Diallylthiosulfinate (Allicin), a Volatile Antimicrobial from Garlic (Allium sativum), Kills Human Lung Pathogenic Bacteria, Including MDR Strains, as a Vapor
Abstract
:1. Introduction
2. Results
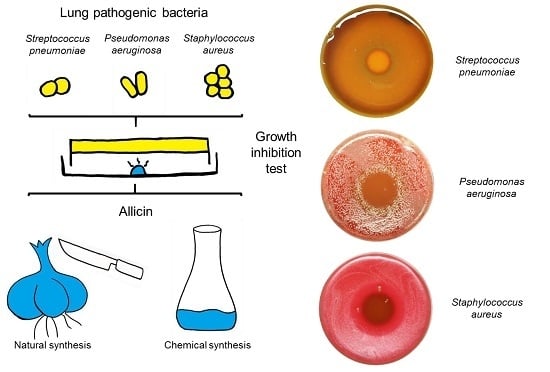
2.1. Allicin Vapor Inhibits Lung-Pathogenic Bacteria
2.2. MIC and MBC
2.3. Cytotoxicity of Allicin to Mammalian Cells
2.4. Cytotoxicity of Allicin to Rat PCLS
3. Discussion
4. Materials and Methods
4.1. Allicin Synthese
4.2. Bacteria
4.3. Antibiotic Activity of Allicin Vapor
4.4. MIC and MBC Determination
4.5. Effect of allicin on Mammalian Cells
4.6. Effect of Allicin on Rat PCLS
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cavallito, C.J.; Bailey, H.J. Allicin, the antibacterial principle of Allium sativum I. Isolation, physical properties and antibacterial action. J. Am. Chem. Soc. 1944, 66, 1950–1951. [Google Scholar] [CrossRef]
- Cavallito, C.J.; Buck, J.S.; Suter, C.M. Allicin, the antibacterial principle of Allium sativum II. Determination of the chemical structure. J. Am. Chem. Soc. 1944, 66, 1952–1954. [Google Scholar] [CrossRef]
- Rabinkov, A.; Miron, T.; Konstantinovski, L.; Wilchek, M.; Mirelman, D.; Weiner, L. The mode of action of allicin: Trapping of radicals and interaction with thiol containing proteins. Biochim. Biophys. Acta 1998, 1379, 233–244. [Google Scholar] [CrossRef]
- Ankri, S.; Mirelman, D. Antimicrobial properties of allicin from garlic. Microbes Infect. 1999, 1, 125–129. [Google Scholar] [CrossRef]
- Arora, D.S.; Kaur, J. Antimicrobial activity of spices. Int. J. Antimicrob. Agents 1999, 12, 257–262. [Google Scholar] [CrossRef]
- Curtis, H.; Noll, U.; Störmann, J.; Slusarenko, A.J. Broad-spectrum activity of the volatile phytoanticipin allicin in extracts of garlic (Allium sativum L.) against plant pathogenic bacteria, fungi and oomycetes. Physiol. Mol. Plant Pathol. 2004, 65, 79–89. [Google Scholar] [CrossRef]
- Block, E. Garlic and the Other Alliums. The Lore and the Science, 1st ed.; RSC Publishing: Cambridge, UK, 2010; ISBN 10:1849731802. [Google Scholar]
- Ilić, D.P.; Nikolić, V.D.; Nikolić, L.B.; Stanković, M.Z.; Stanojević, L.P.; Cakić, M.D. Allicin and related compounds: Biosynthesis, synthesis and pharmacological activity. FU Phys. Chem. Technol. 2011, 9, 9–20. [Google Scholar] [CrossRef]
- Müller, A.; Eller, J.; Albrecht, F.; Prochnow, P.; Kuhlmann, K.; Bandow, J.E.; Slusarenko, A.J.; Leichert, L.I.O. Allicin induces thiol stress in bacteria through S-allylmercapto modification of protein cysteines. J. Biol. Chem. 2016, 291, 11477–11490. [Google Scholar] [CrossRef] [PubMed]
- Giles, G.I.; Tasker, K.M.; Jacob, C. Hypothesis: The role of reactive sulfur species in oxidative stress. Free Radic. Biol. Med. 2001, 31, 1279–1283. [Google Scholar] [CrossRef]
- Giles, G.I.; Jacob, C. Reactive sulfur species: An emerging concept in oxidative stress. Biol. Chem. 2002, 383, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Gruhlke, M.C.H.; Slusarenko, A.J. The biology of reactive sulfur species (RSS). Plant Physiol. Biochem. 2012, 59, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Wills, E.D. Enzyme inhibition by allicin, the active principle of garlic. Biochem. J. 1956, 63, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Rabinkov, A.; Miron, T.; Mirelman, D.; Wilchek, M.; Weiner, L. S-Allylmercaptoglutathione: The reaction product of allicin with glutathione possesses SH-modifying and antioxidant properties. Biochim. Biophys. Acta 2000, 1499, 144–153. [Google Scholar] [CrossRef]
- Gruhlke, M.C.H.; Portz, D.; Stitz, M.; Anwar, A.; Schneider, T.; Jacob, C.; Schlaich, N.L.; Slusarenko, A.J. Allicin disrupts the cell’s electrochemical potential and induces apoptosis in yeast. Free Radic. Biol. Med. 2010, 49, 1916–1924. [Google Scholar] [CrossRef] [PubMed]
- Virtual Computational Chemistry Laboratory. Available online: http://www.vcclab.org (accessed on 23 August 2017).
