Emerging Anti-Mitotic Activities and Other Bioactivities of Sesquiterpene Compounds upon Human Cells
Abstract
:1. Introduction
2. Sesquiterpene Lactones as Natural Products and Their Sources
3. Biological Activities of Sesquiterpene Lactones
3.1. Effects upon Insects and Grazing Herbivores
3.2. Effects of Sesquiterpene Lactones upon Humans
3.3. Medicinal Properties of Sesquiterpene Lactones
3.4. Anti-Inflammatory Effects
3.5. Anti-Tumour Activities
3.6. Clinical Trials of Sesquiterpene Lactones
4. Anti-Mitotic Activities of Sesquiterpenes
4.1. 6-O-Angeloylplenolin (6-OAP)
4.2. 9β-Acetoxycostunolide and Santamarine
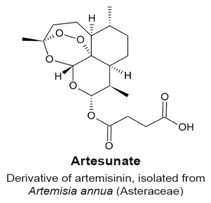
4.3. Artemisinin and its Derivatives Artesunate and Dihydroartemisinin
4.4. Coronopilin
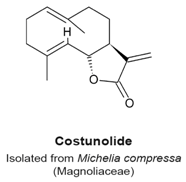
4.5. Costunolide
4.6. Dehydroleucodine
4.7. NP136
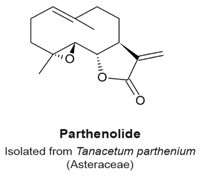
4.8. Parthenolide
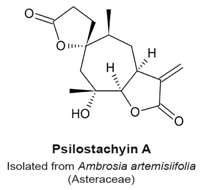
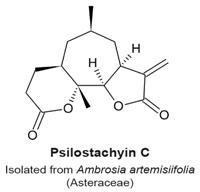
4.9. Psilostachyin A and Psilostachyin C
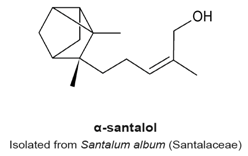
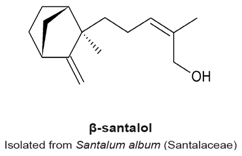
4.10. α- and β-Santalols
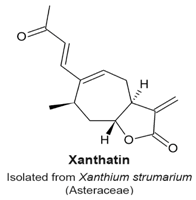
4.11. Xanthatin
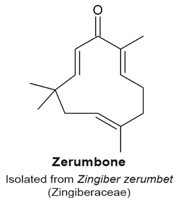
4.12. Zerumbone
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Yu, F.; Utsumi, R. Diversity, regulation, and genetic manipulation of plant mono- and sesquiterpenoid biosynthesis. Cell. Mol. Life Sci. 2009, 66, 3043–3052. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, E.; Towers, G.; Mitchell, J.C. Biological activities of sesquiterpene lactones. Phytochemistry 1976, 15, 1573–1580. [Google Scholar] [CrossRef]
- Zhang, S.; Won, Y.K.; Ong, C.N.; Shen, H.M. Anti-cancer potential of sesquiterpene lactones: Bioactivity and molecular mechanisms. Curr. Med. Chem. Anticancer Agents 2005, 5, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Fraga, B.M. Natural sesquiterpenoids. Nat. Prod. Rep. 2012, 29, 1334–1366. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, M.; Trewin, H.; Gawthrop, F.; Wagstaff, C. Sesquiterpenoids lactones: Benefits to plants and people. Int. J. Mol. Sci. 2013, 14, 12780–12805. [Google Scholar] [CrossRef] [PubMed]
- Kupchan, S.M.; Fessler, D.C.; Eakin, M.A.; Giacobbe, T.J. Reactions of alpha methylene lactone tumor inhibitors with model biological nucleophiles. Science 1970, 168, 376–378. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.J. Helenanolide-type sesquiterpene lactones—III. Rates and stereochemistry in the reaction of helenalin and related helenanolides with sulfhydryl containing biomolecules. Bioorg. Med. Chem. 1997, 5, 645–653. [Google Scholar] [CrossRef]
- Scotti, M.T.; Fernandes, M.B.; Ferreira, M.J.; Emerenciano, V.P. Quantitative structure-activity relationship of sesquiterpene lactones with cytotoxic activity. Bioorg. Med. Chem. 2007, 15, 2927–2934. [Google Scholar] [CrossRef] [PubMed]
- Miseta, A.; Csutora, P. Relationship between the occurrence of cysteine in proteins and the complexity of organisms. Mol. Biol. Evol. 2000, 17, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.; Robles, M.; West, J.E.; Ortiz de Montellano, B.R.; Rodriguez, E. Ethnopharmacology of mexican asteraceae (compositae). Annu. Rev. Pharmacol. Toxicol. 1998, 38, 539–565. [Google Scholar] [CrossRef] [PubMed]
- Robles, M.; Aregullin, M.; West, J.; Rodriguez, E. Recent studies on the zoopharmacognosy, pharmacology and neurotoxicology of sesquiterpene lactones. Planta Med. 1995, 61, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Ivie, G.W.; Witzel, D.A.; Rushing, D.D. Toxicity and milk bittering properties of tenulin, the major sesquiterpene lactone constituent of Helenium amarum (bitter sneezeweed). J. Agric. Food. Chem. 1975, 23, 845–849. [Google Scholar] [CrossRef] [PubMed]
- Seaman, F.C. Sesquiterpene lactones as taxonomic characters in the asteraceae. Brittonia 1983, 35, 121–594. [Google Scholar] [CrossRef]
- Schnee, C.; Kollner, T.G.; Gershenzon, J.; Degenhardt, J. The maize gene terpene synthase 1 encodes a sesquiterpene synthase catalyzing the formation of (E)-β-farnesene, (E)-nerolidol, and (E,E)-farnesol after herbivore damage. Plant Physiol. 2002, 130, 2049–2060. [Google Scholar] [CrossRef] [PubMed]
