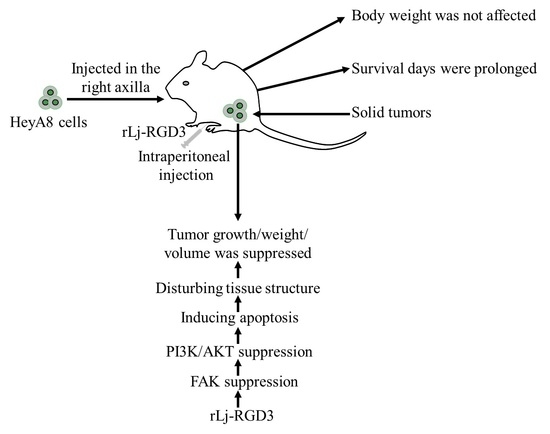
rLj-RGD3 Suppresses the Growth of HeyA8 Cells in Nude Mice
Abstract
:1. Introduction
2. Results
2.1. rLj-RGD3 Suppressed the Tumor Growth in HeyA8 Xenografted Mice without Affecting Their Body Weight
2.2. rLj-RGD3 Improved the Survival Days in the HeyA8 Xenografted Mice
2.3. rLj-RGD3 Changed the Histological Characteristics and Induced Apoptosis in the Tumors of HeyA8 Xenografted Mice
2.4. rLj-RGD3 Suppressed the FAK/PI3K/AKT Pathway in the Tumors of HeyA8 Xenografted Mice
3. Discussion
4. Materials and Methods
4.1. The Preparation of rLj-RGD3
4.2. HeyA8 Cells Culture
4.3. Animals
4.4. Xenograft Models
4.5. Tumor Weight and Volume
4.6. H&E, Hoechst 33258 and TUNEL Staining
4.7. Western Blot
4.8. Survival Assays
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AKT | Protein kinase B |
| BCA | Bicinchoninic acid |
| DMEM | Dulbecco’s modified Eagle medium |
| ECM | Extracellular matrix |
| FAK | Focal adhesion kinase |
| FBS | Fetal bovine serum |
| H&E | Hematoxylin and eosin |
| HRP | Horseradish peroxidase |
| IR | Inhibitory rate |
| L. japonica | Lampetra japonica |
| PBS | Phosphate buffered saline |
| PBST | Phosphate buffered saline tween |
| PI3K | Phosphoinositide 3-kinase |
| PMSF | Phenylmethanesulfonyl fluoride |
| PVDF | Polyvinylidene difluoride |
| RGD | Arg-Gly-Asp |
| SD | Sprague Dawley |
| SDS-PAGE | Sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| TME | Tumor microenvironment |
References
- Rashed, M.H.; Kanlikilicer, P.; Rodriguez-Aguayo, C.; Pichler, M.; Bayraktar, R.; Bayraktar, E.; Ivan, C.; Filant, J.; Silva, A.; Aslan, B.; et al. Exosomal miR-940 maintains SRC-mediated oncogenic activity in cancer cells: A possible role for exosomal disposal of tumor suppressor miRNAs. Oncotarget 2017, 8, 20145–20164. [Google Scholar] [CrossRef] [PubMed]
- Palmirotta, R.; Silvestris, E.; D’Oronzo, S.; Cardascia, A.; Silvestris, F. Ovarian cancer: Novel molecular aspects for clinical assessment. Crit. Rev. Oncol. Hematol. 2017, 117, 12–29. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.T. Fertility drugs and ovarian cancer. Curr. Cancer Drug Targets 2017. [Google Scholar] [CrossRef]
- Matulonis, U.A.; Sood, A.K.; Fallowfield, L.; Howitt, B.E.; Sehouli, J.; Karlan, B.Y. Ovarian cancer. Nat. Rev. Dis. Primers 2016, 2, 16061. [Google Scholar] [CrossRef] [PubMed]
- Desgrosellier, J.S.; Cheresh, D.A. Integrins in cancer: Biological implications and therapeutic opportunities. Nat. Rev.Cancer 2010, 10, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Sawada, K.; Mitra, A.K.; Radjabi, A.R.; Bhaskar, V.; Kistner, E.O.; Tretiakova, M.; Jagadeeswaran, S.; Montag, A.; Becker, A.; Kenny, H.A.; et al. Loss of E-cadherin promotes ovarian cancer metastasis via α5-integrin, which is a therapeutic target. Cancer Res. 2008, 68, 2329–2339. