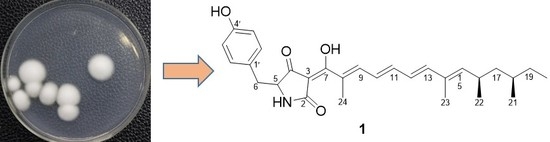
PTP1B Inhibitors from the Entomogenous Fungi Isaria fumosorosea
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification of Compounds 1–6
2.2. PTP1B Inhibitory Activity of Compounds 1–6
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material and Cultivation Conditions
3.3. Extraction and Isolation
3.4. Spectroscopic Data
3.5. PTP Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hunter, T. Signaling—2000 and beyond. Cell 2000, 100, 113–127. [Google Scholar] [CrossRef]
- Huijsduijnen, R.H.V.; Sauer, W.H.B.; Bombrun, A.; Swinnen, D. Prospects for inhibitors of protein tyrosine phosphatase 1B as antidiabetic drugs. J. Med. Chem. 2004, 47, 4142–4146. [Google Scholar] [CrossRef] [PubMed]
- Barr, A.J. Protein tyrosine phosphatases as drug targets: Strategies and challenges of inhibitor development. Future Med. Chem. 2010, 2, 1563–1576. [Google Scholar] [CrossRef] [PubMed]
- Liu, G. Protein tyrosine phosphatase 1B inhibition: Opportunities and challenges. Curr. Med. Chem. 2003, 10, 1407–1421. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.X.; Zhang, J.; Chen, C.; Teng, J.T.; Wang, C.S.; Luo, D.Q. Structure and biosynthesis of fumosorinone, a new protein tyrosine phosphatase 1B inhibitor firstly isolated from the entomogenous fungus isaria fumosorosea. Fungal Genet. Biol. 2015, 81, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Liu, T.; Chen, C.; Li, M.Y.; Wang, Z.Y.; Chen, R.S.; Wei, G.X.; Wang, X.Y.; Luo, D.Q. Fumosorinone, a novel PTP1B inhibitor, activates insulin signaling in insulin-resistance HepG2 cells and shows anti-diabetic effect in diabetic KKAy mice. Toxicol. Appl. Pharmacol. 2015, 285, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.; Riese, U.; Li, Z.Z.; Hamburger, M. Novel tetramic acids and pyridine alkaloids, militarinones B, C, and D, from the insect pathogenic fungus paecilomyces militaris. J. Nat. Prod. 2003, 66, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Stahl, M.; Schopfer, U.; Frenking, G.; Hoffmann, R.W. Assignment of relative configuration to acyclic compounds based on 13C NMR shifts. A density functional and molecular mechanics study. J. Org. Chem. 1996, 61, 8083–8088. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.J.; Ellard, J.M. Synthesis of the C9-C25 fragment of L-755, 807. Evidence for the relative configuration of the side-chain. Tetrahedron Lett. 1998, 39, 6033–6036. [Google Scholar] [CrossRef]
- Ackland, M.J.; Hanson, J.R.; Hitchcock, P.B. Structure of the Cephalosporolides B-F, A Group of C10 Lactones from cephalosporium aphidicola. J. Chem. Soc. Perkin Trans. 1 1985, 843–847. [Google Scholar] [CrossRef]