- González, A.G.; Jiménez, I.A.; Ravelo, A.G.; Sazatornil, J.G.; Bazzocchi, I.L. New sesquiterpenes with antifeedant activity from Maytenus canariensis (celastraceae). Tetrahedron 1993, 49, 697–702. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef] [PubMed]
- Cifuente, D.A.; Borkowski, E.J.; Sosa, M.E.; Gianello, J.C.; Giordano, O.S.; Tonn, C.E. Clerodane diterpenes from Baccharis sagittalis: Insect antifeedant activity. Phytochemistry 2002, 61, 899–905. [Google Scholar] [CrossRef]
- Picaud, S.; Olsson, M.E.; Brodelius, P.E. Improved conditions for production of recombinant plant sesquiterpene synthases in Escherichia coli. Protein Expr. Purif. 2007, 51, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.H.; Mansfield, J.W.; Lewis, M.J.; Beale, M.H. Cloning and expression of sesquiterpene synthase genes from lettuce (Lactuca sativa L.). Phytochemistry 2002, 60, 255–261. [Google Scholar] [CrossRef]
- Cheng, A.X.; Xiang, C.Y.; Li, J.X.; Yang, C.Q.; Hu, W.L.; Wang, L.J.; Lou, Y.G.; Chen, X.Y. The rice (E)-β-caryophyllene synthase (ostps3) accounts for the major inducible volatile sesquiterpenes. Phytochemistry 2007, 68, 1632–1641. [Google Scholar] [CrossRef] [PubMed]
- Little, D.B.; Croteau, R.B. Alteration of product formation by directed mutagenesis and truncation of the multiple-product sesquiterpene synthases delta-selinene synthase and gamma-humulene synthase. Arch. Biochem. Biophys. 2002, 402, 120–135. [Google Scholar] [CrossRef]
- Lange, G.L.; Lee, M. Synthesis of four sesquiterpenoid lactone skeletons germacranolide, elemanolide, cadinanolide, and guaianolide, from a single photoadduct. J. Org. Chem. 1987, 52, 325–331. [Google Scholar] [CrossRef]
- Lee, K.H.; Ibuka, T.; Rong-Yang, W.; Geissman, T.A. Structure-antimicrobial activity relationships among the sesquiterpene lactones and related compounds. Phytochemistry 1977, 16, 1177–1181. [Google Scholar]
- Picman, A.K. Biological activities of sesquiterpene lactones. Biochem. Syst. Ecol. 1986, 14, 255–281. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [PubMed]
- Barrero, A.F.; Quílez del Moral, J.F.; Lara, A.; Herrador, M.M. Antimicrobial activity of sesquiterpenes from the essential oil of Juniperus thurifera. Planta Med. 2005, 71, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Barrero, A.F.; Oltra, J.E.; Álvarez, M.; Raslan, D.S.; Saúde, D.A.; Akssira, M. New sources and antifungal activity of sesquiterpene lactones. Fitoterapia 2000, 71, 60–64. [Google Scholar] [CrossRef]
- Portillo, A.; Freixa, B.; Ferro, E.; Parella, T.; Casanova, J.; Cañigueral, S. Antifungal sesquiterpene from the root of Vernonanthura tweedieana. J. Ethnopharmacol. 2005, 97, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Burnett, W.C.; Jones, S.B.; Mabry, T.J.; Padolina, W.G. Sesquiterpene lactones—insect feeding deterrents in vernonia. Biochem. Syst. Ecol. 1974, 2, 25–29. [Google Scholar] [CrossRef]
- Rossiter, M.; Gershenzon, J.; Mabry, T.J. Behavioral and growth responses of specialist herbivore, Homoeosoma electellum, to major terpenoid of its host, Helianthus SPP. J. Chem. Ecol. 1986, 12, 1505–1521. [Google Scholar] [CrossRef] [PubMed]
- Irmisch, S.; Jiang, Y.; Chen, F.; Köllner, T.G. Terpene synthases and their contribution to herbivore-induced volatile emission in western balsam poplar (Populus trichocarpa). BMC Plant Biol. 2014, 14, 270–285. [Google Scholar] [CrossRef] [PubMed]
- Bleeker, P.M.; Mirabella, R.; Diergaarde, P.J.; VanDoorn, A.; Tissier, A.; Kant, M.R.; Prins, M.; de Vos, M.; Haring, M.A.; Schuurink, R.C. Improved herbivore resistance in cultivated tomato with the sesquiterpene biosynthetic pathway from a wild relative. Proc. Natl. Acad. Sci. USA 2012, 109, 20124–20129. [Google Scholar] [CrossRef] [PubMed]
- Killiny, N.; Jones, S.E. Profiling of volatile organic compounds released from individual intact juvenile and mature citrus leaves. J. Plant Physiol. 2016, 208, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Holopainen, J.K. Multiple functions of inducible plant volatiles. Trends Plant Sci. 2004, 9, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Burnett, W.C.; Jones, S.B.; Mabry, T.J. Evolutionary implications of sesquiterpene lactones in Vernonia (cCompositae) and mammalian herbivores. Taxon 1977, 26, 203–207. [Google Scholar] [CrossRef]
