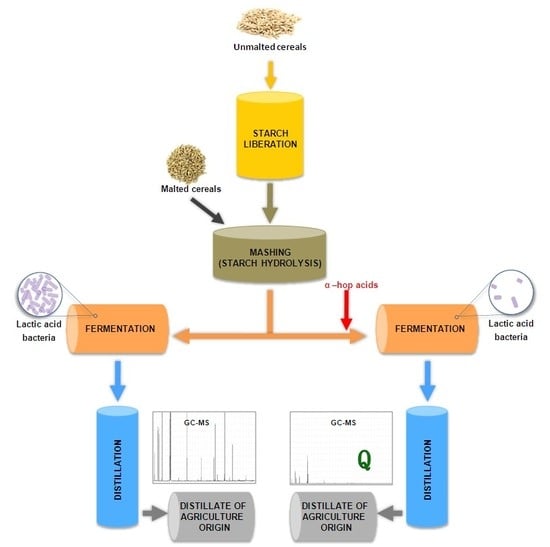
The Effect of Different Starch Liberation and Saccharification Methods on the Microbial Contaminations of Distillery Mashes, Fermentation Efficiency, and Spirits Quality
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition of Raw Materials
2.2. Chemical and Microbiological Characteristic of Mashes
The Discriminant Function Analysis of Microbial Analysis Results
2.3. Chemical Composition of the Obtained Distillates
Principal Component Analysis of Volatile Compounds
- −
- PCA1: (Acetaldehyde (−), Isovaleraldehyde (+), Phenylacetaldehyde (+), 2,3-butanedione (−), Acetaldehyde diethyl acetal (−), Ethyl acetate (+), Ethyl decanoate (+), 2-Phenylethyl isobutyrate (+), 1-propanol (+), 2-methyl-1-propanol (−), 3-methylbutanol (−), 2-methylbutanol (−), 2-phenylethanol).
- −
- PCA2: (Isobutyraldehyde diethyl acetal (+), Ethyl hexanoate (−), 1-butanol).
- −
- PCA3: (Isoamyl acetate (−), Ethyl octanoate (−), Ethyl hexadecanoate (−), Methanol (+)).
- −
- PCA4: (Furfural (+), Isobutyraldehyde (+), Isovaleraldehyde diethyl acetal (+), 2-Methylbutyraldehyde (+), Ethyl 2-hydroxypropanoate (+)).
3. Materials and Methods
3.1. Materials
- −
- barley grain of the Karakan variety (“Danko” Plant Breeding Ltd., Choryń, Poland);
- −
- malted grain of Munich malt type 2 spring barley (Weyermann®, Bamberg, Germany);
- −
- dry distillery yeast (Saccharomyces cerevisiae) Ethanol Red (Fermentis, a division of S.I. Lesaffre, Marcq en Baroeul Cedex, France) at a dose of 0.5 g d.m./L;
- −
- enzyme preparations: Termamyl S.C. α-amylase preparation was used for liquefaction at a dose of 0.13 mL per 1 kg starch and SAN Extra glucoamylase preparation was used for saccharification at 0.6 mL per 1 kg starch (Novozymes, Bagsværd, Denmark);
- −
- mineral nutrient for yeast—an aqueous solution of (NH4)2HPO4 at a dose of 0.2 g/L mash;
- −
- IsoStab® hop α-acid preparation (BetaTec GmbH, Nürnberg, Germany) as an antimicrobial agent at a dose of 80 ppm.
3.2. Analytical Methods
3.3. Preparation of Sweet Mashes
- −
- mashing with malt enzymes—0.6 kg of barley grain and 0.6 kg of Munich malt type 2 grain was ground and mixed (1:1) with water (3.5 L per 1 kg). The mixture was continuously stirred by an overhead stirrer (CAT, R50) and heated to 53–56 °C. The mash was kept at this temperature for 60 min to conduct starch liquefaction and saccharification (pH was kept at 5.3), and then cooled down to 30 °C.
- −
- mashing with enzyme preparations—1.2 kg of barley grain was ground and mixed with water (3.5 L per 1 kg) previously heated to 50 °C. The mixture was continuously stirred by an overhead stirrer and heated to 90 °C, and then treated with the liquefying Termamyl S.C. preparation. The mixture was kept for 60 min at this temperature (pH was kept at 5.5), then cooled to 65 °C and treated with the saccharifying SAN Extra preparation. Directly after the addition of SAN Extra, the mash was cooled down to 30 °C.
- −
- The steamed mass was continuously stirred by an overhead stirrer and cooled down to 53–56 °C. At the same time, barley Munich malt type 2 was ground and mixed with warm water (heated to 53–56 °C), and the obtained mixture was added to the mashing vessel in a ratio of 1:1 (1 part unmalted grain to 1 part malted grain, w/w). The mixture was kept at 53–56 °C for 60 min to conduct starch liquefaction and saccharification (pH was kept at 5.3), and then cooled to 30 °C.
- −
- The steamed mass was continuously stirred by an overhead stirrer and cooled to 90 °C, then treated with the liquefying Termamyl S.C. preparation. The mixture was kept for 60 min at this temperature (pH was kept at 5.5), then cooled down to 65 °C and treated with the saccharifying SAN Extra preparation. Immediately after the addition of SAN Extra, the mash was cooled down to 30 °C.
