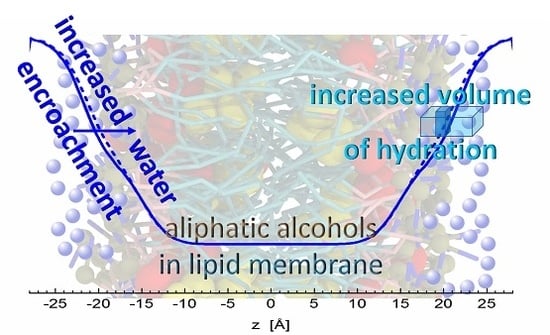
Alcohol Interactions with Lipid Bilayers §
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Small-Angle Neutron Diffraction
4.2. Molecular Dynamics Simulations
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Robinson, D.H.; Toledo, A.H. Historical development of modern anesthesia. J. Investig. Surg. 2012, 25, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Mullins, L.J. Some Physical Mechanisms in Narcosis. Chem. Rev. 1954, 54, 289–323. [Google Scholar] [CrossRef]
- Lee, A.G. Model for action of local anaesthetics. Nature 1976, 262, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Franks, N.P.; Lieb, W.R. Molecular mechanisms of general anaesthesia. Nature 1982, 300, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Pringle, M.J.; Brown, K.B.; Miller, K.W. Can the lipid theories of anesthesia account for the cutoff in anesthetic potency in homologous series of alcohols? Mol. Pharmacol. 1981, 19, 49–55. [Google Scholar] [PubMed]
- Klacsová, M.; Bulacu, M.; Kučerka, N.; Uhríková, D.; Teixeira, J.; Marrink, S.J.; Balgavý, P. The effect of aliphatic alcohols on fluid bilayers in unilamellar DOPC vesicles-a small-angle neutron scattering and molecular dynamics study. Biochim. Biophys. Acta 2011, 1808, 2136–2146. [Google Scholar] [CrossRef] [PubMed]
- Balgavý, P.; Devínsky, F. Cut-off effects in biological activities of surfactants. Adv. Colloid Interface Sci. 1996, 66, 23–63. [Google Scholar] [CrossRef]
- Franks, N.P.; Lieb, W.R. Partitioning of long-chain alcohols into lipid bilayers: Implications for mechanisms of general anesthesia. Proc. Natl. Acad. Sci. USA 1986, 83, 5116–5120. [Google Scholar] [CrossRef] [PubMed]
- Heimburg, T.; Jackson, A.D. The thermodynamics of general anesthesia. Biophys. J. 2007, 92, 3159–3165. [Google Scholar] [CrossRef] [PubMed]
- Graesboll, K.; Sasse-Middelhoff, H.; Heimburg, T. The thermodynamics of general and local anesthesia. Biophys. J. 2014, 106, 2143–2156. [Google Scholar] [CrossRef] [PubMed]
- Pope, J.M.; Walker, L.W.; Dubro, D. On the Ordering of N-Alkane and N-Alcohol Solutes in Phospholipid-Bilayer Model Membrane Systems. Chem. Phys. Lipids 1984, 35, 259–277. [Google Scholar] [CrossRef]
- Petrenko, V.I.; Klacsová, M.; Beskrovnyy, A.I.; Uhríková, D.; Balgavý, P. Interaction of long-chain n-alcohols with fluid DOPC bilayers: A neutron diffraction study. Gen. Phys. Biophys. 2010, 29, 355–361. [Google Scholar] [CrossRef]
- Griepernau, B.; Leis, S.; Schneider, M.F.; Sikor, M.; Steppich, D.; Bockmann, R.A. 1-Alkanols and membranes: A story of attraction. Biochim. Biophys. Acta 2007, 1768, 2899–2913. [Google Scholar] [CrossRef] [PubMed]
- Griepernau, B.; Bockmann, R.A. The influence of 1-alkanols and external pressure on the lateral pressure profiles of lipid bilayers. Biophys. J. 2008, 95, 5766–5778. [Google Scholar] [CrossRef] [PubMed]
- Cantor, R.S. The lateral pressure profile in membranes: A physical mechanism of general anesthesia. Biochemistry 1997, 36, 2339–2344. [Google Scholar] [CrossRef] [PubMed]