- Witzel, D.A.; Ivie, W.; Dollahite, J.W. Mammalian toxicity of helenalin, the toxic principle of Helenium microcephalum CD (smallhead sneezeweed). Am. J. Vet. Res. 1976, 37, 859–861. [Google Scholar] [PubMed]
- Arlette, J.; Mitchell, J.C. Compositae dermatitis. Current aspects. Contact Derm. 1981, 7, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Burry, J.N. Dermatitis from Gaillardia aristata: Compositae dermatitis in south australia. Contact Derm. 1980, 6, 157. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, E. Compositae dermatitis: A survey. Contact Derm. 1992, 26, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.C.; Dupuis, G. Allergic contact dermatitis from sesquiterpenoids of the compositae family of plants. Br. J. Dermatol. 1971, 84, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Jack, A.R.; Norris, P.L.; Storrs, F.J. Allergic contact dermatitis to plant extracts in cosmetics. Semin. Cutan. Med. Surg. 2013, 32, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Spettoli, E.; Silvani, S.; Lucente, P.; Guerra, L.; Vincenzi, C. Contact dermatitis caused by sesquiterpene lactones. Am. J. Contact Dermat. 1998, 9, 49–50. [Google Scholar] [CrossRef] [PubMed]
- Rozas-Munoz, E.; Lepoittevin, J.P.; Pujol, R.M.; Gimenez-Arnau, A. Allergic contact dermatitis to plants: Understanding the chemistry will help our diagnostic approach. Actas Dermosifiliogr. 2012, 103, 456–477. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.R.; Menendez, I.Y. Sesquiterpene lactones inhibit inducible nitric oxide synthase gene expression in cultured rat aortic smooth muscle cells. Biochem. Biophys. Res. Commun. 1999, 262, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Giordano, O.S.; Guerreiro, E.; Pestchanker, M.J.; Guzman, J.; Pastor, D.; Guardia, T. The gastric cytoprotective effect of several sesquiterpene lactones. J. Nat. Prod. 1990, 53, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Ahlemeyer, B.; Möwes, A.; Krieglstein, J. Inhibition of serum deprivation-and staurosporine-induced neuronal apoptosis by ginkgo biloba extract and some of its constituents. Eur. J. Pharmacol. 1999, 367, 423–430. [Google Scholar] [CrossRef]
- Wesołowska, A.; Nikiforuk, A.; Michalska, K.; Kisiel, W.; Chojnacka-Wójcik, E. Analgesic and sedative activities of lactucin and some lactucin-like guaianolides in mice. J. Ethnopharmacol. 2006, 107, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Canales, M.; Hernandez, T.; Caballero, J.; Romo de Vivar, A.; Avila, G.; Duran, A.; Lira, R. Informant consensus factor and antibacterial activity of the medicinal plants used by the people of San Rafael Coxcatlan, Puebla, México. J. Ethnopharmacol. 2005, 97, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Sülsen, V.P.; Puente, V.; Papademetrio, D.; Batlle, A.; Martino, V.S.; Frank, F.M.; Lombardo, M.E. Mode of Action of the Sesquiterpene Lactones Psilostachyin and Psilostachyin C on Trypanosoma cruzi. PLoS ONE 2016, 11, e0150526. [Google Scholar] [CrossRef] [PubMed]
- Sülsen, V.P.; Frank, F.M.; Cazorla, S.I.; Anesini, C.A.; Malchiodi, E.L.; Freixa, B.; Vila, R.; Muschietti, L.V.; Martino, V.S. Trypanocidal and Leishmanicidal Activities of Sesquiterpene Lactones from Ambrosia tenuifolia Sprengel (Asteraceae). Antimicrob. Agents Chemother. 2008, 52, 2415–2419. [Google Scholar] [CrossRef] [PubMed]
- Klayman, D.L. Qinghaosu (artemisinin): An antimalarial drug from China. Science 1985, 228, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y. The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat. Med. 2011, 17, 1217–1220. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y. Artemisinin-a gift from traditional Chinese medicine to the world (Nobel lecture). Angew. Chem. Int. Ed. 2016, 55, 10210–10226. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Good Procurement Practices for Artemisinin-Based Antimalarial Medicines; WHO Press: Geneva, Switzerland, 2010. [Google Scholar]
- World Health Organization. WHO Position Statement (June 2012). Effectiveness of Non-Pharmaceutical Forms of Artemisia annua L. against Malaria. Available online: http://www.who.int/malaria/diagnosis_treatment/position_statement_herbal_remedy_artemisia_annua_l.pdf?ua=1 (accessed on 1 November 2016).