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Sawada, K.; Kimura, T. Potential of Integrin Inhibitors for Treating Ovarian Cancer: A Literature Review. Cancers (Basel) 2017, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Han, X.; Yang, H.; Lu, L.; Wu, Y.; Liu, X.; Guo, R.; Zhang, Y.; Zhang, Y.; Li, Q. A novel RGD-toxin protein, Lj-RGD3, from the buccal gland secretion of Lampetra japonica impacts diverse biological activities. Biochimie 2010, 92, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, J.; Liu, X.; Chu, D.; Li, Q. Expression and bioactivity effects to Hela of recombinant toxin protein rLj-RGD3 from Lampetra japonica. Sheng Wu Gong Cheng Xue Bao 2009, 25, 686–694. [Google Scholar] [PubMed]
- Jin, M.; Wang, J.; Xiao, R.; Liu, X.; Wu, F.; Pang, Y.; Feng, B.; Yang, D.; Li, Q. Effects of the recombinant toxin protein rLj-RGD3 in multidrug-resistant human breast carcinoma cells. Acta Biochim. Biophys. Sin. 2012, 44, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Xiao, R.; Wang, J.; Liu, X.; Liu, Y.; Xue, Z.; Lv, L.; Zheng, Y.; Li, Q. Low concentrations of the recombinant toxin protein rLj-RGD3 suppress TNF-α-induced human renal carcinoma cell invasion. Acta Bioch. Biophys. Sin. 2013, 45, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zheng, Y.; Tu, Z.; Dai, Y.; Xu, H.; Lv, L.; Wang, J. The anti-tumor effects of the recombinant toxin protein rLj-RGD3 from Lampetra japonica on pancreatic carcinoma Panc-1 cells in nude mice. Peptides 2017, 88, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Li, Q.; Han, J.; Gou, M.; Zheng, Y.; Li, B.; Xiao, R.; Wang, J. rLj-RGD3 induces apoptosis via the mitochondrial-dependent pathway and inhibits adhesion, migration and invasion of human HeyA8 cells via FAK pathway. Int. J. Biol. Macromol. 2017, 96, 652–668. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tong, Y.; Cao, R.; Tian, Z.; Yang, B.; Yang, P. In vivo enhancement of anticancer therapy using bare or chemotherapeutic drug-bearing nanodiamond particles. Int. J. Nanomed. 2014, 9, 1065–1082. [Google Scholar] [CrossRef] [PubMed]
- Nieberler, M.; Reuning, U.; Reichart, F.; Notni, J.; Wester, H.J.; Schwaiger, M.; Weinmüller, M.; Räder, A.; Steiger, K.; Kessler, H. Exploring the Role of RGD-Recognizing Integrins in Cancer. Cancers (Basel) 2017, 9, 116. [Google Scholar] [CrossRef] [PubMed]
- Barczyk, M.; Carracedo, S.; Gullberg, D. Integrins. Cell Tissue Res. 2010, 339, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Longmate, W.; DiPersio, C.M. Beyond adhesion: Emerging roles for integrins in control of the tumor microenvironment. F1000Res 2017, 6, 1612. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Wang, Q.; Lau, W.B.; Lau, B.; Xu, L.; Zhao, L.; Yang, H.; Feng, M.; Xuan, Y.; Yang, Y.; et al. Tumor microenvironment: The culprit for ovarian cancer metastasis? Cancer Lett. 2016, 377, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Scaringi, C.; Minniti, G.; Caporello, P.; Enrici, R.M. Integrin inhibitor cilengitide for the treatment of glioblastoma: A brief overview of current clinical results. Anticancer Res. 2012, 32, 4213–4223. [Google Scholar] [PubMed]