3.4. Fermentation Process
3.5. Distillation
3.6. Calculations
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- European Union (EU). Council Regulation (EC) No 834/2007 of 28 June 2007 on Organic Production and Labelling of Organic Products and Repealing Regulation (EEC) No 2092/91; The Publications Office of the European Union: Luxembourg, 2007; p. L189/1. [Google Scholar]
- European Union (EU). Regulation (EC) No 110/2008 of the European Parliament and of the Council of 15 January 2008 on the Definition, Description, Presentation, Labelling and the Protection of Geographical Indications of Spirit Drinks and Repealing Council Regulation (EEC) No 1576/89; The Publications Office of the European Union: Luxembourg, 2008. [Google Scholar]
- De Souza, P.M.; de Oliveira, M.P. Application of microbial α-amylase in industry—A review. Braz. J. Microbiol. 2010, 41, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Gurung, N.; Ray, S.; Bose, S.; Rai, V. A broader view: Microbial enzymes and their relevance in industries, medicine, and beyond. BioMed Res. Int. 2013, 2013. Article ID 329121. [Google Scholar] [CrossRef]
- Muller, R. The effects of mashing temperature and mash thickness on wort carbohydrate composition. J. Inst. Brew. 1991, 97, 85–92. [Google Scholar] [CrossRef]
- Donga, L.; Piaoc, Y.; Zhangd, X.; Zhaob, C.; Houb, Y.; Shia, Z. Analysis of volatile compounds from a malting process using headspace solid-phase micro-extraction and GC–MS. Food Res. Int. 2013, 51, 783–789. [Google Scholar] [CrossRef]
- Skinner, K.A.; Leathers, T.D. Bacterial contaminants of fuel ethanol production. J. Ind. Microbiol. Biotechnol. 2004, 31, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Broda, M.; Grajek, W. Microbial contaminations during bioethanol production. Sci. Tech. Mag. Ferment. Fruit Veg. Ind. 2009, 7-8, 58–60. [Google Scholar]
- Beckner, M.; Ivey, M.L.; Phister, T.G. Microbial contamination of fuel ethanol fermentations. Lett. Appl. Microbiol. 2011, 53, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Muthaiyan, A.; Limayem, A.; Rücke, S. Antimicrobial strategies for limiting bacterial contaminants in fuel bioethanol fermentations. Prog. Energy Combust. Sci. 2011, 37, 351–370. [Google Scholar] [CrossRef]
- Broda, M.; Leja, K. The microbiological situation of distilleries in Poland. Pol. J. Environ. Stud. 2010, 19, 901–906. [Google Scholar]
- O’Sullivan, T.F.; Walsh, Y.; O’Mahonyi, A.; Fitzgerald, G.F.; van Sinderen, D. A comparative study of malthouse and brewhouse microflora. J. Inst. Brew. 1999, 105, 55–61. [Google Scholar] [CrossRef]
- Narendranath, N.V. Bacterial contamination and control in ethanol production. In The Alcohol Textbook, 4th ed.; Jacques, K.A., Lyons, T.P., Kelsall, D.R., Eds.; Nottingham University: Nottingham, UK, 2003; Volume 20, pp. 287–298. ISBN 1-897676-13-1. [Google Scholar]
- Narendranath, N.V.; Power, R. Relationship between pH and medium dissolved solids in terms of growth and metabolism of Lactobacilli and Saccharomyces cerevisiae during ethanol production. Appl. Environ. Microbiol. 2005, 71, 2239–2243. [Google Scholar] [CrossRef] [PubMed]
- Sakamotoa, K.; Konings, W.N. Beer spoilage bacteria and hop resistance. Int. J. Food Microbiol. 2003, 89, 105–124. [Google Scholar] [CrossRef]
- Faour, S. Bacterial Inhibition without Antibiotics. Ethanol Producer Magazine, 2012. Available online: http://www.ethanolproducer.com/articles/9014/bacterial-inhibition-without-antibiotics (accessed on 12 June 2017).
- Rückle, L.; Senn, T. Hop acids can efficiently replace antibiotics in ethanol production. Int. Sugar J. 2006, 108, 139–147. [Google Scholar]
- Blümelhuber, G. Cereals, malts and hops. Brauwelt Int. 2012, 2, 75–83. [Google Scholar]
- Pyler, R.E.; Thomas, D.A. Malted cereals: Their production and use. In Handbook of Cereal Science and Technology, 2nd ed.; Kulp, K., Ponte, J.G., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2000; Volume 22, pp. 688–691. ISBN 9780824782948. [Google Scholar]
- Briggs, D.E.; Boulton, C.A.; Brookes, P.A.; Stevens, R. Brewing: Science and Practice; Woodhead Publishing Limited: Cambridge, UK, 2004; p. 28. ISBN 9780849325472. [Google Scholar]
- Montanuci, F.D.; de Matos Jorge, L.M.; Matos Jorge, R.M. Kinetic, thermodynamic properties, and optimization of barley hydration. Food Sci. Technol. 2013, 33. No. 4. [Google Scholar] [CrossRef]
- Baks, T.; Bruins, M.E.; Matser, A.M.; Janssen, A.E.M.; Boom, R.M. Effect of gelatinization and hydrolysis conditions on the selectivity of starch hydrolysis with α-amylase from Bacillus licheniformis. J. Agric. Food Chem. 2008, 56, 488–495. [Google Scholar] [CrossRef]
- Bao, J. The functionality of rice starch. In Starch in Food: Structure, Function and Applications; Eliasson, A.C., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2004; Volume 9, p. 282. ISBN 978-1-85573-731-0. [Google Scholar]