- World Health Organization. Guidelines for the treatment of malaria, 3rd ed.; WHO Press: Geneva, Switzerland, 2015. [Google Scholar]
- Corey, V.C.; Lukens, A.K.; Istvan, E.S.; Lee, M.C.; Franco, V.; Magistrado, P.; Coburn-Flynn, O.; Sakata-Kato, T.; Fuchs, O.; Gnädig, N.F.; et al. A broad analysis of resistance development in the malaria parasite. Nat. Commun. 2016, 7, 11901–11910. [Google Scholar] [CrossRef] [PubMed]
- Winzeler, E.A.; Manary, M.J. Drug resistance genomics of the antimalarial drug artemisinin. Genome Biol. 2014, 15, 544–556. [Google Scholar] [CrossRef] [PubMed]
- Cravo, P.; Napolitano, H.; Culleton, R. How genomics is contributing to the fight against artemisinin-resistant malaria parasites. Acta Trop. 2015, 148, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Meshnick, S.R. Artemisinin: Mechanisms of action, resistance and toxicity. Int. J. Parasitol. 2002, 32, 1655–1660. [Google Scholar] [CrossRef]
- Cui, L.W.; Su, X.Z. Discovery, mechanisms of action and combination therapy of artemisinin. Expert Rev. Anti Infect Ther. 2009, 7, 999–1013. [Google Scholar] [CrossRef] [PubMed]
- Rüngeler, P.; Castro, V.; Mora, G.; Gören, N.; Vichnewski, W.; Pahl, H.L.; Merfort, I.; Schmidt, T.J. Inhibition of transcription factor NF-κB by sesquiterpene lactones: A proposed molecular mechanism of action. Bioorg. Med. Chem. 1999, 7, 2343–2352. [Google Scholar] [CrossRef]
- Siedle, B.; García-Piñeres, A.J.; Murillo, R.; Schulte-Mönting, J.; Castro, V.; Rüngeler, P.; Klaas, C.A.; Da Costa, F.B.; Kisiel, W.; Merfort, I. Quantitative structure-activity relationship of sesquiterpene lactones as inhibitors of the transcription factor NF-κB. J. Med. Chem. 2004, 47, 6042–6054. [Google Scholar] [CrossRef] [PubMed]
- Bork, P.M.; Schmitz, M.L.; Kuhnt, M.; Escher, C.; Heinrich, M. Sesquiterpene lactone containing mexican indian medicinal plants and pure sesquiterpene lactones as potent inhibitors of transcription factor NF-κB. FEBS Lett. 1997, 402, 85–90. [Google Scholar] [CrossRef]
- Gilmore, T.D. Introduction to NF-κB: Players, pathways, perspectives. Oncogene 2006, 25, 6680–6684. [Google Scholar] [CrossRef] [PubMed]
- Kretz-Remy, C.; Mehlen, P.; Mirault, M.-E.; Arrigo, A.-P. Inhibition of I kappa B-alpha phosphorylation and degradation and subsequent NF-kappa B activation by glutathione peroxidase overexpression. J. Cell Biol. 1996, 133, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, J.; Goldberg, A.L. The proteasome (multicatalytic protease) is a component of the 1500-kDa proteolytic complex which degrades ubiquitin-conjugated proteins. J. Biol. Chem. 1990, 265, 4789–4792. [Google Scholar] [PubMed]
- Napetschnig, J.; Wu, H. Molecular basis of NF-κB signaling. Annu. Rev. Biophys. 2013, 42, 443–468. [Google Scholar] [CrossRef] [PubMed]
- Takada, Y.; Murakami, A.; Aggarwal, B.B. Zerumbone abolishes NF-κB and IκBα kinase activation leading to suppression of antiapoptotic and metastatic gene expression, upregulation of apoptosis, and downregulation of invasion. Oncogene 2005, 24, 6957–6969. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.L.; Rossi, R.M.; Karnischky, L.; Li, X.; Peterson, D.R.; Howard, D.S.; Jordan, C.T. The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells. Blood 2005, 105, 4163–4169. [Google Scholar] [CrossRef] [PubMed]
- Kwok, B.H.; Koh, B.; Ndubuisi, M.I.; Elofsson, M.; Crews, C.M. The anti-inflammatory natural product parthenolide from the medicinal herb feverfew directly binds to and inhibits IκB kinase. Chem. Biol. 2001, 8, 759–766. [Google Scholar] [CrossRef]
- Ghantous, A.; Gali-Muhtasib, H.; Vuorela, H.; Saliba, N.A.; Darwiche, N. What made sesquiterpene lactones reach cancer clinical trials? Drug Discov. Today 2010, 15, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Huang, E.S.; Piantadosi, C.; Pagano, J.S.; Geissman, T.A. Cytotoxicity of sesquiterpene lactones. Cancer Res. 1971, 31, 1649–1654. [Google Scholar] [PubMed]
- Huang, P.R.; Yeh, Y.M.; Wang, T.V. Potent inhibition of human telomerase by helenalin. Cancer Lett. 2005, 227, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Furukawa, H. Antitumor agents. 3. Synthesis and cytotoxic activity of helenalin amine adducts and related derivatives. J. Med. Chem. 1972, 15, 609–611. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Meck, R.; Piantadosi, C.; Huang, E.S. Antitumor agents. 4. Cytotoxicity and in vitro activity of helenalin esters and related derivatives. J. Med. Chem. 1973, 16, 299–301. [Google Scholar] [CrossRef] [PubMed]