- Cantor, R.S. Breaking the Meyer-Overton rule: Predicted effects of varying stiffness and interfacial activity on the intrinsic potency of anesthetics. Biophys. J. 2001, 80, 2284–2297. [Google Scholar] [CrossRef]
- Murugova, T.N.; Klacsová, M.; Pullmannová, P.; Karlovská, J.; Balgavý, P. Study of interaction of long-chain n-alcohols with fluid DOPC bilayers by a lateral pressure sensitive fluorescence probe. Gen. Physiol. Biophys. 2012, 31, 225–227. [Google Scholar] [CrossRef] [PubMed]
- Katsaras, J.; Yang, D.S.; Epand, R.M. Fatty-acid chain tilt angles and directions in dipalmitoyl phosphatidylcholine bilayers. Biophys. J. 1992, 63, 1170–1175. [Google Scholar] [CrossRef]
- Tristram-Nagle, S.; Zhang, R.; Suter, R.M.; Worthington, C.R.; Sun, W.J.; Nagle, J.F. Measurement of chain tilt angle in fully hydrated bilayers of gel phase lecithins. Biophys. J. 1993, 64, 1097–1109. [Google Scholar] [CrossRef]
- Nagle, J.F.; Katsaras, J. Absence of a vestigial vapor pressure paradox. Phys. Rev. E 1999, 59, 7018–7024. [Google Scholar] [CrossRef]
- Harroun, T.A.; Balai-Mood, K.; Hauss, T.; Otomo, T.; Bradshaw, J.P. Neutron diffraction with an excess-water cell. J. Biolphys. 2005, 31, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Kondela, T.; Gallová, J.; Hauß, T.; Ivankov, O.; Kučerka, N.; Balgavý, P. Effect of alkan-1-ols on the structure of dopc model membrane. Eur. Biopharm. J. 2017. [Google Scholar] [CrossRef]
- Chu, N.; Kučerka, N.; Liu, Y.; Tristram-Nagle, S.; Nagle, J.F. Anomalous swelling of lipid bilayer stacks is caused by softening of the bending modulus. Phys. Rev. E 2005, 71, 041904. [Google Scholar] [CrossRef] [PubMed]
- Kučerka, N.; Nieh, M.P.; Pencer, J.; Sachs, J.N.; Katsaras, J. What determines the thickness of a biological membrane. Gen. Phys. Biophys. 2009, 28, 117–125. [Google Scholar] [CrossRef]
- Tristram-Nagle, S.; Petrache, H.I.; Nagle, J.F. Structure and interactions of fully hydrated dioleoylphosphatidylcholine bilayers. Biophys. J. 1998, 75, 917–925. [Google Scholar] [CrossRef]
- Klacsová, M.; Westh, P.; Balgavý, P. Molecular and component volumes of saturated n-alkanols in DOPC+DOPS bilayers. Chem. Phys. Lipids 2010, 163, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Kučerka, N.; Heberle, F.A.; Pan, J.; Katsaras, J. Structural Significance of Lipid Diversity as Studied by Small Angle Neutron and X-ray Scattering. Membranes 2015, 5, 454–472. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Wassenaar, T.A.; Ingolfsson, H.I.; Bockmann, R.A.; Tieleman, D.P.; Marrink, S.J. Computational Lipidomics with insane: A Versatile Tool for Generating Custom Membranes for Molecular Simulations. J. Chem. Theory Comput. 2015, 11, 2144–2155. [Google Scholar] [CrossRef] [PubMed]
- Tanford, C. The Hydrophobic Effect: Formation of Micelles and Biological Membranes, 2nd ed.; John Wiley and Sons: New York, NY, USA, 1980. [Google Scholar]
