Natural Deep Eutectic Solvents (NADES) to Enhance Berberine Absorption: An In Vivo Pharmacokinetic Study
Abstract
:1. Introduction
2. Results
2.1. Berberine Solubility in NADES
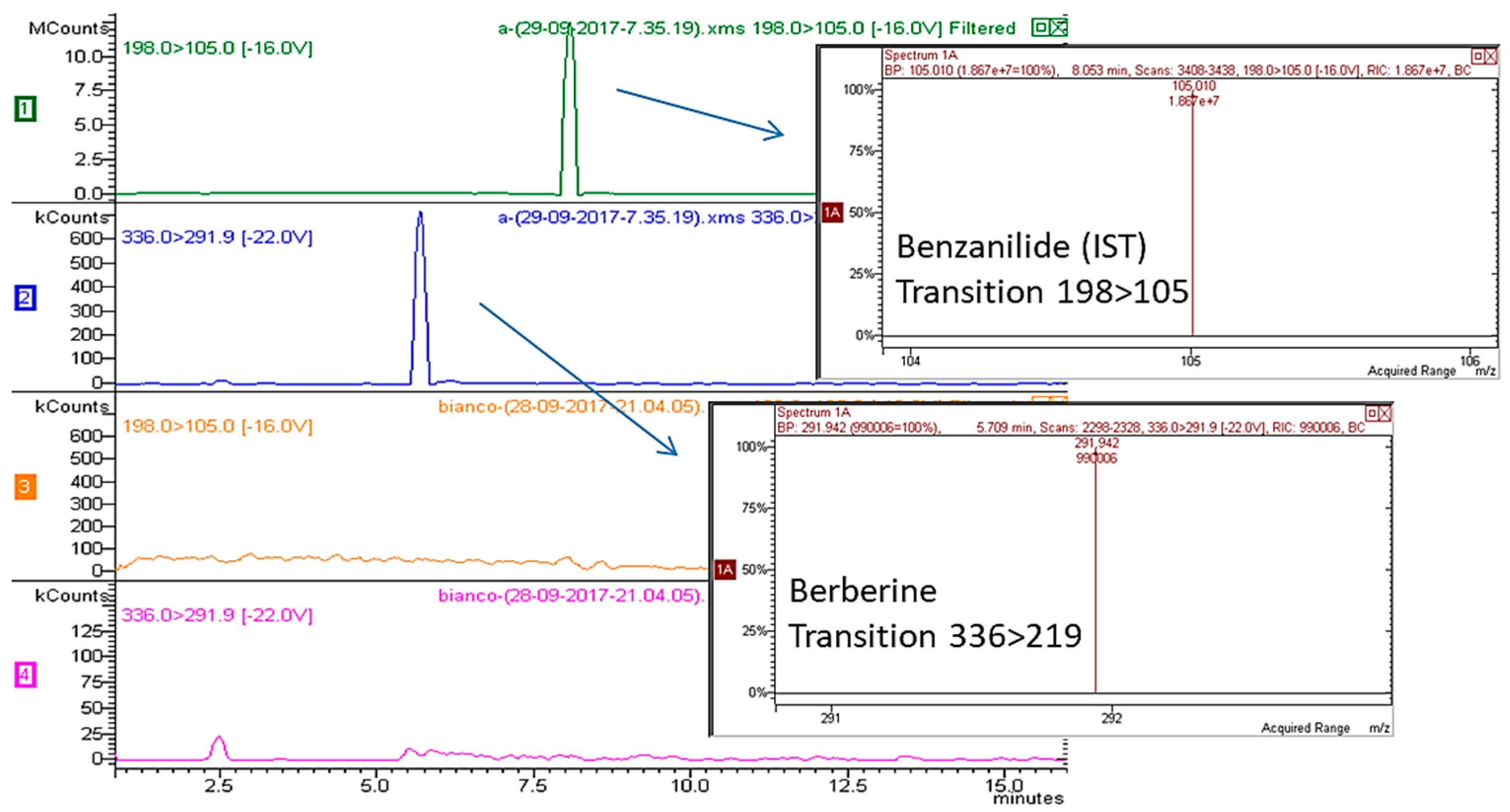
2.2. HPLC-MS/MS Method Validation
2.2.1. Specificity, Linearity, LOQ and LOD
2.2.2. Accuracy and Precision
2.3. Pharmacokinetics of Selected Eutectic Solubilised Berberin and Berberine Hydrochloride Water Suspensions in Mice
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. NADES Preparation
4.3. Solubility Trials and Quantification of Solubilized Berberine in the NADES by HPLC-DAD
4.4. Animals Blood Collection and Extraction
4.5. HPLC-MS Plasma Analysis
4.6. Method Validation
4.7. Pharmacokinetic Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kumar, A.; Ekavali; Chopra, K.; Mukherjee, M.; Pottabathini, R.; Dhull, D.K. Current knowledge and pharmacological profile of berberine: An update. Eur. J. Pharmacol. 2015, 761, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Wei, J.; Abidi, P.; Lin, M.; Inaba, S.; Li, C.; Wang, Y.; Wang, Z.; Si, S.; Pan, H.; et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat. Med. 2004, 10, 1344–1351. [Google Scholar] [CrossRef] [PubMed]
- Birdsall, T.C. Berberine: Therapeutic potential of an alkaloid found in several medicinal plants. Altern. Med. Rev. 1997, 2, 94–103. [Google Scholar]
- Battu, S.K.; Repka, M.A.; Maddineni, S.; Chittiboyina, A.G.; Avery, M.A.; Majumdar, S. Physicochemical characterization of berberine chloride: A perspective in the development of a solution dosage form for oral delivery. AAPS PharmSciTech 2010, 11, 1466–1475. [Google Scholar] [CrossRef] [PubMed]
- Imenshahidi, M.; Hosseinzadeh, H. Berberis Vulgaris and Berberine: An Update Review. Phytother. Res. 2016, 30, 1745–1764. [Google Scholar] [CrossRef] [PubMed]
- Tillhon, M.; Ortiz, L.M.G.; Lombardi, P.; Scovassi, A.I. Berberine: New perspectives for old remedies. Biochem. Pharmacol. 2012, 84, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Caliceti, C.; Franco, P.; Spinozzi, S.; Roda, A.; Cicero, A.F.G. Berberine: New insights from pharmacological aspects to clinical evidences in the management of metabolic disorders. Curr. Med. Chem. 2016, 23, 1460–1476. [Google Scholar] [CrossRef] [PubMed]
- Spinozzi, S.; Colliva, C.; Camborata, C.; Roberti, M.; Ianni, C.; Neri, F.; Calvarese, C.; Lisotti, A.; Mazzella, G.; Roda, A. Berberine and its metabolites: Relationship between physicochemical properties and plasma levels after administration to human subjects. J. Nat. Prod. 2014, 77, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zheng, Y.; Zhang, Y.; Long, X. Research progress on berberine with a special focus on its oral bioavailability. Fitoterapia 2016, 109, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Sut, S.; Baldan, V.; Faggian, M.; Peron, G.; Dall’Acqua, S. Nutraceuticals, a New Challenge for Medicinal Chemistry. Curr. Med. Chem. 2016, 23, 1–26. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.; Verpoorte, R.; Choi, Y.H. Ionic liquids and deep eutectic solvents in natural products research: Mixtures of solids as extraction solvents. J. Nat. Prod. 2013, 76, 2162–2173. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Witkamp, G.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as a new extraction media for phenolic metabolites in carthamus tinctorius L. Anal. Chem. 2013, 85, 6272–6278. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; van Spronsen, J.; Witkamp, G.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Witkamp, G.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Dou, L.; Guo, L.; Li, P.; Liu, E. Comprehensive Evaluation of Deep Eutectic Solvents in Extraction of Bioactive Natural Products. ACS Sustain. Chem. Eng. 2016, 4, 2405–2411. [Google Scholar] [CrossRef]
- Bakirtzi, C.; Triantafyllidou, K.; Makris, D.P. Novel lactic acid-based natural deep eutectic solvents: Efficiency in the ultrasound-assisted extraction of antioxidant polyphenols from common native Greek medicinal plants. J. Appl. Res. Med. Aromat. Plants 2015, 3, 120–127. [Google Scholar] [CrossRef]
- Espino, M.; Fernández, D.M.; Gomez, F.J.V.; Silva, M.F. Natural designer solvents for greening analytical chemistry. TrAC Trends Anal. Chem. 2016, 76, 126–136. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents formed between choline chloride and carboxylic acids: Versatile alternatives to ionic liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef] [PubMed]
- Aroso, I.M.; Silva, J.C.; Mano, F.; Ferreira, A.S.D.; Dionísio, M.; Sá-Nogueira, I.; Barreiros, S.; Reis, R.L.; Paiva, A.; Duarte, A.R.C. Dissolution enhancement of active pharmaceutical ingredients by therapeutic deep eutectic systems. Eur. J. Pharm. Biopharm. 2016, 98, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Faggian, M.; Sut, S.; Perissutti, B.; Baldan, V.; Grabnar, I.; Dall’Acqua, S. Natural Deep Eutectic Solvents (NADES) as a tool for bioavailability improvement: Pharmacokinetics of rutin dissolved in proline/glycine after oral administration in rats: Possible application in nutraceuticals. Molecules 2016, 21, 1531. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Taguchi, G.; Fukui, H.; Tabata, M. Role of malic acid in solubilizing excess berberine accumulating in vacuoles of Coptis japonica. Phytochemistry 1992, 31, 3451–3454. [Google Scholar] [CrossRef]