- Rukachaisirikul, V.; Pramjit, S.; Pakawatchai, C.; Isaka, M.; Supothina, S. 10-membered macrolides from the insect pathogenic fungus cordyceps militaris BBC 2816. J. Nat. Prod. 2004, 67, 1953–1955. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.X.; Burns, A.M.; Liu, M.X.; Faeth, S.H.; Gunatilaka, A.A. Search for cell motility and angiogenesis inhibitors with potential anticancer activity: Beauvericin and other constituents of two endophytic strains of fusarium oxysporum. J. Nat. Prod. 2007, 70, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Yang, C.G.; Wei, P.Y.; Li, L.; Luo, D.Q.; Zheng, Z.H.; Lu, X.H. Penostatin derivatives, a novel kind of protein phosphatase 1B inhibitors isolated from solid cultures of the entomogenous fungus Isaria tenuipes. Molecules 2014, 19, 1663–1671. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |


| No. | δC (Dept) | δH (Mult, J in Hz) | No. | δC (Dept) | δH (Mult, J in Hz) |
|---|---|---|---|---|---|
| 2 | 174.8, C | 17a | 44.6, CH2 | 1.16 a, m | |
| 3 | 99.7, C | 17b | 1.34 a, m | ||
| 4 | 194.1, C | 18 | 32.3, CH | 1.34 a, m | |
| 5 | 61.6, CH | 4.05, t (4.8) | 19a | 29.8, CH2 | 1.16 a, m |
| 6a | 36.5, CH2 | 2.89, dd (14.1, 6.1) | 19b | 1.34 a, m | |
| 6b | 3.03, dd (14.1, 4.1) | 20 | 10.3, CH3 | 0.88, t (7.4) | |
| 7 | 185.0, C | 21 | 18.2, CH3 | 0.88, d (6.9) | |
| 8 | 128.5, C | 22 | 20.4, CH3 | 0.98, d (6.6) | |
| 9 | 142.7, CH | 7.67, d (9.5) | 23 | 11.4, CH3 | 1.84, s |
| 10 | 126.4, CH | 6.70, dd (15.2, 9.5) | 24 | 11.2, CH3 | 2.06, s |
| 11 | 142.8, CH | 6.70, dd (15.2, 9.5) | 1′ | 126.4, C | |
| 12 | 126.2, CH | 6.42, dd (15.2, 9.5) | 2′ | 130.4, CH | 7.03, d (8.2) |
| 13 | 142.8, CH | 6.54, d (15.2) | 3′ | 114.7, CH | 6.71, d (8.2) |
| 14 | 132.7, C | 4′ | 155.9, C | ||
| 15 | 143.3, CH | 5.45, d (9.8) | 5′ | 114.7, CH | 6.71, d (8.2) |
| 16 | 30.5, CH | 2.68, m | 6′ | 130.4, CH | 7.03, d (8.2) |
| Compounds | IC50 (μM) |
|---|---|
| 1 | 3.24 ± 0.37 |
| 2 | >1000 |
| 3 | >1000 |
| 4 | >1000 |
| 5 | >1000 |
| 6 | 0.59 ± 0.15 |
| Sodium orthovanadate | 11.3 ± 0.87 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Meng, L.-L.; Wei, J.-J.; Fan, P.; Liu, S.-S.; Yuan, W.-Y.; Zhao, Y.-X.; Luo, D.-Q. PTP1B Inhibitors from the Entomogenous Fungi Isaria fumosorosea. Molecules 2017, 22, 2058. https://doi.org/10.3390/molecules22122058
Zhang J, Meng L-L, Wei J-J, Fan P, Liu S-S, Yuan W-Y, Zhao Y-X, Luo D-Q. PTP1B Inhibitors from the Entomogenous Fungi Isaria fumosorosea. Molecules. 2017; 22(12):2058. https://doi.org/10.3390/molecules22122058
Chicago/Turabian StyleZhang, Jun, Lin-Lin Meng, Jing-Jing Wei, Peng Fan, Sha-Sha Liu, Wei-Yu Yuan, You-Xing Zhao, and Du-Qiang Luo. 2017. "PTP1B Inhibitors from the Entomogenous Fungi Isaria fumosorosea" Molecules 22, no. 12: 2058. https://doi.org/10.3390/molecules22122058
APA StyleZhang, J., Meng, L. -L., Wei, J. -J., Fan, P., Liu, S. -S., Yuan, W. -Y., Zhao, Y. -X., & Luo, D. -Q. (2017). PTP1B Inhibitors from the Entomogenous Fungi Isaria fumosorosea. Molecules, 22(12), 2058. https://doi.org/10.3390/molecules22122058