun, Y.; Lin, X.; Chen, B.; Tan, C.; Cao, G.; Wang, Z. Protective effects of salidroside on hydrogen peroxide-induced apoptosis in SH-SY5Y human neuroblastoma cells. Eur. J. Pharmacol. 2007, 564, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Chetsawang, B.; Putthaprasart, C.; Phansuwan-Pujito, P.; Govitrapong, P. Melatonin protects against hydrogen peroxide-induced cell death signaling in SH-SY5Y cultured cells: Involvement of nuclear factor kappa b, bax and bcl-2. J. Pineal Res. 2006, 41, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.S.; Lee, J.Y.; Choi, Y.K.; Kim, G.S.; Han, B.H. Neuroprotective effects of flavones on hydrogen peroxide-induced apoptosis in sh-sy5y neuroblostoma cells. Bioorg. Med. Chem. Lett. 2004, 14, 2261–2264. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.S.; Lee, J.Y.; Choi, Y.K.; Song, S.S.; Kim, J.S.; Jeon, S.J.; Han, Y.N.; Son, K.H.; Han, B.H. Neuroprotective effects of naturally occurring biflavonoids. Bioorg. Med. Chem. Lett. 2005, 15, 3588–3591. [Google Scholar] [CrossRef] [PubMed]
- Ruffels, J.; Griffin, M.; Dickenson, J.M. Activation of ERK1/2, jnk and pkb by hydrogen peroxide in human SH-SY5Y neuroblastoma cells: Role of ERK1/2 in H2O2-induced cell death. Eur. J. Pharmacol. 2004, 483, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Lee, M.Y.; Seo, C.S.; Yoo, S.R.; Jeon, W.Y.; Shin, H.K. Acute and subacute toxicity of an ethanolic extract of Melandrii Herba in Crl:CD sprague dawley rats and cytotoxicity of the extract in vitro. BMC Complement. Altern. Med. 2016, 16, 370. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.C. Bcl-2 family proteins. Oncogene 1998, 17, 3225–3236. [Google Scholar] [CrossRef] [PubMed]
- Spicer, Z.; Millhorn, D.E. Oxygen sensing in neuroendocrine cells and other cell types: Pheochromocytoma (pc12) cells as an experimental model. Endocr. Pathol. 2003, 14, 277–291. [Google Scholar] [CrossRef]
- Suzaki, Y.; Yoshizumi, M.; Kagami, S.; Koyama, A.H.; Taketani, Y.; Houchi, H.; Tsuchiya, K.; Takeda, E.; Tamaki, T. Hydrogen peroxide stimulates c-src-mediated big mitogen-activated protein kinase 1 (BMK1) and the MEF2C signaling pathway in pc12 cells: Potential role in cell survival following oxidative insults. J. Biol. Chem. 2002, 277, 9614–9621. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.L.; Liu, X.H.; Jia, Y.L.; Wu, D.; Xiong, Q.H.; Gong, Q.H.; Wang, Y.; Zhu, Y.Z. A novel compound derived from danshensu inhibits apoptosis via upregulation of heme oxygenase-1 expression in SH-SY5Y cells. Biochim. Biophys. Acta 2013, 1830, 2861–2871. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Choi, B.J.; Chang, M.S.; Park, S.K. Nelumbo nucifera semen extract improves memory in rats with scopolamine-induced amnesia through the induction of choline acetyltransferase expression. Neurosci. Lett. 2009, 461, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Long, Y.; Han, M.; Wang, T.; Chen, Q.; Wang, R. Water-soluble derivative of propolis mitigates scopolamine-induced learning and memory impairment in mice. Pharmacol. Biochem. Behav. 2008, 90, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Bartus, R.T.; Flicker, C.; Dean, R.L.; Pontecorvo, M.; Figueiredo, J.C.; Fisher, S.K. Selective memory loss following nucleus basalis lesions: Long term behavioral recovery despite persistent cholinergic deficiencies. Pharmacol. Biochem. Behav. 1985, 23, 125–135. [Google Scholar] [CrossRef]
- Goverdhan, P.; Sravanthi, A.; Mamatha, T. Neuroprotective effects of meloxicam and selegiline in scopolamine-induced cognitive impairment and oxidative stress. Int. J. Alzheimers Dis. 2012, 2012, 974013. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Hu, J.; Li, J.; Yang, Z.; Xin, X.; Wang, J.; Ding, J.; Geng, M. Effect of acidic oligosaccharide sugar chain on scopolamine-induced memory impairment in rats and its related mechanisms. Neurosci. Lett. 2005, 374, 222–226. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbiny, D.A.; Khalifa, A.E.; Attia, A.S.; Eldenshary Eel, D. Hypericum perforatum extract demonstrates antioxidant properties against elevated rat brain oxidative status induced by amnestic dose of scopolamine. Pharmacol. Biochem. Behav. 2003, 76, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.J.; Lee, K.Y.; Kim, S.H.; Sung, S.H.; Kim, Y.C. Cognitive-enhancing and antioxidant activities of iridoid glycosides from Scrophularia buergeriana in scopolamine-treated mice. Eur. J. Pharmacol. 2008, 588, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Dember, W.N.; Fowler, H. Spontaneous alternation behavior. Psychol. Bull. 1958, 55, 412–428. [Google Scholar] [CrossRef] [PubMed]
- Holcomb, L.; Gordon, M.N.; McGowan, E.; Yu, X.; Benkovic, S.; Jantzen, P.; Wright, K.; Saad, I.; Mueller, R.; Morgan, D.; et al. Accelerated alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat. Med. 1998, 4, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Senechal, Y.; Kelly, P.H.; Dev, K.K. Amyloid precursor protein knockout mice show age-dependent deficits in passive avoidance learning. Behav. Brain Res. 2008, 186, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, L.H.; Kelly, P.H.; Bettler, B.; Kaupmann, K.; Cryan, J.F. Specific roles of gaba(b(1)) receptor isoforms in cognition. Behav. Brain Res. 2007, 181, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Venable, N.; Kelly, P.H. Effects of nmda receptor antagonists on passive avoidance learning and retrieval in rats and mice. Psychopharmacol. Berl. 1990, 100, 215–221. [Google Scholar] [CrossRef]
Sample Availability: Not available. |




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.M.; Lee, A.S.; Choi, I. Melandrii Herba Extract Attenuates H2O2-Induced Neurotoxicity in Human Neuroblastoma SH-SY5Y Cells and Scopolamine-Induced Memory Impairment in Mice. Molecules 2017, 22, 1646. https://doi.org/10.3390/molecules22101646
Lee KM, Lee AS, Choi I. Melandrii Herba Extract Attenuates H2O2-Induced Neurotoxicity in Human Neuroblastoma SH-SY5Y Cells and Scopolamine-Induced Memory Impairment in Mice. Molecules. 2017; 22(10):1646. https://doi.org/10.3390/molecules22101646
Chicago/Turabian StyleLee, Kwang Min, Ae Sin Lee, and Inwook Choi. 2017. "Melandrii Herba Extract Attenuates H2O2-Induced Neurotoxicity in Human Neuroblastoma SH-SY5Y Cells and Scopolamine-Induced Memory Impairment in Mice" Molecules 22, no. 10: 1646. https://doi.org/10.3390/molecules22101646