- Hall, I.H.; Lee, K.-H.; Mar, E.C.; Starnes, C.O.; Waddell, T.G. Antitumor agents 21. A proposed mechanism for inhibition of cancer growth by tenulin and helenalin and related cyclopentenones. J. Med. Chem. 1977, 20, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Hall, I.H.; Mar, E.C.; Starnes, C.O.; ElGebaly, S.A.; Waddell, T.G.; Hadgraft, R.I.; Ruffner, C.G.; Weidner, I. Sesquiterpene antitumor agents: Inhibitors of cellular metabolism. Science 1977, 196, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Hall, I.H.; Lee, K.H.; Starnes, C.O.; Eigebaly, S.A.; Ibuka, T.; Wu, Y.S.; Kimura, T.; Haruna, M. Antitumor Agents XXX. Evaluation of α-methylene-γ-lactone-Containing agents For Inhibition of Tumor Growth, Respiration, and Nucleic Acid Synthesis. J. Pharm. Sci. 1978, 67, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Kupchan, S.M. Novel natural products with antitumor activity. Fed. Proc. 1974, 33, 2288–2295. [Google Scholar] [PubMed]
- Hanson, R.L.; Lardy, H.A.; Kupchan, S.M. Inhibition of phosphofructokinase by quinone methide and α-methylene lactone tumor inhibitors. Science 1970, 168, 378–380. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.H.; Larner, J.; Thomas, A.M.; Kupchan, S.M. Inactivation of glycogen synthase by the tumor inhibitor vernolepin. Biochim. Biophys. Acta 1972, 276, 94–104. [Google Scholar] [CrossRef]
- Hall, I.H.; Williams, J.W.L.; Grippo, A.A.; Lee, K.H.; Holbrook, D.J.; Chaney, S.G. Inhibition of nucleic acid synthesis in P-388 lymphocytic leukemia cells in culture by sesquiterpene lactones. Anticancer Res. 1988, 8, 33–42. [Google Scholar] [PubMed]
- Baud, V.; Karin, M. Is NF-κB a good target for cancer therapy? Hopes and pitfalls. Nat. Rev. Drug Discov. 2009, 8, 33–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravi, R.; Bedi, A. NF-κB in cancer: A friend turned foe. Drug Res. Updat. 2004, 7, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Yu, J.; Kinghorn, A.D. Development of anticancer agents from plant-derived sesquiterpene lactones. Curr. Med. Chem. 2016, 23, 2397–2420. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.; Ganapathi, S.; Ster, I.C.; Saeed, M.E.M.; Cowan, M.; Finlayson, C.; Kovacsevics, H.; Jansen, H.; Kremsner, P.G.; Efferth, T.; et al. A randomised, double blind, placebo-controlled pilot study of oral artesunate therapy for colorectal cancer. EBioMedicine 2015, 2, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Intravaginal Artesunate for the Treatment of HPV+ High Grade Cervical Intraepithelial Neoplasia (CIN2/3). Available online: https://clinicaltrials.gov/ct2/show/NCT02354534 (accessed on 1 November 2016).
- Phase I Study of Intravenous Artesunate for Solid Tumors. Available online: https://clinicaltrials.gov/ct2/show/NCT02353026 (accessed on 1 November 2016).
- Efferth, T.; Dunstan, H.; Sauerbrey, A.; Miyachi, H.; Chitambar, C.R. The anti-malarial artesunate is also active against cancer. Int. J. Oncol. 2001, 18, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zuo, L.F.; Guo, J.W. Reversal of multidrug resistance by the anti-malaria drug artesunate in the esophageal cancer Eca109/ABCG2 cell line. Oncol. Lett. 2013, 6, 1475–1481. [Google Scholar] [PubMed]
- Mahalingam, D.; Wilding, G.; Denmeade, S.; Sarantopoulas, J.; Cosgrove, D.; Cetnar, J.; Azad, N.; Bruce, J.; Kurman, M.; Allgood, V. Mipsagargin, a novel thapsigargin-based PSMA-activated prodrug: Results of a first-in-man phase I clinical trial in patients with refractory, advanced or metastatic solid tumours. Br. J. Cancer 2016, 114, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Thapsigargin Prodrug G-202 in Treating Patients with Recurrent or Progressive Glioblastoma. Available online: https://www.cancer.gov/about-cancer/treatment/clinical-trials/search/view?cdrid=758277&version=HealthProfessional&protocolsearchid=15383069 (accessed on 1 November 2016.
- Doan, N.T.Q.; Paulsen, E.S.; Sehgal, P.; Møller, J.V.; Nissen, P.; Denmeade, S.R.; Isaacs, J.T.; Dionne, C.A.; Christensen, S.B. Targeting thapsigargin towards tumors. Steroids 2015, 97, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Vander Griend, D.J.; Antony, L.; Dalrymple, S.L.; Xu, Y.; Christensen, S.B.; Denmeade, S.R.; Isaacs, J.T. Amino acid containing thapsigargin analogues deplete androgen receptor protein via synthesis inhibition and induce death of prostate cancer cells. Mol. Cancer Ther. 2009, 8, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.L.; Rossi, R.M.; Neelakantan, S.; Li, X.; Corbett, C.A.; Hassane, D.C.; Becker, M.W.; Bennett, J.M.; Sullivan, E.; Lachowicz, J.L.; et al. An orally bioavailable parthenolide analog selectively eradicates acute myelogenous leukemia stem and progenitor cells. Blood 2007, 110, 4427–4435. [Google Scholar] [CrossRef] [PubMed]
- Adis Insight. Drug Profile: LC-1. Available online: http://adisinsight.springer.com/drugs/800029612 (accessed on 14 October 2016).