- Balcerek, M.; Pielech-Przybylska, K.; Strąk, E.; Patelski, P.; Dziekońska, U. Comparison of fermentation results and quality of the agricultural distillates obtained by application of commercial amylolytic preparations and cereal malts. Eur. Food Res. Technol. 2016, 242, 321–335. [Google Scholar] [CrossRef]
- Narendranath, N.V.; Thomas, K.C.; Ingledew, W.M. Effects of acetic acid and lactic acid on the growth of Saccharomyces cerevisiae in a minimal medium. J. Ind. Microbiol. Biotechnol. 2001, 26, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.C.; Hynes, S.H.; Ingledew, W.M. Effect of lactobacilli on yeast growth, viability and batch and semi-continuous alcoholic fermentation of corn mash. J. Appl. Microbiol. 2001, 90, 819–828. [Google Scholar] [CrossRef] [PubMed]
- De Giori, G.; de Valdez, G.; de Ruiz Holgado, A.; Oliver, G. Effect of pH and temperature on the proteolytic activity of lactic acid bacteria. J. Diary Sci. 1985, 68, 2160–2164. [Google Scholar] [CrossRef]
- Jurkowski, M.; Błaszczyk, M. Physiology and biochemistry of lactic acid bacteria. Kosmos 2012, 61, 493–504. [Google Scholar]
- Van Cleemput, M.; Cattoor, K.; de Bosscher, K.; Haegeman, G.; de Keukeleire, D.; Heyerick, A. Hop (Humulus lupulus)-derived bitter acids as multipotent bioactive compounds. J. Nat. Prod. 2009, 72, 1220–1230. [Google Scholar] [CrossRef] [PubMed]
- Buśko, M.; Jeleń, H.; Góral, T.; Chmielewski, J.; Stuper, K.; Szwajkowska-Michałek, L.; Tyrakowska, B.; Perkowski, J. Volatile metabolites in various cereal grains. Food Addit. Contam. 2010, 27, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Dragone, G.; Mussatto, S.I.; Oliveira, J.M.; Teixeira, J.A. Characterisation of volatile compounds in an alcoholic beverage produced by whey fermentation. Food Chem. 2009, 112, 929–935. [Google Scholar] [CrossRef] [Green Version]
- Russell, I. Understanding yeast fundamentals. In The Alcohol Textbook, 4th ed.; Jacques, K.A., Lyons, T.P., Kelsall, D.R., Eds.; Nottingham University: Nottingham, UK, 2003; Volume 9, pp. 85–119. ISBN 1-897676-13-1. [Google Scholar]
- Nykänen, L.; Nykänen, I. Distilled beverages. In Volatile Compounds in Foods and Beverages; Maarse, H., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1991; Volume 16, pp. 548–552. ISBN 0-8247-8390-5. [Google Scholar]
- Hofmann, T.; Schieberle, P. Thermal processing: More than extending the shelf life of foods. In Thermal Processing of Food: Potential Health Benefits and Risks; Eisenbrand, G., Engel, K.H., Grunow, W., Hartwig, A., Knorr, D., Knudsen, I., Schlatter, J., Schreier, P., Steinberg, P., Vieths, S., Eds.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; Volume 4, p. 35. ISBN 9783527319091. [Google Scholar]
- De Clippeleer, J.; de Rouck, G.; de Cooman, L.; Aerts, G. Influence of the hopping technology on the storage-induced appearance of staling aldehydes in beer. J. Inst. Brew. 2010, 116, 381–398. [Google Scholar] [CrossRef]
- Berger, R.G. Application of genetic methods to the generation of volatile flavors. In Food Biotechnology: Microorganisms; Hui, Y.H., Khachatourians, G.G., Eds.; WILEY-VCH, Inc.: Hoboken, NJ, USA, 1995; Volume No. 7, p. 286. ISBN 978-0-471-18570-3. [Google Scholar]
- Barbour, E.A.; Priest, F.G. Some effects of Lactobacillus contamination in Scotch whisky fermentations. J. Inst. Brew. 1988, 94, 89–92. [Google Scholar] [CrossRef]
- Lyons, T.P. Production of scotch and irish whiskies: Their history and evolution. In Alcohol Textbook, 4th ed.; Jacques, K.A., Lyons, T.P., Kelsall, D.R., Eds.; Alltech Inc.: Nicholasville, KY, USA, 2003; Volume 14, pp. 193–206. ISBN 1-897676-13-1. [Google Scholar]
- Polish Committee for Standardization. Polish Standard PN-A-79523:2002. Agricultural Distillate; Polish Committee for Standardization: Warsaw, Poland, 2002; pp. 1–7. [Google Scholar]
- Balcerek, M.; Pielech-Przybylska, K.; Dziekońska-Kubczak, U.; Patelski, P.; Strąk, E. Fermentation results and chemical composition of agricultural distillates obtained from rye and barley grains and the corresponding malts as a source of amylolytic enzymes and starch. Molecules 2016, 21, 1320. [Google Scholar] [CrossRef] [PubMed]
- Kłosowski, G.; Mikulski, D.; Macko, D.; Miklaszewska, B.; Kotarska, K.; Czupryński, B. Influence of various yeast strains and selected starchy raw materials on production of higher alcohols during the alcoholic fermentation process. Eur. Food Res. Technol. 2015, 240, 233–242. [Google Scholar] [CrossRef]
- Adam, L.; Versini, G.A. Study on the Possibilities to Lower the Content of Methyl-Alcohol in Eaux-de-vie de Fruits; European Commission: Brussels, Belgium, 1996; pp. 1–8. ISBN 92-827-7208-X. [Google Scholar]
- British Standards Institution. BS EN ISO 10520:1998. In Native Starch. Determination of Starch Content. Ewers Polarimetric Method; British Standards Institution: London, UK, 1998. [Google Scholar]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Official Methods of Analysis of AOAC International, 16th ed.; Method 960.52; AOAC International: Rockville, MD, USA, 1995.