- Huang, T.F.; Hsu, C.C.; Kuo, Y.J. Anti-thrombotic agents derived from snake venom proteins. Thromb. J. 2016, 14 (Suppl. S1), 18. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Fang, Y.; Han, Y.; Bai, X.; Yan, X.; Zhang, Y.; Lai, R.; Zhang, Z. YY-39, a tick anti-thrombosis peptide containing RGD domain. Peptides 2015, 68, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Gomez, E.A.; Fujita, M.; Ishimaru, Y.; Uezato, H.; Mimori, T.; Iwata, H.; Hashiguchi, Y. Ayadualin, a novel RGD peptide with dual antihemostatic activities from the sand fly Lutzomyia ayacuchensis, a vector of Andean-type cutaneous leishmaniasis. Biochimie 2015, 112, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Xu, X.; An, S.; Liu, H.; Yang, X.; Andersen, J.F.; Wang, Y.; Tokumasu, F.; Ribeiro, J.M.; Francischetti, I.M.; et al. A novel family of RGD-containing disintegrins (Tablysin-15) from the salivary gland of the horsefly Tabanus yao targets αIIbβ3 or αVβ3 and inhibits platelet aggregation and angiogenesis. Thromb. Haemost. 2011, 105, 1032–1045. [Google Scholar] [CrossRef] [PubMed]
- Pirone, L.; Ripoll-Rozada, J.; Leone, M.; Ronca, R.; Lombardo, F.; Fiorentino, G.; Andersen, J.F.; Pereira, P.J.B.; Arcà, B.; Pedone, E. Functional analyses yield detailed insight into the mechanism of thrombin inhibition by the antihemostatic salivary protein cE5 from Anopheles gambiae. J. Biol. Chem. 2017, 292, 12632–12642. [Google Scholar] [CrossRef] [PubMed]
- Seymour, J.L.; Henzel, W.J.; Nevins, B.; Stults, J.T.; Lazarus, R.A. Decorsin. A potent glycoprotein IIb-IIIa antagonist and platelet aggregation inhibitor from the leech Macrobdella decora. J. Biol. Chem. 1990, 265, 10143–10147. [Google Scholar] [PubMed]
- Zheng, Y.; Han, J.; Wang, Y.; Jiang, Q.; Wang, Y.; Lv, L.; Xiao, R.; Wang, J. Data for the effects of rLj-RGD3 on normal tissues of rats and its location in HeyA8 cells. Data Brief 2017, 12, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.L.; Chen, L.C.; Shen, T.L. Emerging roles of focal adhesion kinase in cancer. BioMed Res. Int. 2015, 2015, 690690. [Google Scholar] [CrossRef] [PubMed]
- Faes, S.; Dormond, O. PI3K and AKT: Unfaithful Partners in Cancer. Int. J. Mol. Sci. 2015, 16, 21138–21152. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the proteins rLj-RGD3 are available from the authors. |







© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Lv, L.; Yi, L.; Wu, R.; Xiao, R.; Wang, J. rLj-RGD3 Suppresses the Growth of HeyA8 Cells in Nude Mice. Molecules 2017, 22, 2234. https://doi.org/10.3390/molecules22122234
Zheng Y, Lv L, Yi L, Wu R, Xiao R, Wang J. rLj-RGD3 Suppresses the Growth of HeyA8 Cells in Nude Mice. Molecules. 2017; 22(12):2234. https://doi.org/10.3390/molecules22122234
Chicago/Turabian StyleZheng, Yuanyuan, Li Lv, Longda Yi, Rui Wu, Rong Xiao, and Jihong Wang. 2017. "rLj-RGD3 Suppresses the Growth of HeyA8 Cells in Nude Mice" Molecules 22, no. 12: 2234. https://doi.org/10.3390/molecules22122234
APA StyleZheng, Y., Lv, L., Yi, L., Wu, R., Xiao, R., & Wang, J. (2017). rLj-RGD3 Suppresses the Growth of HeyA8 Cells in Nude Mice. Molecules, 22(12), 2234. https://doi.org/10.3390/molecules22122234