- Miron, T.; Rabinkov, A.; Mirelman, D.; Wilchek, M.; Weiner, L. The mode of action of allicin: Its ready permeability through phospholipid membranes may contribute to its biological activity. Biochim. Biophys. Acta 2000, 1463, 20–30. [Google Scholar] [CrossRef]
- Gruhlke, M.C.H.; Hemmis, B.; Noll, U.; Wagner, R.; Lühring, H.; Slusarenko, A.J. The defense substance allicin from garlic permeabilizes membranes of Beta vulgaris, Rhoeo discolor, Chara corallina and artificial lipid bilayers. Biochim. Biophys. Acta (BBA) Gen. Subj. 2015, 1850, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Ogita, A.; Fujita, K.I.; Taniguchi, M.; Tanaka, T. Enhancement of the fungicidal activity of amphotericin B by allicin, an allyl-sulfur compound from garlic, against the yeast Saccharomyces cerevisiae as a model system. Planta Med. 2006, 72, 1247–1250. [Google Scholar] [CrossRef] [PubMed]
- Ogita, A.; Nagao, Y.; Fujita, K.; Tanaka, T. Amplification of vacuole targeting fungicidial activity of antibacterial antibiotic polymyxin B by allicin, an allyl sulfur compound from garlic. J. Antibiot. 2007, 60, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Minchin, W.D. A Study in Tubercule Virus Polymorphism, and the Treatment of Tuberculosis and Lupus with Oleum Allii; Bailliere, Tindall and Cox: London, UK, 1927. [Google Scholar]
- Cutler, R.R.; Wilson, P. Antibacterial activity of a new, stable, aqueous extract of allicin against methicillin-resistant Staphylococcus aureus. Br. J. Biomed. Sci. 2004, 61, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Bjarnsholt, T.; Jensen, P.Ø.; Rasmussen, T.B.; Christophersen, L.; Calum, H.; Hentzer, M.; Hougen, H.P.; Rygaard, J.; Moser, C.; Eberl, L.; et al. Garlic blocks quorum sensing and promotes rapid clearing of pulmonary Pseudomonas aeruginosa infections. Microbiol. SGM 2005, 151, 3873–3880. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Wang, R.; Pei, F.; Liang, B.B. Antibacterial activity of allicin alone and in combination with beta-lactams against Staphylococcus spp. and Pseudomonas aeruginosa. J. Antibiot. 2007, 60, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Högberg, L.D.; Heddini, A.; Cars, O. The global need for effective antibiotics: Challenges and recent advances. Trends Pharmacol. Sci. 2010, 31, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. P&T 2015, 40, 277–283. [Google Scholar]
- Powers, J.H. Antimicrobial drug development–the past, the present, and the future. Clin. Microbiol. Infect. 2004, 10, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Silver, L.L. Challenges of antibacterial discovery. Clin. Microbiol. Rev. 2011, 24, 71–109. [Google Scholar] [CrossRef] [PubMed]
- Muto, C.A.; Jernigan, J.A.; Ostrowsky, B.E.; Richet, H.M.; Jarvis, W.R.; Boyce, J.M.; Farr, B.M. SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and Enterococcus. Infect. Control Hosp. Epidemiol. 2003, 24, 362–386. [Google Scholar] [CrossRef] [PubMed]
- Georg, A.M.; Jones, P.M.; Middleton, P.G. Cystic fibrosis infections: Treatment strategies and prospects. FEMS Microbiol. Lett. 2009, 300, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rew. 2010, 74, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Croucher, N.J.; Harris, S.R.; Fraser, C.; Quail, M.A.; Burton, J.; van der Linden, M.; McGee, L.; von Gottberg, A.; Song, J.H.; Ko, K.S.; et al. Rapid pneumococcal evolution in response to clinical interventions. Science 2011, 331, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Dini, C.; Fabbri, A.; Geraci, A. The potential role of garlic (Allium sativum) against the multi-drug resistant tuberculosis pandemic: A review. Ann. Ist. Super. Sanità 2011, 47, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Chen, L. Notes from the field: Pan-resistant New Delhi metallo-β-lactamase-producing Klebsiella pneumoniae—Washoe County, Nevada, 2016. Morb. Mortal. Wkly. Rep. 2017, 66. [Google Scholar] [CrossRef] [PubMed]