- International Organization for Standardization. ISO 21527-1:2008. Microbiology of Food and Animal Feeding Stuffs. Horizontal Method for the Enumeration of Yeasts and Moulds. Part 1: Colony Count Technique in Products with Water Activity Greater than 0.95; International Organization for Standardization: Geneva, Switzerland, 2008. [Google Scholar]
- International Organization for Standardization. ISO 4833:2004. Microbiology of Food and Animal Feeding Stuffs. Horizontal Method for the Enumeration of Microorganisms. Colony-Count Technique at 30 Degrees C; International Organization for Standardization: Geneva, Switzerland, 2003. [Google Scholar]
- International Organization for Standardization. ISO 6887-1:1999. Microbiology of Food and Animal Feeding Stuffs—Preparation of Test Samples, Initial Suspension and Decimal Dilutions for Microbiological Examination; International Organization for Standardization: Geneva, Switzerland, 1999. [Google Scholar]
- Chida, M.; Sone, Y.; Tamur, H. Aroma characteristics of stored tobacco cut leaves analyzed by a high vacuum distillation and canister system. J. Agric. Food. Chem. 2004, 52, 7918–7924. [Google Scholar] [CrossRef] [PubMed]
- Mahattanatawee, K.; Goodner, K.L.; Baldwin, E.A. Volatile constituents and character impact compounds of selected Florida’s tropical fruit. Proc. Fla. State Hort. Soc. 2005, 118, 414–418. [Google Scholar]
- Van den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A. 1963, 11, 463–471. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| Components | Content | |
|---|---|---|
| Barley Variety Karakan | Barley Munich Malt Type 2 | |
| Moisture (g/kg) | 113.20 ± 3.61b | 43.40 ± 1.42a |
| Reducing sugars (g/kg) | 71.70 ± 2.40a | 184.30 ± 8.43b |
| Total sugars (g/kg) | 682.71 ± 28.42a | 736.07 ± 25.51a |
| Starch (g/kg) | 549.90 ± 17.71a | 496.52 ± 15.20a |
| Protein (g/kg d.w.) | 97.04 ± 5.13a | 95.60 ± 3.33a |
| Method of Starch Liberation | Source of Amylolytic Enzymes | Extract (g/kg) | pH | Sugars (g/L) | Total Sugars (Glucose) (g/L) | Acids (g/L) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Glucose | Maltose | Maltotriose | Dextrins | Lactic Acid | Acetic Acid | |||||
| PLS | Barley Munich malt type 2 | 190.64 ± 9.51b | 4.8 ± 0.1a | 4.06 ± 0.14a | 31.26 ± 1.17c | 4.88 ± 0.18a | 91.12 ± 2.83b | 164.70 ± 6.29b | 0.04 ± 0.00a | 0.08 ± 0.00b |
| Termamyl S.C. SanExtra | 172.18 ± 7.25a | 4.8 ± 0.1a | 7.64 ± 0.26b | 16.25 ± 0.61b | 20.44 ± 0.72c | 102.84 ± 3.19c | 152.20 ± 3.97a | ND | 0.04 ± 0.00a | |
| Pressure-thermal | Barley Munich malt type 2 | 191.19 ± 11.05b | 4.8 ± 0.1a | 10.88 ± 0.37c | 41.23 ± 1.54d | 7.96 ± 0.29b | 66.13 ± 2.05a | 162.70 ± 4.90b | 0.05 ± 0.00a | 0.12 ± 0.00b |
| Termamyl S.C. SanExtra | 175.89 ± 10.08a | 4.8 ± 0.1a | 35.14 ± 1.20d | 9.68 ± 0.31a | 21.22 ± 0.76c | 87.18 ± 2.82b | 150.40 ± 3.69a | ND | 0.16 ± 0.00c | |
| Method of Starch Liberation | Source of Amylolytic Enzymes/Addition of Hop α-Acids (+ or − *) | Extract (g/kg) | pH | Sugars (g/L) | Total Sugars (Glucose) (g/L) | Acids (g/L) | Ethyl Alcohol (g/L) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucose | Maltose | Maltotriose | Dextrins | Lactic Acid | Acetic Acid | ||||||
| PLS | Barley Munich malt type 2/− | 42.10 ± 1.25f | 3.4 ± 0.0a | 0.62 ± 0.02f | 3.20 ± 0.12c | 12.17 ± 0.44e | 5.49 ± 0.17b | 28.80 ± 0.49ef | 7.10 ± 0.21d | 0.62 ± 0.01e | 53.30 ± 1.82a |
| Barley Munich malt type 2/+ | 29.41 ± 1.09d | 4.1 ± 0.1d | 0.42 ± 0.01e | 3.04 ± 0.11c | 4.80 ± 0.18c | 2.97 ± 0.09a | 9.50 ± 0.36b | 0.26 ± 0.01ab | 0.22 ± 0.01bc | 66.40 ± 2.43d | |
| Termamyl S.C. SanExtra/− | 34.64 ± 1.12e | 3.5 ± 0.1a | 0.04 ± 0.00a | 0.38 ± 0.01b | 1.00 ± 0.04b | 9.48 ± 0.29d | 21.30 ± 0.31cd | 0.43 ± 0.01b | 0.24 ± 0.01c | 59.18 ± 2.19bc | |
| Termamyl S.C. SanExtra/+ | 31.05 ± 0.97d | 3.6 ± 0.1a | 0.04 ± 0.00a | 0.40 ± 0.01b | 0.96 ± 0.03b | 9.99 ± 0.31d | 12.10 ± 0.46de | 0.19 ± 0.01ab | 0.18 ± 0.00b | 63.28 ± 2.36c | |
| Pressure-thermal | Barley Munich malt type 2/− | 22.34 ± 0.86c | 3.3 ± 0.1a | 0.08 ± 0.00b | 0.18 ± 0.01a | 6.34 ± 0.23d | 11.95 ± 0.37e | 17.30 ± 0.52e | 9.58 ± 0.25e | 1.72 ± 0.05f | 57.72 ± 1.63ab |