- Wen, J.; You, K.-R.; Lee, S.-Y.; Song, C.-H.; Kim, D.-G. Oxidative stress-mediated apoptosis the anticancer effect of the sesquiterpene lactone parthenolide. J. Biol. Chem. 2002, 277, 38954–38964. [Google Scholar] [CrossRef] [PubMed]
- Lyss, G.; Knorre, A.; Schmidt, T.J.; Pahl, H.L.; Merfort, I. The anti-inflammatory sesquiterpene lactone helenalin inhibits the transcription factor NF-κB by directly targeting p65. J. Biol. Chem. 1998, 273, 33508–33516. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, X.Q.; Liang, H.X.; Zhang, F.X.; Zhang, B.; Jin, J.; Chen, Y.L.; Cheng, Y.X.; Zhou, G.B. Small compound 6-O-angeloylplenolin induces mitotic arrest and exhibits therapeutic potentials in multiple myeloma. PLoS ONE 2011, 6, e21930. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Sun, Y. SFC E3 Ubiquitin Ligases as Anticancer Targets. Curr. Cancer Drug Targets 2011, 11, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Wang, X.L.; Cheng, X.; Lu, Y.Z.; Wang, G.Z.; Li, X.C.; Zhang, J.; Wen, Z.S.; Huang, Z.L.; Gao, Q.L.; et al. Skp1 in lung cancer: Clinical significance and therapeutic efficacy of its small molecule inhibitors. Oncotarget 2015, 6, 34953–34967. [Google Scholar] [PubMed]
- Cheng, X.; Liu, Y.Q.; Wang, G.Z.; Yang, L.N.; Lu, Y.Z.; Li, X.C.; Zhou, B.; Qu, L.W.; Wang, X.L.; Cheng, Y.X.; et al. Proteomic identification of the oncoprotein STAT3 as a target of a novel Skp1 inhibitor. Oncotarget 2017, 8, 2681–2693. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Chong, L.; Li, Z.; Cheung, A.H.; Tattersall, M.H. Anticancer activities of sesquiterpene lactones from Cyathocline purpurea in vitro. Cancer Chemother. Pharmacol. 2009, 64, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Steinbruck, L.; Pereira, G.; Efferth, T. Effects of artesunate on cytokinesis and G2/M cell cycle progression of tumour cells and budding yeast. Cancer Genom. Proteom. 2010, 7, 337–346. [Google Scholar]
- Luo, J.; Chen, X.; Chen, G.; Zhou, X.; Lu, X.; Ling, Y.; Zhang, S.; Zhu, W.; Cao, J. Dihydroartemisinin induces radiosensitivity in cervical cancer cells by modulating cell cycle progression. Saudi Med. J. 2013, 34, 254–260. [Google Scholar] [PubMed]
- Cotugno, R.; Fortunato, R.; Santoro, A.; Gallotta, D.; Braca, A.; De Tommasi, N.; Belisario, M.A. Effect of sesquiterpene lactone coronopilin on leukaemia cell population growth, cell type-specific induction of apoptosis and mitotic catastrophe. Cell Prolif. 2012, 45, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Whipple, R.A.; Vitolo, M.I.; Boggs, A.E.; Charpentier, M.S.; Thompson, K.; Martin, S.S. Parthenolide and costunolide reduce microtentacles and tumor cell attachment by selectively targeting detyrosinated tubulin independent from NF-κB inhibition. Breast Cancer Res. 2013, 15, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Chang, H.S.; Chen, I.S.; Chen, C.J.; Hsu, M.L.; Fu, S.L.; Chen, Y.J. Costunolide causes mitotic arrest and enhances radiosensitivity in human hepatocellular carcinoma cells. Radiat. Oncol. 2011, 6, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Bocca, C.; Gabriel, L.; Bozzo, F.; Miglietta, A. A sesquiterpene lactone, costunolide, interacts with microtubule protein and inhibits the growth of MCF-7 cells. Chem. Biol. Interact. 2004, 147, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Legault, J.; Gaulin, J.F.; Mounetou, E.; Bolduc, S.; Lacroix, J.; Poyet, P.; Gaudreault, R.C. Microtubule Disruption Induced in Vivo by Alkylation of β-Tubulin by 1-Aryl-3-(2-Chloroethyl)Ureas, a Novel Class of Soft Alkylating Agents. Cancer Res. 2000, 60, 985–992. [Google Scholar] [PubMed]
- Rasul, A.; Bao, R.; Malhi, M.; Zhao, B.; Tsuji, I.; Li, J.; Li, X. Induction of apoptosis by costunolide in bladder cancer cells is mediated through ROS generation and mitochondrial dysfunction. Molecules 2013, 18, 1418–1433. [Google Scholar] [CrossRef] [PubMed]