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.C.H.; Nwachukwu, I.D.; Slusarenko, A.J. Allicin: Chemistry and biological properties. Molecules 2014, 19, 12591–12618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallock-Richards, D.; Doherty, C.J.; Doherty, L.; Clarke, D.J.; Place, M.; Govan, J.R.; Campopiano, D.J. Garlic revisited: Antimicrobial activity of allicin-containing garlic extracts against Burkholderia cepacia complex. PLoS ONE 2014, 9, e112726. [Google Scholar] [CrossRef]
- Smyth, A.R.; Cifelli, P.M.; Ortori, C.A.; Righetti, K.; Lewis, S.; Erskine, P.; Elaine, D.; Holland, E.D.; Givskov, M.; Williams, P.; et al. Garlic as an inhibitor of Pseudomonas aeruginosa quorum sensing in cystic fibrosis—A pilot randomized controlled trial. Pediatr. Pulm. 2010, 45, 315–416. [Google Scholar] [CrossRef]
- Hess, A.; Wang-Lauenstein, L.; Braun, A.; Kolle, S.N.; Landsiedel, R.; Liebsch, M.; Ma-Hock, L.; Pirow, R.; Schneider, X.; Steinfath, M.; et al. Prevalidation of the ex vivo model PCLS for prediction of respiratory toxicity. Toxicol. In Vitro 2016, 32, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; Morrison, D.; Donaldson MacNee, W. Systemic oxidative stress in asthma, COPD, and smokers. Am. J. Respir. Crit. Care Med. 1996, 154, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I. Inflammation and the regulation of glutathione level in lung epithelial cells. Antioxid. Redox Signal. 1999, 1, 425–447. [Google Scholar] [CrossRef] [PubMed]
- Dammeyer, P.; Arnér, E.S.J. Human Protein Atlas of redox systems—What can be learnt? Biochim. Biophys. Acta (BBA) Gen. Subj. 2011, 1810, 111–138. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press: Oxford, UK, 2015; p. 905. ISBN 9780198717485. [Google Scholar]
- Gruhlke, M.C.H.; Schlembach, I.; Leontiev, R.; Uebachs, A.; Gollwitzer, P.U.G.; Weiss, A.; Delaunay, A.; Toledano, M.; Slusarenko, A.J. Yap1p, the central regulator of the S. cerevisiae oxidative stress response, is activated by allicin, a natural oxidant and defence substance of garlic. Free Radic. Biol. Med. 2017, 108, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Cantin, A.M.; North, S.L.; Hubbard, R.C.; Crystal, R.G. Normal alveolar epithelial lining fluid contains high levels of glutathione. J. Appl. Physiol. 1987, 63, 152–157. [Google Scholar] [PubMed]
- Fahy, J.V.; Dickey, B.F. Airway Mucus Function and Dysfunction. N. Engl. J. Med. 2010, 363, 2233–2247. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, F.; Leontiev, R.; Jacob, C.; Slusarenko, A.J. An Optimized Facile Procedure to Synthesize and Purify Allicin. Molecules 2017, 22, 770. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Rieg, A.D.; Rossaint, R.; Uhlig, S.; Martin, C. Cardiovascular agents affect the tone of pulmonary arteries and veins in precision-cut lung slices. PLoS ONE 2011, 6, e29698. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Available online: http://www.who.int/mediacentre/news/releases/2017/bacteria-antibiotics-needed/en/ (accessed on 10 October 2017).