| Barley Munich malt type 2/+ | 14.16 ± 0.54b | 3.9 ± 0.1c | 0.09 ± 0.00b | 0.16 ± 0.01a | 5.10 ± 0.18c | 6.49 ± 0.20c | 7.60 ± 0.25a | 1.56 ± 0.05c | 0.34 ± 0.01d | 66.60 ± 2.94d | |
| Termamyl S.C. SanExtra/− | 23.52 ± 1.04c | 3.7 ± 0.1bc | 0.24 ± 0.01d | 0.16 ± 0.01a | 0.28 ± 0.01a | 14.54 ± 0.45e | 16.70 ± 0.41c | 0.16 ± 0.00ab | 0.19 ± 0.01bc | 59.26 ± 2.53bc | |
| Termamyl S.C. SanExtra/+ | 12.37 ± 0.75a | 3.8 ± 0.1bc | 0.19 ± 0.01c | 0.12 ± 0.00a | 0.22 ± 0.01a | 5.83 ± 0.18bc | 6.90 ± 0.22a | 0.04 ± 0.00a | 0.12 ± 0.00a | 66.71 ± 2.27d | |
| Method of Starch Liberation | Source of Amylolytic Enzymes/Addition of Hop α-Acids (+ or − *) | Time of Fermentation (h) | Yeast (Y) Count log (cfu/mL) | Lactic Acid Bacteria (LAB) Count log (cfu/mL) | Total Mesophilic Bacteria (TMB) Count log (cfu/mL) |
|---|---|---|---|---|---|
| PLS | Barley Munich malt type 2/− | 0 | 6.85 ± 0.44Ca | 2.00 ± 0.10Ab | 2.72 ± 0.15Ac |
| 24 | 8.41 ± 0.40Da | 8.12 ± 0.46Be | 7.47 ± 0.32Bd | ||
| 48 | 4.18 ± 0.35Ba | 7.82 ± 0.62Bf | 7.32 ± 0.58Bc | ||
| 72 | 2.54 ± 0.25Aa | 7.08 ± 0.63Bc | 7.11 ± 0.63Bc | ||
| Barley Munich malt type 2/+ | 0 | 6.85 ± 0.44Aa | 2.00 ± 0.10Ab | 2.72 ± 0.15Ac | |
| 24 | 8.32 ± 0.41Ba | 3.08 ± 0.15Bc | 2.61 ± 0.22Aa | ||
| 48 | 8.08 ± 0.34Bb | 3.61 ± 0.30Be | 3.85 ± 0.30Bb | ||
| 72 | 8.40 ± 0.25Bd | 3.34 ± 0.33Bc | 3.54 ± 0.31Bb | ||
| Termamyl S.C.; SanExtra/− | 0 | 6.85 ± 0.44Aa | 1.00 ± 0.04Aa | 1.18 ± 0.06Ab | |
| 24 | 8.04 ± 0.90Aa | 3.85 ± 0.22Bd | 4.11 ± 0.22Bb | ||
| 48 | 8.62 ± 0.36Bb | 3.30 ± 0.27Be | 4.26 ± 0.34Bbc | ||
| 72 | 8.98 ± 0.82Bd | 3.04 ± 0.30Bc | 3.58 ± 0.31Bb | ||
| Termamyl S.C.; SanExtra/+ | 0 | 6.85 ± 0.44Aa | 1.00 ± 0.04Aa | 1.18 ± 0.06Ab | |
| 24 | 8.18 ± 0.27Ba | 1.78 ± 0.11Bb | 2.30 ± 0.13Ca | ||
| 48 | 7.95 ± 0.25Bb | <1.00Aa | 1.48 ± 0.11Ba | ||
| 72 | 8.28 ± 0.34Bd | <1.00Aa | 1.30 ± 0.11ABa | ||
| Pressure-thermal | Barley Munich malt type 2/− | 0 | 6.85 ± 0.44Aa | 2.00 ± 0.12Ab | 2.46 ± 0.12Ac |
| 24 | 8.53 ± 0.70Ba | 4.34 ± 0.26Bd | 5.11 ± 0.26Bc | ||
| 48 | 7.46 ± 0.52ABb | 6.63 ± 0.55Cf | 6.71 ± 0.53Cd | ||
| 72 | 6.15 ± 0.47Abc | 7.18 ± 0.71Cd | 7.87 ± 0.69Cc | ||
| Barley Munich malt type 2/+ | 0 | 6.85 ± 0.44Aa | 2.00 ± 0.12Cb | 2.46 ± 0.12Cc | |
| 24 | 8.56 ± 0.47Ba | 1.12 ± 0.05Ba | 2.15 ± 0.12BCa | ||
| 48 | 8.62 ± 0.40Bb | 1.48 ± 0.12Bbc | 1.90 ± 0.15ABa | ||
| 72 | 8.48 ± 0.42Bd | <1.00Aa | 1.70 ± 0.15ABa | ||
| Termamyl S.C.; SanExtra/− | 0 | 6.85 ± 0.40Aa | <1.00Aa | <1.00Aa | |
| 24 | 7.20 ± 0.27Aa | 1.00 ± 0.05Aa | 4.40 ± 0.24Cb | ||
| 48 | 8.18 ± 0.27Bb | 2.40 ± 0.20Bd | 3.32 ± 0.21Bb | ||
| 72 | 7.95 ± 0.37Bcd | 3.18 ± 0.31Cc | 3.30 ± 0.29Bb | ||
| Termamyl S.C.; SanExtra/+ | 0 | 6.85 ± 0.49Aa | <1.00Aa | <1.00Aa | |
| 24 | 8.43 ± 0.20Ba | <1.00Aa | 2.11 ± 0.13Ca | ||
| 48 | 8.34 ± 0.19Bb | 1.30 ± 0.11Bb | 1.85 ± 0.15BCa | ||
| 72 | 8.04 ± 0.22Bcd | 1.18 ± 0.12Bb | 1.60 ± 0.13Ba |
| Method of Starch Liberation | Source of Amylolytic Enzymes/Addition of Hop α-Acids (+ or − *) | Intake of Total Sugars (%) | Fermentation Efficiency (% of Theoretical) |
|---|---|---|---|
| PLS | Barley Munich malt type 2/− | 92.23 ± 2.77a | 63.32 ± 2.16a |
| Barley Munich malt type 2/+ | 94.23 ± 2.84a | 78.88 ± 2.69bc | |
| Termamyl S.C.; SanExtra/− | 92.58 ± 3.21a | 76.08 ± 2.59bc | |
| Termamyl S.C.; SanExtra/+ | 92.05 ± 3.78a | 81.35 ± 2.77d | |
| Pressure-thermal | Barley Munich malt type 2/− | 91.58 ± 3.29a | 69.41 ± 2.37a |
| Barley Munich malt type 2/+ | 95.33 ± 4.21a | 80.09 ± 3.53cd | |
| Termamyl S.C.; SanExtra/− | 93.07 ± 3.45a | 75.09 ± 3.20bc | |
| Termamyl S.C.; SanExtra/+ | 95.53 ± 3.21a | 84.53 ± 2.88d |
| Variable | Microorganisms log (cfu/mL) | Significance of the Model and Discrimination | Wilks’ Lambda * | Wilks’ Part. * | F = 1.92 * | p * |
|---|---|---|---|---|---|---|
| Method of starch liberation | Y | Wilks’ Lambda: 0.89085 F(3.92) = 3.7573 p < 0.0135 | 0.900 | 0.990 | 0.902 | 0.345 |
| LAB | 0.979 | 0.910 | 9.134 | 0.003 | ||
| TMB | 0.950 | 0.937 | 6.138 | 0.015 | ||