- Bajkacz, S.; Adamek, J. Evaluation of new natural deep eutectic solvents for the extraction of isoflavones from soy products. Talanta 2017, 168, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Feng, F.; Jiang, J.; Qiao, Y.; Wu, T.; Voglmeir, J.; Chen, Z. Green and efficient extraction of rutin from tartary buckwheat hull by using natural deep eutectic solvents. Food Chem. 2017, 221, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Gil-Chávez, G.J.; Villa, J.A.; Ayala-Zavala, J.F.; Heredia, J.B.; Sepulveda, D.; Yahia, E.M.; González-Aguilar, G.A. Technologies for Extraction and Production of Bioactive Compounds to be Used as Nutraceuticals and Food Ingredients: An Overview. Compr. Rev. Food Sci. Food Saf. 2013, 12, 5–23. [Google Scholar] [CrossRef]
- Morrison, H.G.; Sun, C.C.; Neervannan, S. Characterization of thermal behavior of deep eutectic solvents and their potential as drug solubilization vehicles. Int. J. Pharm. 2009, 378, 136–139. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |

| NADES Type | Composition (w/w) | Berberine Solubility at 22 °C (mg/mL) | |
|---|---|---|---|
| Water | 2.10 ± 0.05 | ||
| Ethanol | 2.75 ± 0.05 | ||
| Urea-based | 1 | Proline–urea 1:1 | 15.0 ± 0.05 |
| 2 | Proline–urea 2:1 | 12.3 ± 0.07 (B) | |
| Sugar and organic acid-based | 3 | Citric Acid–glucose 1:1 | 0.54 ± 0.02 |
| Organic acid and amino acids-based | 4 | Proline–Malic Acid 1:1 | 2.50 ± 0.06 |
| 5 | Proline–Malic Acid 1:2 | 2.80 ± 0.05 (A) | |
| 6 | Proline–Malic Acid 1:3 | 6.00 ± 0.09 | |
| 7 | Proline–Malic Acid–Water 1:2:3 | 1.57 ± 0.02 * | |
| 8 | Proline–Malic Acid–Water 1:2:6 | 0.74 ± 0.01 * | |
| 9 | Proline–Citric Acid 1:1 | 1.82 ± 0.04 * | |
| 10 | Proline–Citric Acid 1:2 | 2.45 ± 0.03 * | |
| 11 | Proline–Tartaric Acid–Citric–Acid 1:1:1 | 1.75 ± 0.05 * | |
| 12 | Proline–Glutamic Acid 1:1 | 0.45 ± 0.01 | |
| 13 | Proline–Glutamic Acid 1:2 | 0.78 ± 0.02 * | |
| 14 | Proline–Malic Acid–Lactic Acid–Water 1:0.2:0.3:0.5 | 25.0 ± 0.05 (C) | |
| Choline chloride-based | 15 | Proline–Choline Chloride 1:1 | 1.24 ± 0.02 |
| 16 | Proline–Choline Chloride 1:2 | 1.05 ± 0.02 * | |
| 17 | Proline–Choline Chloride 2:1 | 0.80 ± 0.02 * | |
| 18 | Choline Chloride–Malic Acid 1:1 | 0.30 ± 0.01 * | |
| 19 | Choline Chloride–Malic Acid 1:2 | 1.35 ± 0.06 * | |
| 20 | Choline Chloride–Malic Acid 1:3 | 1.56 ± 0.05 * | |
| 21 | Choline Chloride–Malic Acid 2:1 | 1.42 ± 0.06* | |
| 22 | Choline Chloride–Malic Acid 3:1 | 0.87 ± 0.05 * | |
| 23 | Choline Chloride–Citric Acid 2:1 | 0.75 ± 0.02 * | |
| 24 | Choline Chloride–Citric Acid 3:1 | 0.64 ± 0.03 | |
| 25 | Carnitine–Choline Chloride 1:1 | 0.41 ± 0.02 | |
| Carnitine-based | 26 | Acetylcarnitine–Choline Chloride 1:1 | 0.89 ± 0.05 |
| 27 | Carnitine–Proline 1:1 | 1.07 ± 0.04 * | |
| 28 | Acetylcarnitine–Proline 1:1 | 0.85 ± 0.03 * | |
| 29 | Carnitine–Proline 1:1 | 1.48 ± 0.07 * | |
| 30 | Carnitine–sorbitole 1:1 | 0.35 ± 0.01 | |
| 31 | Acetylcarnitine–sorbitole 1:1 | 0.27 ± 0.01 | |
| 32 | Carnitine–xylitole 1:1 | 0.38 ± 0.01 | |
| 33 | Acetylcarnitine–xylitole 1:1 | 0.25 ± 0.01 | |
| 34 | Acetylcarnitine–proline–Lactic Acid 1:1:2 | 1.24 ± 0.05 * | |
| 35 | Acetylcarnitine–proline–Citric Acid 1:1:2 | 1.36 ± 0.04 * | |
