Anti-Trypanosomatid Elemanolide Sesquiterpene Lactones from Vernonia lasiopus O. Hoffm †
Abstract
:1. Introduction
2. Results and Discussion
2.1. Antiprotozoal Activity of V. lasiopus Crude Extracts
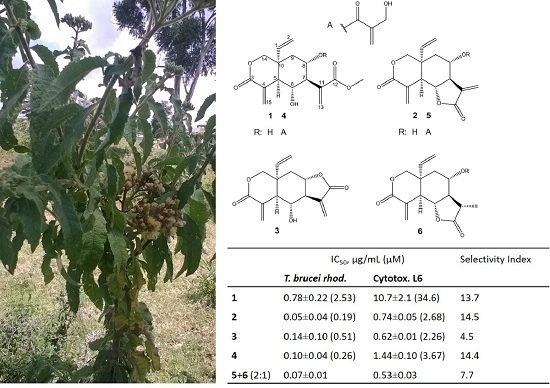
2.2. Bioassay-Guided Fractionation and Isolation of Elemanolide Sesquiterpene Lactones
2.3. Anti-Trypanosomal Activity of Isolated Elemanolides
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.3.1. Small-Scale Extraction for Initial Bioassays
3.3.2. Soxhlet Extraction for Preparative Isolation
3.3.3. Purification by Preparative High Performance Liquid Chromatography (HPLC/UV-DAD)
3.4. Analysis of the Plant Extracts and Isolated Compound by UHPLC/+ESI-QTOF-MS/MS
3.5. Structural Analysis of Isolated Compounds
3.6. Analytical Data
3.7. In Vitro Bioassays
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization (WHO) Neglected Tropical Diseases. Available online: http://www.who.int/neglected_diseases/diseases/summary/en/ (accessed on 19 October 2016).
- Barrett, M.P.; Boykin, D.W.; Brun, R.; Tidwell, R.R. Human African trypanosomiasis: Pharmacological re-engagement with a neglected disease. Br. J. Pharm. 2007, 152, 1155–1171. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.J.; Khalid, S.A.; Romanha, A.J.; Alves, T.M.; Biavatti, M.W.; Brun, R.; Da Costa, F.B.; de Castro, S.L.; Ferreira, V.F.; de Lacerda, M.V. The Potential of Secondary Metabolites from Plants as Drugs or Leads against Protozoan Neglected Diseases—Part I. Curr. Med. Chem. 2012, 19, 2128–2175. [Google Scholar] [PubMed]
- Hotez, P.J.; Yamey, G. The evolving scope of PLoS Neglected Tropical Diseases. PLoS Negl. Trop. Dis. 2009, 3, 2–3. [Google Scholar] [CrossRef]
- World Health Organization Trypanosomiasis, Human African (Sleeping Sickness). Available online: http://www.who.int/mediacentre/factsheets/fs259/en/ (accessed on 19 October 2016).
- Franco, J.R.; Simarro, P.P.; Diarra, A.; Jannin, J.G. Epidemiology of human African trypanosomiasis. Clin. Epidemiol. 2014, 6, 257–275. [Google Scholar] [PubMed]
- Hoet, S.; Opperdoes, F.; Brun, R.; Quetin-Leclercq, J. Natural products active against African trypanosomes: A step towards new drugs. Nat. Prod. Rep. 2004, 21, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.J.; Khalid, S.A.; Romanha, A.J.; Alves, T.M.; Biavatti, M.W.; Brun, R.; Da Costa, F.B.; de Castro, S.L.; Ferreira, V.F.; de Lacerda, M.V. The potential of secondary metabolites from plants as drugs or leads against protozoan neglected diseases—Part II. Curr Med. Chem. 2012, 19, 2176–2228. [Google Scholar] [CrossRef] [PubMed]
- Althaus, J.B.; Jerz, G.; Winterhalter, P.; Kaiser, M.; Brun, R.; Schmidt, T.J. Antiprotozoal activity of Buxus sempervirens and activity-guided isolation of O-Tigloylcyclovirobuxeine-B as the main constituent active against Plasmodium falciparum. Molecules 2014, 19, 6184–6201. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.J.; Da Costa, F.B.; Lopes, N.P.; Kaiser, M.; Brun, R. Silico prediction and experimental evaluation of furanoheliangolide sesquiterpene lactones as potent agents against Trypanosoma brucei rhodesiense. Antimicrob. Agents Chemother. 2014, 58, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, M.; Da Costa, F.; Brun, R.; Kaiser, M.; Schmidt, T. ent-Pimarane and ent-Kaurane Diterpenes from Aldama discolor (Asteraceae) and Their Antiprotozoal Activity. Molecules 2016, 21, 1237. [Google Scholar] [CrossRef] [PubMed]