| Source of amylolytic enzyme | Y | Wilks’ Lambda: 0.01323 F(45.232) = 16.992 p < 0.0135 | 0.036 | 0.365 | 9.035 | <0.01 |
| LAB | 0.026 | 0.505 | 5.100 | <0.01 | ||
| TMB | 0.056 | 0.238 | 16.656 | <0.01 | ||
| Time of fermentation | Y | Wilks’ Lambda: 0.525 F (9.219) = 7.3776 p < 0.01 | 0.807 | 0.650 | 16.121 | <0.01 |
| LAB | 0.532 | 0.988 | 0.374 | 0.772 | ||
| TMB | 0.597 | 0.880 | 4.108 | 0.009 | ||
| Method of starch liberation × Source of amylolytic enzymes | Y | Wilks’ Lambda: 0.21241 F(21.247) = 8.4290 p < 0.01 | 0.292 | 0.726 | 4.629 | <0.01 |
| LAB | 0.341 | 0.623 | 7.436 | <0.01 | ||
| TMB | 0.274 | 0.775 | 3.573 | 0.002 | ||
| Method of starch liberation × Time of fermentation | Y | Wilks’ Lambda: 0.31851 F(21.247) = 5.7690 p < 0.01 | 0.504 | 0.631 | 7.170 | <0.01 |
| LAB | 0.503 | 0.633 | 7.118 | <0.01 | ||
| TMB | 0.511 | 0.623 | 7.425 | <0.01 | ||
| Source of amylolytic enzymes × Time of fermentation | Y | Wilks’ Lambda: 0.01323 F(45.232) = 16.992 p < 0.01 | 0.036 | 0.365 | 9.035 | <0.01 |
| LAB | 0.026 | 0.505 | 5.100 | <0.01 | ||
| TMB | 0.056 | 0.238 | 16.656 | <0.01 | ||
| Method of starch liberation × Source of amylolytic enzymes × Time of fermentation | Y | Wilks’ Lambda: 0.00001 F(93.186) = 114.75 p < 0.01 | 0.000 | 0.074 | 24.856 | <0.01 |
| LAB | 0.001 | 0.004 | 554.449 | <0.01 | ||
| TMB | 0.001 | 0.004 | 505.327 | <0.01 |
| Volatile Compounds | Method of Starch Liberation & Source of Enzymes | |||||||
|---|---|---|---|---|---|---|---|---|
| PLS | Pressure-thermal | |||||||
| Enzymes: Munich Malt Type 2 without Addition of Hop α-Acids | Enzymes: Munich Malt Type 2 with Addition of Hop α-Acids | Enzymes: Termamyl S.C.; SanExtra without Addition of Hop α-Acids | Enzymes: Termamyl S.C.; SanExtra with Addition of Hop α-Acids | Enzymes: Munich Malt Type 2 without Addition of Hop α-Acids | Enzymes: Munich Malt Type 2 with Addition of Hop α-Acids | Enzymes: Termamyl S.C.; SanExtra without Addition of Hop α-Acids | Enzymes: Termamyl S.C.; SanExtra with Addition of Hop α-Acids | |
| CARBONYL COMPOUNDS | (mg/L of absolute alcohol) | |||||||
| Acetaldehyde | 34.247 ± 0.982e | 12.842 ± 0.357b | 41.952 ± 1.187f | 34.247 ± 0.997e | 17.123 ± 0.511c | 10.098 ± 0.239a | 29.966 ± 0.758d | 15.103 ± 0.444bc |
| Furfural | 225.807 ± 6.475e | 79.700 ± 2.213b | 43.532 ± 1.231a | 49.774 ± 1.448a | 271.249 ± 8.090f | 120.366 ± 2.851d | 95.501 ± 2.416c | 50.400 ± 1.482a |
| Isobutyraldehyde | 4.214 ± 0.121d | 2.536 ± 0.070b | 3.567 ± 0.101c | 1.866 ± 0.054a | 6.233 ± 0.186f | 2.107 ± 0.050a | 5.611 ± 0.142e | 1.754 ± 0.052a |
| Isovaleraldehyde | 14.632 ± 0.420c | 10.302 ± 0.286b | ND | ND | 61.866 ± 1.845e | 40.214 ± 0.952d | ND | ND |
| 2-Methylbutyraldehyde | 5.348 ± 0.153c | 3.268 ± 0.091b | 8.211 ± 0.232d | 5.765 ± 0.168c | 21.898 ± 0.653f | 9.412 ± 0.223e | 3.416 ± 0.086b | 1.997 ± 0.059a |
| Phenylacetaldehyde | 6.766 ± 0.194 | 3.819 ± 0.106b | ND | ND | 11.322 ± 0.338e | 7.895 ± 0.187d | ND | ND |
| 2,3-Butanedione | 8.416 ± 0.241c | 6.188 ± 0.172ab | 53.537 ± 1.514e | 40.783 ± 1.187d | 6.722 ± 0.200ab | 3.455 ± 0.082a | 64.029 ± 1.620f | 30.841 ± 0.907c |
| ACETALS | (mg/L of absolute alcohol) | |||||||
| Acetaldehyde diethyl acetal | 70.334 ± 2.017a | 63.823 ± 1.772a | 214.836 ± 6.077e | 176.780 ± 5.145d | 112.472 ± 3.355b | 101.720 ± 2.409b | 154.424 ± 3.907c | 109.086 ± 3.207b |
| Isobutyraldehyde diethyl acetal | 1.872 ± 0.054e | 0.917 ± 0.025b | 3.487 ± 0.099d | 3.494 ± 0.102d | 6.153 ± 0.184f | 1.296 ± 0.031c | ND | ND |
| Isovaleraldehyde diethyl acetal | 4.276 ± 0.123b | 4.233 ± 0.118b | ND | ND | 9.915 ± 0.296c | ND | ND | ND |
| ESTERS | (mg/L of absolute alcohol) | |||||||
| Ethyl acetate | 502.371 ± 14.405c | 498.964 ± 13.854c | 282.430 ± 7.989a | 306.258 ± 8.913a | 953.045 ± 28.425d | 962.153 ± 22.787d | 417.976 ± 10.576b | 410.906 ± 12.080b |
| Isoamyl acetate | 3.448 ± 0.099e | 3.792 ± 0.105d | 3.618 ± 0.102c | 3.822 ± 0.111c | 2.983 ± 0.089c | 2.912 ± 0.069b | 2.811 ± 0.071b | 2.735 ± 0.080a |