- Cevc, G.; Marsh, D. Phospholipid Bilayers. In Physical Principles and Models; John Wiley & Sons, Inc.: New York, NY, USA, 1987; Volume 5. [Google Scholar]
- Yeagle, P. The Structure of Biological Membranes; CRC Press: Boca Raton, FL, USA, 1992. [Google Scholar]
- Petrache, H.I.; Dodd, S.W.; Brown, M.F. Area per lipid and acyl length distributions in fluid phosphatidylcholines determined by 2H NMR spectroscopy. Biophys. J. 2000, 79, 3172–3192. [Google Scholar] [CrossRef]
- Kučerka, N.; Nieh, M.P.; Katsaras, J. Fluid phase lipid areas and bilayer thicknesses of commonly used phosphatidylcholines as a function of temperature. Biochim. Biophys. Acta 2011, 1808, 2761–2771. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.J. Coarse-grained simulations of lipid bilayers. J. Chem. Phys. 2004, 121, 11942–11948. [Google Scholar] [CrossRef] [PubMed]
- Kučerka, N.; Gallová, J.; Uhríková, D.; Balgavý, P.; Bulacu, M.; Marrink, S.J.; Katsaras, J. Areas of monounsaturated diacylphosphatidylcholines. Biophys. J. 2009, 97, 1926–1932. [Google Scholar] [CrossRef] [PubMed]
- Kučerka, N.; Pencer, J.; Sachs, J.N.; Nagle, J.F.; Katsaras, J. Curvature effect on the structure of phospholipid bilayers. Langmuir 2007, 23, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Fábián, B.; Sega, M.; Voloshin, V.P.; Medvedev, N.N.; Jedlovszky, P. Lateral Pressure Profile and Free Volume Properties in Phospholipid Membranes Containing Anesthetics. J. Phys. Chem. B 2017, 121, 2814–2824. [Google Scholar] [CrossRef] [PubMed]
- Tristram-Nagle, S.A. Preparation of oriented, fully hydrated lipid samples for structure determination using X-ray scattering. Methods Mol. Biol. 2007, 400, 63–75. [Google Scholar] [PubMed]
- Hauß, T. V1: Membrane Diffractometer at BER II. J. Large Scale Res. Facil. 2016, 2, 94. [Google Scholar] [CrossRef]
- Nagle, J.F.; Akabori, K.; Treece, B.W.; Tristram-Nagle, S. Determination of mosaicity in oriented stacks of lipid bilayers. Soft Matter 2016, 12, 1884–1891. [Google Scholar] [CrossRef] [PubMed]
- Katsaras, J.; Kučerka, N.; Nieh, M.P. Structure from substrate supported lipid bilayers (Review). Biointerphases 2008, 3, FB55–FB63. [Google Scholar] [CrossRef] [PubMed]
- Franks, N.P.; Lieb, W.R. The structure of lipid bilayers and the effects of general anaesthetics: An X-ray and neutron diffraction study. J. Mol. Biol. 1979, 133, 469–500. [Google Scholar] [CrossRef]
- Léonard, A.; Escrive, C.; Laguerre, M.; Pebay-Peyroula, E.; Néri, W.; Pott, T.; Katsaras, J.; Dufourc, E.J. Location of Cholesterol in DMPC Membranes: A Comparative Study by Neutron Diffraction and Molecular Mechanics Simulation. Langmuir 2001, 17, 2019–2030. [Google Scholar] [CrossRef]
- Harroun, T.A.; Katsaras, J.; Wassall, S.R. Cholesterol is found to reside in the center of a polyunsaturated lipid membrane. Biochemistry 2008, 47, 7090–7096. [Google Scholar] [CrossRef] [PubMed]
- Nagle, J.F.; Wiener, M.C. Relations for lipid bilayers. Connection of electron density profiles to other structural quantities. Biophys. J. 1989, 55, 309–313. [Google Scholar] [CrossRef]