- Costantino, V.V.; Mansilla, S.F.; Speroni, J.; Amaya, C.; Cuello-Carrion, D.; Ciocca, D.R.; Priestap, H.A.; Barbieri, M.A.; Gottifredi, V.; Lopez, L.A. The sesquiterpene lactone dehydroleucodine triggers senescence and apoptosis in association with accumulation of DNA damage markers. PLoS ONE 2013, 8, e53168. [Google Scholar] [CrossRef] [PubMed]
- Graciotti, M.; Fang, Z.; Johnsson, K.; Gönczy, P. Chemical genetic screen identifies natural products that modulate centriole number. ChemBioChem 2016, 17, 2063–2074. [Google Scholar] [CrossRef] [PubMed]
- Fonrose, X.; Ausseil, F.; Soleilhac, E.; Masson, V.; David, B.; Pouny, I.; Cintrat, J.C.; Rousseau, B.; Barette, C.; Massiot, G.; et al. Parthenolide inhibits tubulin carboxypeptidase activity. Cancer Res. 2007, 67, 3371–3378. [Google Scholar] [CrossRef] [PubMed]
- Lafanechère, L.; Job, D. The third tubulin pool. Neurochem. Res. 2000, 25, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Barra, H.S.; Arce, C.A.; Argaraña, C.E. Posttranslational tyrosination/detyrosination of tubulin. Mol. Neurobiol. 1988, 2, 133–153. [Google Scholar] [CrossRef] [PubMed]
- Lafanechère, L.; Courtay-Cahen, C.; Kawakami, T.; Jacrot, M.; Rüdiger, M.; Wehland, J.; Job, D.; Margolis, R.L. Suppression of tubulin tyrosine ligase during tumor growth. J. Cell Sci. 1998, 111, 171–181. [Google Scholar] [PubMed]
- Tang, T.K.; Chiu, S.C.; Lin, C.W.; Su, M.J.; Liao, M.H. Induction of Survivin Inhibition, G2/M Cell Cycle Arrest and Autophagic on Cell Death in Human Malignant Glioblastoma Cells. Chin. J. Physiol. 2015, 58, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Sturgeon, C.M.; Craig, K.; Brown, C.; Rundle, N.T.; Andersen, R.J.; Roberge, M. Modulation of the G2 Cell Cycle Checkpoint by Sesquiterpene Lactones Psilostachyins A and C Isolated from the Common Ragweed Ambrosia artemisiifolia. Planta Med. 2005, 71, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Bohmann, J.; Reeves, T.; Levenson, C.; Risinger, A.L. α- and β-Santalols Directly Interact with Tubulin and Cause Mitotic Arrest and Cytotoxicity in Oral Cancer Cells. J. Nat. Prod. 2015, 78, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, W.; Guillermo, R.; Chandrasekher, G.; Kaushik, R.S.; Young, A.; Fahmy, H.; Dwivedi, C. Alpha-santalol, a chemopreventive agent against skin cancer, causes G2/M cell cycle arrest in both p53-mutated human epidermoid carcinoma A431 cells and p53 wild-type human melanoma UACC-62 cells. BMC Res. Notes 2010, 3, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Santha, S.; Bommareddy, A.; Rule, B.; Guillermo, R.; Kaushik, R.S.; Young, A.; Dwivedi, C. Antineoplastic effects of α-santalol on estrogen receptor-positive and estrogen receptor-negative breast cancer cells through cell cycle arrest at G2/M phase and induction of apoptosis. PLoS ONE 2013, 8, e56982. [Google Scholar] [CrossRef]
- Zhang, L.; Ruan, J.; Yan, L.; Li, W.; Wu, Y.; Tao, L.; Zhang, F.; Zheng, S.; Wang, A.; Lu, Y. Xanthatin induces cell cycle arrest at G2/M checkpoint and apoptosis via disrupting NF-κB pathway in A549 non-small-cell lung cancer cells. Molecules 2012, 17, 3736–3750. [Google Scholar] [CrossRef] [PubMed]
- Rahman, H.S.; Rasedee, A.; Abdul, A.B.; Zeenathul, N.A.; Othman, H.H.; Yeap, S.K.; How, C.W.; Hafiza, W.A. Zerumbone-loaded nanostructured lipid carrier induces G2/M cell cycle arrest and apoptosis via mitochondrial pathway in a human lymphoblastic leukemia cell line. Int. J. Nanomed. 2014, 9, 527–538. [Google Scholar]
- Chan, M.L.; Liang, J.W.; Hsu, L.C.; Chang, W.L.; Lee, S.S.; Guh, J.H. Zerumbone, a ginger sesquiterpene, induces apoptosis and autophagy in human hormone-refractory prostate cancers through tubulin binding and crosstalk between endoplasmic reticulum stress and mitochondrial insult. Naunyn Schmiedeberg Arch. Pharmacol. 2015, 388, 1223–1236. [Google Scholar] [CrossRef] [PubMed]