Sample Availability: Samples of the compounds are not available from the authors. |





| Organism | MIC (µg/mL) | MBC (µg/mL) |
|---|---|---|
| S. pneumoniae Spain23F-1 | 64 | 128 |
| S. pneumoniae CSR14-10 | 64 | 128 |
| S. pneumoniae S.Africa19A-13 | 64 | 128 |
| S. pneumoniae Poland23F-16 | 64 | 64 |
| S. pneumoniae SNo 67715 | 64 | 64 |
| S. pneumoniae SNo 68665 | 64 | 64 |
| S. pneumoniae SNo 68668 | 32 | 64 |
| K. pneumonia SNo 45412 | 128 | 128 |
| K. pneumonia SNo 45413 | 128 | 128 |
| A. baumannii SNo 45541 | 16 | 32 |
| A. baumannii SNo 45757 | 16 | 32 |
| A. baumannii SNo 45760 | 16 | 32 |
| P. aeruginosa PAO1 SBUG8 | 64 | 128 |
| P. aeruginosa PAO25 | 64 | 256 |
| P. aeruginosa DSM2659 | 512 | 1024 |
| S. aureus SNo 68709 | 64 | 256 |
| S. aureus ATCC 43300 | 32 | 512 |
| S. pyogenes SNo 67467 | 32 | 64 |
| S. dysgalactiae SNo 67799 | 64 | 1024 |
| S. dysgalactiae SNo 73742 | 64 | 1024 |
| S. agalactiae SNo 67764 | 64 | 128 |
| S. agalactiae SNo 69235 | 64 | 64 |
| MIC (µg/mL) | Allicin | PEN | FOT | ERY | CLIN | TET | CHL | SXT | AMOX | LEVO | MXF | VAN | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Organism | |||||||||||||
| S. pneumoniae Spain23F-1 | 64 | 1 | 1.5 | 0.025 | 0.19 | 64 | 16 | 2 | NT | NT | NT | NT | |
| S. pneumoniae CSR14-10 | 64 | 8 | 1 | ≥256 | ≥256 | 48 | 32 | 0.25 | NT | NT | NT | NT | |
| S. pneumoniae S.Africa19A-13 | 64 | 1.5 | 0.5 | ≥256 | ≥256 | 48 | 24 | 4 | NT | NT | NT | NT | |
| S. pneumoniae Poland23F-16 | 64 | 8 | 4 | ≥256 | ≥256 | 64 | 16 | 1.5 | NT | NT | NT | NT | |
| S. pneumoniae SNo 67715 | 64 | 4 | 2 | ≤0.12 | ≤0.12 | 16 | ≤2 | 1 | 4 | 1 | ≤0.25 | ≤0.5 | |
| S. pneumoniae SNo 68668 | 32 | ≤0.015 | ≤0.015 | ≤0.12 | ≤0.12 | 0.25 | ≤2 | ≤0.25 | ≤0.015 | 1 | ≤0.25 | ≤0.5 | |
| S. pneumoniae SNo 68665 | 64 | ≤0.015 | ≤0.015 | ≤0.12 | ≤0.12 | 0.25 | ≤2 | ≤0.25 | ≤0.015 | 1 | ≤0.25 | ≤0.5 | |
| S. pyogenes SNo 67467 | 32 | ≤0.015 | ≤0.015 | ≥256 | 64 | 64 | ≤2 | ≤0.25 | ≤0.015 | 1 | ≤0.25 | ≤0.5 | |
| S. dysgalactiae SNo 67799 | 64 | ≤0.015 | ≤0.015 | ≤0.12 | ≤0.12 | 16 | ≤2 | ≤0.25 | 0.03 | 1 | ≤0.25 | ≤0.5 | |
| S. agalactiae SNo 67764 | 64 | 0.03 | 0.06 | ≤0.12 | ≤0.12 | 64 | ≤2 | ≤0.25 | 0.06 | 1 | ≤0.25 | ≤0.5 | |
| drug concentration (mg/L) | |||||||||||||
| 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | |
| EC50 Allicin | ||
|---|---|---|
| without GSH | with 1 mM GSH | |
| LDH-test | 14.7 µM (2.4 µg/mL) | 655.9 µM (106.4 µg/mL) |
| WST-test | 14.4 µM (2.3 µg/mL) | 280.5 µM (45.5 µg/mL) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reiter, J.; Levina, N.; Van der Linden, M.; Gruhlke, M.; Martin, C.; Slusarenko, A.J. Diallylthiosulfinate (Allicin), a Volatile Antimicrobial from Garlic (Allium sativum), Kills Human Lung Pathogenic Bacteria, Including MDR Strains, as a Vapor. Molecules 2017, 22, 1711. https://doi.org/10.3390/molecules22101711
Reiter J, Levina N, Van der Linden M, Gruhlke M, Martin C, Slusarenko AJ. Diallylthiosulfinate (Allicin), a Volatile Antimicrobial from Garlic (Allium sativum), Kills Human Lung Pathogenic Bacteria, Including MDR Strains, as a Vapor. Molecules. 2017; 22(10):1711. https://doi.org/10.3390/molecules22101711
Chicago/Turabian StyleReiter, Jana, Natalja Levina, Mark Van der Linden, Martin Gruhlke, Christian Martin, and Alan J. Slusarenko. 2017. "Diallylthiosulfinate (Allicin), a Volatile Antimicrobial from Garlic (Allium sativum), Kills Human Lung Pathogenic Bacteria, Including MDR Strains, as a Vapor" Molecules 22, no. 10: 1711. https://doi.org/10.3390/molecules22101711