| Ethyl hexanoate | 2.916 ± 0.084b | 3.038 ± 0.084b | 2.234 ± 0.063a | 1.977 ± 0.058a | 2.035 ± 0.061a | 1.989 ± 0.047a | 6.205 ± 0.157d | 5.289 ± 0.155c |
| Ethyl octanoate | 11.512 ± 0.330d | 10.616 ± 0.295b | 8.393 ± 0.237c | 7.627 ± 0.222b | 7.907 ± 0.236a | 6.746 ± 0.160a | 6.349 ± 0.161b | 5.103 ± 0.150b |
| Ethyl decanoate | 1.708 ± 0.049b | 1.725 ± 0.048b | 1.399 ± 0.040a | 1.356 ± 0.039a | 2.022 ± 0.060c | 1.921 ± 0.045c | 1.334 ± 0.034a | 1.256 ± 0.037a |
| Ethyl hexadecanoate | 8.642 ± 0.248d | 3.669 ± 0.102b | 6.202 ± 0.175c | 3.909 ± 0.114b | 1.179 ± 0.035a | 1.202 ± 0.028a | 3.535 ± 0.089b | 3.518 ± 0.103b |
| 2-Phenylethyl isobutyrate | 2.178 ± 0.062d | 2.055 ± 0.057d | 1.527 ± 0.043b | 1.481 ± 0.043b | 2.044 ± 0.061d | 1.879 ± 0.045c | 1.153 ± 0.029a | 1.088 ± 0.032a |
| Ethyl 2-hydroxypropanoate | 96.540 ± 2.768c | ND | ND | ND | 140.889 ± 4.202d | ND | 4.028 ± 0.102b | ND |
| ALCOHOLS | (mg/L of absolute alcohol) | |||||||
| 1-Propanol | 1065.428 ± 30.551b | 1416.376 ± 39.326c | 674.938 ± 19.093a | 589.248 ± 17.148a | 10,536.890 ± 314.267d | 10,521.101 ± 249.172d | 1648.119 ± 41.702c | 1625.719 ± 47.792c |
| 2-Methyl-1-propanol | 5287.157 ± 151.606b | 5626.924 ± 156.231b | 10,590.344 ± 299.582d | 10,111.506 ± 294.259d | 3220.649 ± 96.057a | 3204.551 ± 75.894a | 7071.816 ± 178.935c | 7013.669 ± 206.185c |
| 1-Butanol | 34.869 ± 1.000c | 39.943 ± 1.109d | 15.869 ± 0.449b | 12.614 ± 0.367a | 30.819 ± 0.919b | 30.623 ± 0.725b | 36.664 ± 0.928c | 36.702 ± 1.079c |
| 3-Methylbutanol | 6936.398 ± 198.897b | 7324.607 ± 203.367b | 12,883.438 ± 364.449d | 13,562.804 ± 394.696d | 4980.179 ± 148.536a | 4892.402 ± 115.867a | 11,712.660 ± 296.361c | 11,710.791 ± 344.270c |
| 2-Methylbutanol | 3398.718 ± 97.456b | 3634.696 ± 100.917b | 5058.071 ± 143.084c | 5199.437 ± 151.311c | 1926.010 ± 57.444a | 1900.361 ± 45.006a | 4969.325 ± 125.737c | 4971.144 ± 146.140c |
| 2-Phenylethanol | 803.119 ± 23.029b | 941.135 ± 26.131b | 2603.190 ± 73.640d | 2629.700 ± 76.528d | 266.199 ± 7.939a | 262.343 ± 6.213a | 1150.156 ± 29.102c | 1117.587 ± 32.854c |
| Methanol | 57.906 ± 1.660b | 55.679 ± 1.546b | 35.635 ± 1.008a | 33.408 ± 0.972a | 80.178 ± 2.391d | 80.185 ± 1.899d | 73.497 ± 1.860c | 73.483 ± 2.160c |
| PCA Factor | Own Value | % of Variance | The Cumulated Own Value | Cumulative % |
|---|---|---|---|---|
| PCA1 | 13.50 | 53.99 | 13.50 | 53.99 |
| PCA2 | 4.89 | 19.56 | 18.39 | 73.56 |
| PCA3 | 3.14 | 12.57 | 21.53 | 86.13 |
| PCA4 | 2.35 | 9.40 | 23.88 | 95.53 |
| Compound | PCA1 | PCA2 | PCA3 | PCA4 |
|---|---|---|---|---|
| Acetaldehyde | −0.701 | 0.319 | −0.501 | 0.248 |
| Furfural | 0.545 | −0.068 | −0.034 | 0.810 |
| Isobutyraldehyde | −0.083 | −0.150 | 0.063 | 0.931 |
| Isovaleraldehyde | 0.721 | 0.210 | 0.415 | 0.513 |
| 2-Methylbutyraldehyde | 0.370 | 0.486 | 0.371 | 0.688 |
| Phenylacetaldehyde | 0.833 | 0.096 | 0.134 | 0.519 |
| 2,3-Butanedione | −0.971 | −0.001 | 0.085 | −0.035 |
| Acetaldehyde diethyl acetal | −0.793 | 0.544 | 0.182 | 0.009 |
| Isobutyraldehyde diethyl acetal | 0.131 | 0.761 | 0.035 | 0.596 |
| Isovaleraldehyde diethyl acetal | 0.547 | 0.057 | −0.105 | 0.717 |
| Ethyl acetate | 0.780 | 0.041 | 0.519 | 0.317 |
| Isoamyl acetate | −0.002 | 0.547 | −0.755 | −0.230 |
| Ethyl hexanoate | −0.493 | −0.843 | 0.176 | −0.041 |
| Ethyl octanoate | 0.400 | 0.101 | −0.869 | 0.202 |
| Ethyl decanoate | 0.887 | 0.178 | 0.101 | 0.377 |
| Ethyl hexadecanoate | −0.305 | −0.099 | −0.856 | 0.081 |
| 2-Phenylethyl isobutyrate | 0.818 | 0.225 | −0.419 | 0.299 |
| Ethyl 2-hydroxypropionate | 0.386 | 0.042 | −0.098 | 0.874 |
| 1-Propanol | 0.643 | 0.183 | 0.664 | 0.306 |
| 2-Methyl−1-propanol | −0.834 | 0.343 | −0.301 | −0.300 |
| 1-Butanol | 0.420 | −0.881 | 0.033 | 0.079 |
| 3-Methylbutanol | −0.927 | 0.113 | −0.107 | −0.307 |
| 2-Methylbutanol | −0.891 | −0.071 | −0.254 | −0.331 |
| 2-Phenylethanol | −0.744 | 0.507 | −0.316 | −0.292 |
| Methanol | 0.439 | −0.575 | 0.630 | 0.272 |
| Dimension | Compound | Mean | Median | Minimum | Maximum | Standard Deviation |
|---|---|---|---|---|---|---|
| PCA1 | Acetaldehyde | 24.45 | 23.54 | 10.10 | 41.95 | 12.01 |