- Klauda, J.B.; Kučerka, N.; Brooks, B.R.; Pastor, R.W.; Nagle, J.F. Simulation-Based Methods for Interpreting X-Ray Data From Lipid Bilayers. Biophys. J. 2006, 90, 2796–2807. [Google Scholar] [CrossRef] [PubMed]
- Barnoud, J.; Monticelli, L. Coarse-Grained Force Fields for Molecular Simulations. In Molecular Modeling of Proteins; Kukol, A., Ed.; Springer: New York, NY, USA, 2015; pp. 125–149. [Google Scholar]
- Bulacu, M.; Periole, X.; Marrink, S.J. In silico design of robust bolalipid membranes. Biomacromolecules 2012, 13, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.N.; Arnarez, C.; Sikkema, H.; Kumar, N.; Walko, M.; Berendsen, H.J.; Kocer, A.; Marrink, S.J.; Ingolfsson, H.I. High-Throughput Simulations Reveal Membrane-Mediated Effects of Alcohols on MscL Gating. J. Am. Chem. Soc. 2017, 139, 2664–2671. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, H.J.C.; van der Spoel, D.; van Drunen, R. GROMACS: A message-passing parallel molecular dynamics implementation. Comput. Phys. Commun. 1995, 91, 43–56. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- De Jong, D.H.; Baoukina, S.; Ingólfsson, H.I.; Marrink, S.J. Martini straight: Boosting performance using a shorter cutoff and GPUs. Comput. Phys. Commun. 2016, 199, 1–7. [Google Scholar] [CrossRef]
- Michaud-Agrawal, N.; Denning, E.J.; Woolf, T.B.; Beckstein, O. MDAnalysis: A toolkit for the analysis of molecular dynamics simulations. J. Comput. Chem. 2011, 32, 2319–2327. [Google Scholar] [CrossRef] [PubMed]
- Gowers, R.J.; Linke, M.; Barnoud, J.; Reddy, T.J.E.; Melo, M.N.; Seyler, S.L.; Dotson, D.L.; Domanski, J.; Buchoux, S.; Kenney, I.M.; et al. MDAnalysis: A Python Package for the Rapid Analysis of Molecular Dynamics Simulations. In Proceedings of the 15th Python in Science Conference, Austin, TX, USA, 11–17 July 2016; pp. 102–109. [Google Scholar]
- Castillo, N.; Monticelli, L.; Barnoud, J.; Tieleman, D.P. Free energy of WALP23 dimer association in DMPC, DPPC, and DOPC bilayers. Chem. Phys. Lipids 2013, 169, 95–105. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |










© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondela, T.; Gallová, J.; Hauß, T.; Barnoud, J.; Marrink, S.-J.; Kučerka, N. Alcohol Interactions with Lipid Bilayers. Molecules 2017, 22, 2078. https://doi.org/10.3390/molecules22122078
Kondela T, Gallová J, Hauß T, Barnoud J, Marrink S-J, Kučerka N. Alcohol Interactions with Lipid Bilayers. Molecules. 2017; 22(12):2078. https://doi.org/10.3390/molecules22122078
Chicago/Turabian StyleKondela, Tomáš, Jana Gallová, Thomas Hauß, Jonathan Barnoud, Siewert-J. Marrink, and Norbert Kučerka. 2017. "Alcohol Interactions with Lipid Bilayers" Molecules 22, no. 12: 2078. https://doi.org/10.3390/molecules22122078
APA StyleKondela, T., Gallová, J., Hauß, T., Barnoud, J., Marrink, S.-J., & Kučerka, N. (2017). Alcohol Interactions with Lipid Bilayers. Molecules, 22(12), 2078. https://doi.org/10.3390/molecules22122078