- Althaus, J.B.; Kaiser, M.; Brun, R.; Schmidt, T.J. Antiprotozoal activity of Achillea ptarmica (Asteraceae) and its main alkamide constituents. Molecules 2014, 19, 6428–6438. [Google Scholar] [CrossRef] [PubMed]
- Dharani, N.; Rukunga, G.; Yeneser, A.; Mbora, A.; Mwaura, L.; Dawson, I.; Jamnadass, R. Common Antimalarial Trees and Shrubs of East Africa: A Description of Species and a Guide to Cultivation and Conservation Through Use; Dawson, I., Ed.; The World Agroforestry Centre (ICRAF): Nairobi, Kenya, 2010. [Google Scholar]
- Koul, J.L.; Koul, S.; Singh, C.; Taneja, S.C.; Shanmugavel, M.; Kampasi, H.; Saxena, A.K.; Qazi, G.N. In vitro cytotoxic elemanolides from Vernonia lasiopus. Planta Med. 2003, 69, 164–166. [Google Scholar] [CrossRef] [PubMed]
- Njenga, D.; Irungu, B.; Mbaria, J.; Mutai, C. Antiplasmodial, Cytotoxic and Acute Toxicity Activities of Vernonia lasiopus O. Hoffman. Afr. J. Pharmacol. Ther. 2015, 4, 16–20. [Google Scholar]
- Katuura, E.; Waako, P.; Ogwal-Okeng, J.; Bukenya-Ziraba, R. Traditional treatment of malaria in Mbarara District, western Uganda. Afr. J. Ecol. 2007, 45, 48–51. [Google Scholar] [CrossRef]
- Muregi, F.W.; Ishih, A.; Miyase, T.; Suzuki, T.; Kino, H.; Amano, T.; Mkoji, G.M.; Terada, M. Antimalarial activity of methanolic extracts from plants used in Kenyan ethnomedicine and their interactions with chloroquine (CQ) against a CQ-tolerant rodent parasite, in mice. J. Ethnopharmacol. 2007, 111, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Rachuonyo, H.O.; Ogola, P.E.; Arika, W.; Nyamai, D.; Wambani, J. In Vitro Antimicrobial Activity of Crude Leaf Extracts from Aloe secundiflora, Bulbine frutescens, Vernonia lasiopus and Tagetes minuta against Salmonella typhi. J. Tradit. Mad. Clin. Naturop. 2016, 5, 2–4. [Google Scholar]
- Irungu, B.N.; Rukunga, G.M.; Mungai, G.M.; Muthaura, C.N. In vitro antiplasmodial and cytotoxicity activities of 14 medicinal plants from Kenya. S. Afr. J. Bot. 2007, 73, 204–207. [Google Scholar] [CrossRef]
- Katuura, E.; Waako, P.; Tabuti, J.R.S.; Bukenya-Ziraba, R.; Ogwal-Okeng, J. Antiplasmodial activity of extracts of selected medicinal plants used by local communities in western Uganda for treatment of malaria. Afr. J. Ecol. 2007, 45, 94–98. [Google Scholar] [CrossRef]
- Ganjian, I.; Kubo, I.; Fludzinski, P. Insect antifeedant elemanolide lactones from Vernonia amagydalina. Phytochemistry 1983, 22, 2515–2526. [Google Scholar] [CrossRef]
- Jakupovic, J.; Baruah, R.N.; Thi, T.V.; Bohlmann, F.; Msonthi, J.D.; Schmeda-Hirschmann, G. New Vernolepin Derivatives from Vernonia glabra and Glaucolides from Vernonia scorpioides. Planta Med. 1985, 51, 378–380. [Google Scholar] [CrossRef] [PubMed]
- Looi, C.Y.; Arya, A.; Cheah, F.K.; Muharram, B.; Leong, K.H.; Mohamad, K.; Wong, W.F.; Rai, N.; Mustafa, M.R. Induction of Apoptosis in Human Breast Cancer Cells via Caspase Pathway by Vernodalin Isolated from Centratherum anthelminticum (L.) Seeds. PLoS ONE 2013, 8, e56643. [Google Scholar] [CrossRef] [PubMed]