| Isovaleraldehyde | 15.88 | 5.15 | 0.00 | 61.87 | 23.14 | |
| Phenylacetaldehyde | 3.73 | 1.91 | 0.00 | 11.32 | 4.47 | |
| 2,3-Butanedione | 26.75 | 19.63 | 3.46 | 64.03 | 23.98 | |
| Acetaldehyde diethyl acetal | 125.43 | 110.78 | 63.82 | 214.84 | 52.51 | |
| Ethyl acetate | 541.76 | 458.47 | 282.43 | 962.15 | 268.44 | |
| Ethyl decanoate | 1.59 | 1.55 | 1.26 | 2.02 | 0.29 | |
| 2-Phenylethyl isobutyrate | 1.68 | 1.70 | 1.09 | 2.18 | 0.42 | |
| 1-Propanol | 3509.73 | 1521.05 | 589.25 | 10,536.89 | 4350.33 | |
| 2-Methyl-1-propanol | 6515.83 | 6320.30 | 3204.55 | 10,590.34 | 2782.86 | |
| 3-Methylbutanol | 9250.41 | 9517.70 | 4892.40 | 13,562.80 | 3589.71 | |
| 2-Methylbutanol | 3882.22 | 4302.01 | 1900.36 | 5199.44 | 1390.52 | |
| 2-Phenylethanol | 1221.68 | 1029.36 | 262.34 | 2629.70 | 925.37 | |
| PCA2 | Isobutyraldehyde diethyl acetal | 2.15 | 1.58 | 0.00 | 6.15 | 2.11 |
| Ethyl hexanoate | 3.21 | 2.58 | 1.98 | 6.21 | 1.64 | |
| 1-Butanol | 29.76 | 32.84 | 12.61 | 39.94 | 10.10 | |
| PCA3 | Isoamyl acetate | 3.27 | 3.22 | 2.74 | 3.82 | 0.45 |
| Ethyl octanoate | 8.03 | 7.77 | 5.10 | 11.51 | 2.14 | |
| Ethyl hexadecanoate | 3.98 | 3.60 | 1.18 | 8.64 | 2.47 | |
| Methanol | 61.25 | 65.69 | 33.41 | 80.19 | 18.86 | |
| PCA4 | Furfural | 117.04 | 87.60 | 43.53 | 271.25 | 86.06 |
| Isobutyraldehyde | 3.49 | 3.05 | 1.75 | 6.23 | 1.73 | |
| 2-Methylbutyraldehyde | 7.41 | 5.56 | 2.00 | 21.90 | 6.37 | |
| Isovaleraldehyde diethyl acetal | 2.30 | 0.00 | 0.00 | 9.92 | 3.63 | |
| Ethyl 2-hydroxypropionate | 30.18 | 0.00 | 0.00 | 140.89 | 55.93 |
| Method of Starch Liberation & Saccharification | PCA1 | PCA2 | PCA3 | PCA4 |
|---|---|---|---|---|
| PLS (source of enzymes—malt) Without addition of hop α-acids | 0.146 | 0.176 | 0.451 | 0.163 |
| PLS (source of enzymes—malt) With addition of hop α-acids | 0.047 | 0.001 | 0.695 | 0.097 |
| PLS (source of enzymes—enzyme preparations) Without addition of hop α-acids | 0.661 | 0.264 | 0.052 | 0.000 |
| PLS (source of enzymes—enzyme preparations) With addition of hop α-acids | 0.666 | 0.198 | 0.033 | 0.080 |
| Thermal-pressure (source of enzymes—malt) Without addition of hop α-acids | 0.836 | 0.035 | 0.096 | 0.021 |
| Thermal-pressure (source of enzymes—malt) With addition of hop α-acids | 0.557 | 0.127 | 0.012 | 0.247 |
| Thermal-pressure (source of enzymes—enzyme preparations) Without addition of hop α-acids | 0.308 | 0.347 | 0.039 | 0.260 |
| Thermal-pressure (source of enzymes—enzyme preparations) With addition of hop α-acids | 0.273 | 0.641 | 0.000 | 0.000 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pielech-Przybylska, K.; Balcerek, M.; Nowak, A.; Wojtczak, M.; Czyżowska, A.; Dziekońska-Kubczak, U.; Patelski, P. The Effect of Different Starch Liberation and Saccharification Methods on the Microbial Contaminations of Distillery Mashes, Fermentation Efficiency, and Spirits Quality. Molecules 2017, 22, 1647. https://doi.org/10.3390/molecules22101647
Pielech-Przybylska K, Balcerek M, Nowak A, Wojtczak M, Czyżowska A, Dziekońska-Kubczak U, Patelski P. The Effect of Different Starch Liberation and Saccharification Methods on the Microbial Contaminations of Distillery Mashes, Fermentation Efficiency, and Spirits Quality. Molecules. 2017; 22(10):1647. https://doi.org/10.3390/molecules22101647
Chicago/Turabian StylePielech-Przybylska, Katarzyna, Maria Balcerek, Agnieszka Nowak, Maciej Wojtczak, Agata Czyżowska, Urszula Dziekońska-Kubczak, and Piotr Patelski. 2017. "The Effect of Different Starch Liberation and Saccharification Methods on the Microbial Contaminations of Distillery Mashes, Fermentation Efficiency, and Spirits Quality" Molecules 22, no. 10: 1647. https://doi.org/10.3390/molecules22101647
APA StylePielech-Przybylska, K., Balcerek, M., Nowak, A., Wojtczak, M., Czyżowska, A., Dziekońska-Kubczak, U., & Patelski, P. (2017). The Effect of Different Starch Liberation and Saccharification Methods on the Microbial Contaminations of Distillery Mashes, Fermentation Efficiency, and Spirits Quality. Molecules, 22(10), 1647. https://doi.org/10.3390/molecules22101647