- Tanida, I.; Ueno, T.; Kominami, E. LC3 and Autophagy. Methods Mol. Biol. 2008, 445, 77–88. [Google Scholar] [PubMed]
- Xian, M.; Ito, K.; Nakazato, T.; Shimizu, T.; Chen, C.K.; Yamato, K.; Murakami, A.; Ohigashi, H.; Ikeda, Y.; Kizaki, M. Zerumbone, a bioactive sesquiterpene, induces G2/M cell cycle arrest and apoptosis in leukemia cells via a fas- and mitochondria-mediated pathway. Cancer Sci. 2007, 98, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, S.I.; Abdul, A.B.; Zain, Z.N.M.; Hadi, A.H.A. Zerumbone inhibits interleukin-6 and induces apoptosis and cell cycle arrest in ovarian and cervical cancer cells. Int. Immunopharmacol. 2012, 12, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Rundle, N.T.; Xu, L.; Andersen, R.J.; Roberge, M. G2 DNA Damage Checkpoint Inhibition and Antimitotic Activity of 13-Hydroxy-15-oxozoapatlin. J. Biol. Chem. 2001, 276, 48231–48236. [Google Scholar] [PubMed]
- Kubara, P.M.; Kerneis-Golsteyn, S.; Studeny, A.; Lanser, B.B.; Meijer, L.; Golsteyn, R.M. Human cells enter mitosis with damaged DNA after treatment with pharmacological concentrations of genotoxic agents. Biochem. J. 2012, 446, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Swift, L.H.; Golsteyn, R.M. The relationship between checkpoint adaptation and mitotic catastrophe in genomic changes in cancer cells. In Genome Stability; Kovalchuck, I., Kovalchuk, O., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; p. 712. [Google Scholar]
- Swift, L.H.; Golsteyn, R.M. Cytotoxic amounts of cisplatin induce either checkpoint adaptation or apoptosis in a concentration-dependent manner in cancer cells: Cisplatin and checkpoint adaptation. Biol. Cell 2016, 108, 127–148. [Google Scholar] [CrossRef] [PubMed]



| Plant Family | Common Name | Reference |
|---|---|---|
| Acanthaceae | Acanthus family | [24] |
| Anacardiaceae | Cashew family | [11] |
| Apiaceae | Celery family | [24] |
| Araceae | Aroids family | [5,48] |
| Asteraceae | Sunflower family | [5,10] |
| Cactaceae | Cactus family | [5,48] |
| Euphorbiaceae | Spurge family | [5,48] |
| Lauraceae | Laurel family | [24] |
| Magnoliaceae | Magnolia family | [24] |
| Menispermaceae | Moonseed family | [24] |
| Rutaceae | Citrus family | [11] |
| Solanaceae | Nightshades family | [5,48] |
| Winteraceae | Winter’s Bark family | [24] |
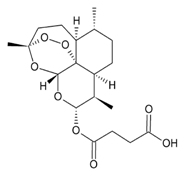 | Artesunate |
| |
 | Dimethylaminoparthenolide (LC-1) |
| |
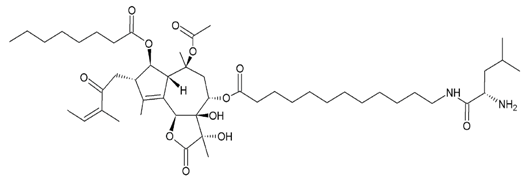 L12ADT (8-O-(12-{l-leucinoylamino}dodecanoyl)-8-O-debutanoyl-thapsigargin) | |
| |
| Compound | Anti-Mitotic Activity | Reference(s) |
|---|---|---|
 |
| [100,102,103] |
 |
| [104] |
 |
| [105,106] |
 |
| [107] |
 |
| [108,109,110,112] |
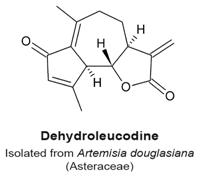 |
| [113] |
 |
| [106] |
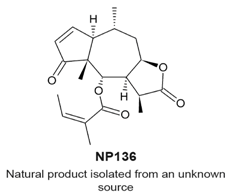 |
| [114] |
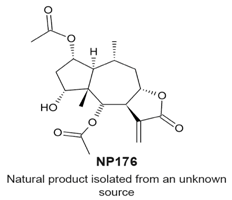 |
| [114] |
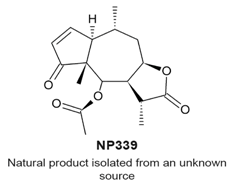 |
| [114] |
 |
| [108] |
 |
| [120] |
 |
| [120] |
 |
| [121,122,123] |
 |
| [121] |
 |
| [104] |
 |
| [124] |
 |
| [125,126,128,129] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bosco, A.; Golsteyn, R.M. Emerging Anti-Mitotic Activities and Other Bioactivities of Sesquiterpene Compounds upon Human Cells. Molecules 2017, 22, 459. https://doi.org/10.3390/molecules22030459
Bosco A, Golsteyn RM. Emerging Anti-Mitotic Activities and Other Bioactivities of Sesquiterpene Compounds upon Human Cells. Molecules. 2017; 22(3):459. https://doi.org/10.3390/molecules22030459
Chicago/Turabian StyleBosco, Alessandra, and Roy M. Golsteyn. 2017. "Emerging Anti-Mitotic Activities and Other Bioactivities of Sesquiterpene Compounds upon Human Cells" Molecules 22, no. 3: 459. https://doi.org/10.3390/molecules22030459
APA StyleBosco, A., & Golsteyn, R. M. (2017). Emerging Anti-Mitotic Activities and Other Bioactivities of Sesquiterpene Compounds upon Human Cells. Molecules, 22(3), 459. https://doi.org/10.3390/molecules22030459