- Laekeman, G.M.; Mertens, J.; Totté, J.; Bult, H.; Vlietinck, A.J.; Herman, A.G. Isolation and Pharmacological Characterization of Vernolepin. J. Nat. Prod. 1983, 46, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Chukwujekwu, J.C.; Lategan, C.A.; Smith, P.J.; Van Heerden, F.R.; Van Staden, J. Antiplasmodial and cytotoxic activity of isolated sesquiterpene lactones from the acetone leaf extract of Vernonia colorata. S. Afr. J. Bot. 2009, 75, 176–179. [Google Scholar] [CrossRef]
- Ohigashi, H.; Huffman, M.A.; Izutsu, D.; Koshimizu, K.; Kawanaka, M.; Sugiyama, H.; Kirby, G.C.; Warhurst, D.C.; Allen, D.; Wright, C.W.; et al. Toward the chemical ecology of medicinal plant use in chimpanzees: The case of Vernonia amygdalina, a plant used by wild chimpanzees possibly for parasite-related diseases. J. Chem. Ecol. 1994, 20, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Kraft, C.; Jenett-Siems, K.; Siems, K.; Jakupovic, J.; Mavi, S.; Bienzle, U.; Eich, E. In vitro antiplasmodial evaluation of medicinal plants from Zimbabwe. Phytother. Res. 2003, 17, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.J.; Nour, A.M.M.; Khalid, S.A.; Kaiser, M.; Brun, R. Quantitative structure—Antiprotozoal activity relationships of sesquiterpene lactones. Molecules 2009, 14, 2062–2076. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 2 and 5 + 6 are available from the authors. |

| Extract | Tbr | Tcr | Ldon | Pf | Cytotox. L6 |
|---|---|---|---|---|---|
| Hex | 5.57 ± 0.750 | 55.0 a | 5.91 a | 11.8 a | nd |
| DCM | 0.170 ± 0.100 | 2.18 a | 1.88 ± 1.07 | 1.09 a | 0.800 ± 0.340 |
| EtOAC | 1.30 ± 0.830 | 17.4 a | 11.9 a | 7.01 a | 4.79 ± 0.260 |
| BuOH | 2.81 ± 1.29 | 27.4 a | 14.9 a | 14.4 a | nd |
| MeOH | 9.25 ± 3.27 | 65.8 a | 41.2 a | 27.0 a | nd |
| H2O | 46.3 ± 6.80 | 75.5 a | >100 a | >50 a | nd |
| Pos. Control | 0.003 ± 0.001 | 0.382 a | 0.091 ± 0.070 | 0.002 ± 0.000 | 0.007 ± 0.001 |
| Fraction | Tbr | Cytotox. L6 |
|---|---|---|
| F1 | 30.1 a | nd |
| F3 | 14.2 a | nd |
| F5 | 0.673 ± 0.170 | 2.90 a |
| F7 | 0.523 ± 0.400 | 1.16 ± 0.590 |
| F8 | 0.414 ± 0.200 | 0.769 a |
| F9 | 0.312 ± 0.240 | 2.84 ± 1.35 |
| F11 | 14.1 a | nd |
| F12 | 4.37 a | nd |
| Compound | Tbr | Cytotox. L6 | SI |
|---|---|---|---|
| 1 | 0.779 ± 0.220 (2.53) | 10.7 ± 2.05 (34.6) | 13.7 |
| 2 | 0.051 ± 0.035 (0.185) | 0.735 ± 0.052 (2.68) | 14.5 |
| 3 | 0.140 ± 0.100 (0.508) | 0.620 ± 0.013 (2.26) | 4.50 |
| 4 | 0.100 ± 0.041 (0.255) | 1.44 ± 0.099 (3.67) | 14.4 |
| 5 + 6 (2:1) | 0.069 ± 0.005 | 0.532 ± 0.029 | 7.70 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimani, N.M.; Matasyoh, J.C.; Kaiser, M.; Brun, R.; Schmidt, T.J. Anti-Trypanosomatid Elemanolide Sesquiterpene Lactones from Vernonia lasiopus O. Hoffm. Molecules 2017, 22, 597. https://doi.org/10.3390/molecules22040597
Kimani NM, Matasyoh JC, Kaiser M, Brun R, Schmidt TJ. Anti-Trypanosomatid Elemanolide Sesquiterpene Lactones from Vernonia lasiopus O. Hoffm. Molecules. 2017; 22(4):597. https://doi.org/10.3390/molecules22040597
Chicago/Turabian StyleKimani, Njogu M., Josphat C. Matasyoh, Marcel Kaiser, Reto Brun, and Thomas J. Schmidt. 2017. "Anti-Trypanosomatid Elemanolide Sesquiterpene Lactones from Vernonia lasiopus O. Hoffm" Molecules 22, no. 4: 597. https://doi.org/10.3390/molecules22040597
APA StyleKimani, N. M., Matasyoh, J. C., Kaiser, M., Brun, R., & Schmidt, T. J. (2017). Anti-Trypanosomatid Elemanolide Sesquiterpene Lactones from Vernonia lasiopus O. Hoffm. Molecules, 22(4), 597. https://doi.org/10.3390/molecules22040597