| 36 | Lactic Acid | 9.00 ± 0.05 | |
| Lactic Acid-based | 37 | Lactic Acid–Water–Citric Acid 1:1:0.1 | 10.00 ± 0.06 |
| 38 | Lactic Acid–Water–Ossalic Acid 1:1:0.1 | 10.00 ± 0.09 |
| Nominal Concentration ng/mL | Measured ng/mL | RSD (%) | Accuracy (%) | |
|---|---|---|---|---|
| Intra day (n = 5) | 0.9 | 0.947 ± 0.081 | 1.6 | 105.2 |
| 4.5 | 4.61 ± 0.34 | 6.8 | 102.4 | |
| 9 | 8.92 ± 0.13 | 2.6 | 99.1 | |
| 22.5 | 23.2 ± 0.68 | 13.7 | 103.2 | |
| Inter day (n = 5) | 0.9 | 0.934 ± 0.088 | 1.9 | 103.7 |
| 4.5 | 4.56 ± 0.24 | 7.0 | 101.3 | |
| 9 | 8.93 ± 0.17 | 3.8 | 99.2 | |
| 22.5 | 23.0 ± 0.51 | 11.0 | 102.2 |
| Time (Min) | Water | A | B | C |
|---|---|---|---|---|
| ng/mL | ng/mL | ng/mL | ng/mL | |
| 10 | 3.83 ± 0.51 | 4.75 ± 0.49 | 11.33 ± 1.10 | 21.80 ± 2.88 |
| 30 | 6.40 ± 0.32 | 20.71 ± 3.63 | 46.94 ± 6.00 | 51.21 ± 9.20 |
| 60 | 4.66 ± 0.36 | 4.73 ± 1.81 | 10.36 ± 2.00 | 32.23 ± 8.50 |
| 180 | 8.50 ± 1.70 | 1.10 ± 0.09 | 7.60 ± 0.90 | 22.72 ± 5.00 |
| 360 | 4.68 ± 0.37 | 0.74 ± 3.00 | 4.50 ± 0.50 | 14.62 ± 6.91 |
| Suspension | A | B | C | |
|---|---|---|---|---|
| tmax (min) | 180 | 30 | 30 | 30 |
| Cmax (ng/mL) | 8.5 | 20.8 | 46.9 | 51.2 |
| t1/2 λz (min) | 208 | 268 | 248 | 265 |
| AUClast (ng min/mL) | 2226 | 1170 | 3499 | 8640 |
| AUC (ng min/mL) | 3630 | 1904 | 5112 | 14,219 |
| AUC (%Extrapolated) | 39 | 39 | 31 | 39 |
| MRT (min) | 371 | 354 | 315 | 384 |
| Fr (%) | 100 | 52 | 141 | 392 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sut, S.; Faggian, M.; Baldan, V.; Poloniato, G.; Castagliuolo, I.; Grabnar, I.; Perissutti, B.; Brun, P.; Maggi, F.; Voinovich, D.; et al. Natural Deep Eutectic Solvents (NADES) to Enhance Berberine Absorption: An In Vivo Pharmacokinetic Study. Molecules 2017, 22, 1921. https://doi.org/10.3390/molecules22111921
Sut S, Faggian M, Baldan V, Poloniato G, Castagliuolo I, Grabnar I, Perissutti B, Brun P, Maggi F, Voinovich D, et al. Natural Deep Eutectic Solvents (NADES) to Enhance Berberine Absorption: An In Vivo Pharmacokinetic Study. Molecules. 2017; 22(11):1921. https://doi.org/10.3390/molecules22111921
Chicago/Turabian StyleSut, Stefania, Marta Faggian, Valeria Baldan, Gabriele Poloniato, Ignazio Castagliuolo, Iztok Grabnar, Beatrice Perissutti, Paola Brun, Filippo Maggi, Dario Voinovich, and et al. 2017. "Natural Deep Eutectic Solvents (NADES) to Enhance Berberine Absorption: An In Vivo Pharmacokinetic Study" Molecules 22, no. 11: 1921. https://doi.org/10.3390/molecules22111921
APA StyleSut, S., Faggian, M., Baldan, V., Poloniato, G., Castagliuolo, I., Grabnar, I., Perissutti, B., Brun, P., Maggi, F., Voinovich, D., Peron, G., & Dall’Acqua, S. (2017). Natural Deep Eutectic Solvents (NADES) to Enhance Berberine Absorption: An In Vivo Pharmacokinetic Study. Molecules, 22(11), 1921. https://doi.org/10.3390/molecules22111921