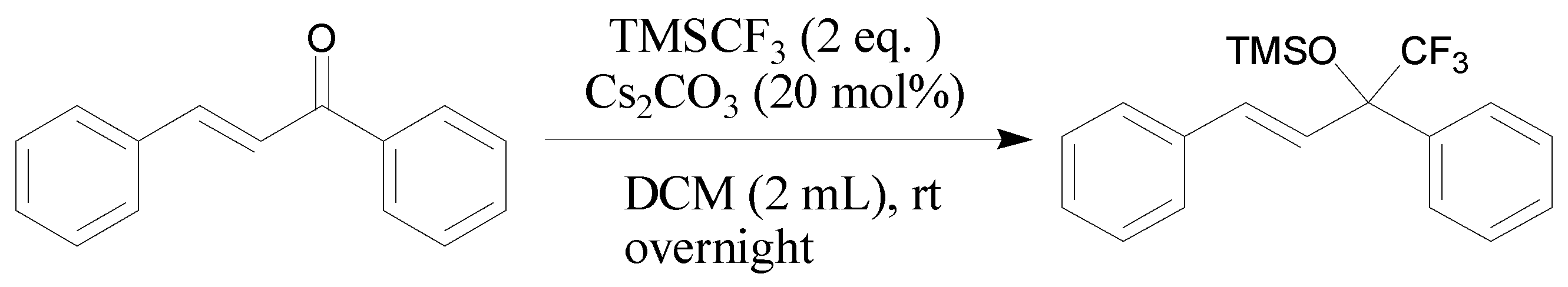Abstract
It was found that 1,2-trifluoromethylation reactions of ketones, enones, and aldehydes were easily accomplished using the Prakash reagent in the presence of catalytic amounts of cesium carbonate, which represents an experimentally convenient, atom-economic process for this anionic trifluoromethylation of non-enolisable aldehydes and ketones.
1. Introduction
The challenge to generate organofluorine molecules featuring a trifluoromethyl motif at a carbon center has increasingly stimulated high interest both in academic and chemical industry research [1,2]. In the past decades, trifluoromethylated compounds have received much attention because of their significant applications as important synthons, biologically active agents, and functional materials that exhibit specific and unique biological and physical features [3,4,5,6,7,8]. Recent reports on the direct introduction of a trifluoromethyl group by electrophilic, nucleophilic, or radical processes have revealed the equally challenging approach of exploiting prochiral trifluoromethylated substrates [4,9,10,11,12,13,14,15,16,17,18,19,20]. In addition, the chemoselective construction of trifluomethylated tertiary alcohols is undoubtedly one of the most fundamental topics in organofluorine chemistry and remains a highly useful process in organic transformations. For this purpose, a direct trifluoromethylation of carbonyl compounds, such as ketones, could be easily completed by nucleophilic addition of TMSCF3 to give trifluomethylated alcohols in the presence of a fluoride catalyst or other Lewis bases, including phosphines, amines, TBD, sodium or lithium acetates, etc., which mediate the silicon-carbon cleavage of TMSCF3 [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47]. Notably, since the first report of Prakash and Olah [23] concerning the trifluoromethylation of benzaldehyde to give organofluorine compounds bearing secondary hydroxyl groups in the presence of fluoride ion reagent there has been a lot of effort devoted to the development of this type of trifluoromethylation reaction, including asymmetric versions of such transformations [22,23,24,25,26,27,28,29,30,31,32,33,34,35]. In this regard, despite the fact that there are several successful synthetic methods in the case of the trifluoromethlytion reactions with TMSCF3, the introduction of commercially available, simple and cheap bases as catalyst precursors for the establishment of a highly efficient and practical trifluomethylation reaction and corresponding one-pot synthesis of trifluoromethylated silyl ethers is still a highly desirable synthetic methodology target.
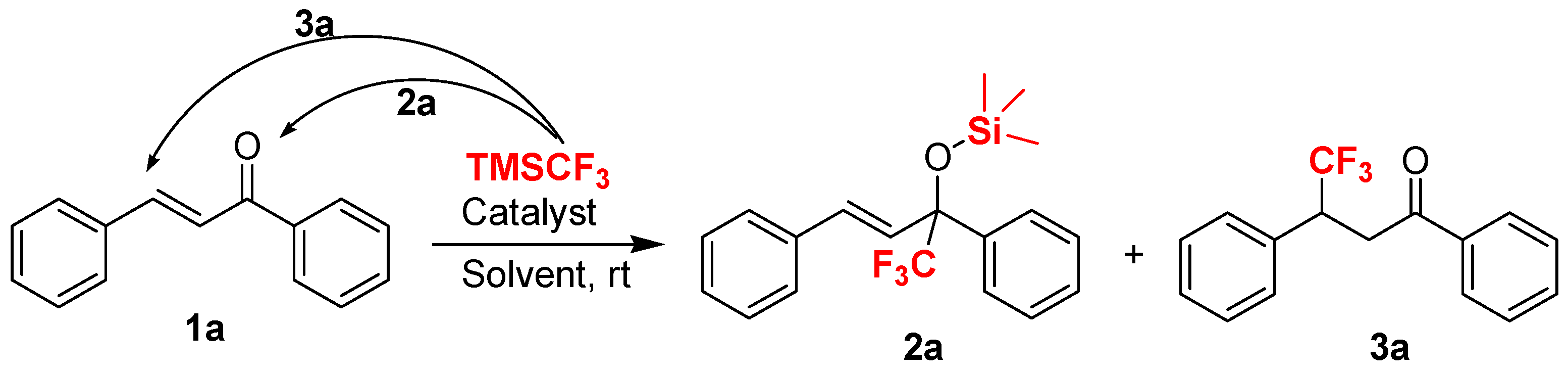
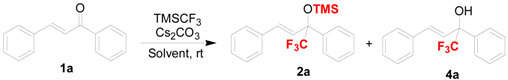
As part of our continuing interest in the catalytic construction of organofluorine molecules [48,49,50,51], we wanted to investigate trifluomethylation reactions of structurally diverse ketones. To evaluate the feasibility of a simple and cheap base-promoted trifluoromethylation in the absence of fluoride reagents, such as tetrabutylammonium fluoride (TBAF), we conducted preliminary experiments on the trifluoromethylation of chalcone 1a with TMSCF3 as a model reaction. In theory, there are two possible pathway for the trifluoromethylation of chalcone with TMSCF3: 1,2-addition and 1,4-conjugate addition [52,53], respectively, in which the chalcone could be converted into two different organofluorine compounds bearing trifluoromethylated groups (Scheme 1).
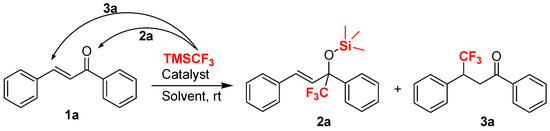
Scheme 1.
The development of new reaction conditions for the trifluoromethylation of chalcone: 1,2-addition versus 1,4-addition.
2. Results and Discussion
Initially, we thought that cupric subcarbonate could be an effective catalyst in the trifluoromethylation of chalcone 1a with TMSCF3 because of the similarity of its copper center and basic carbonate, which could possibly lead to an asymmetric transformation. Unfortunately, cupric subcarbonate has no activity in DCM in this reaction (Table 1, entry 1). Then we optimized the reaction conditions employing various bases to establish a possible copper-catalyzed trifluoro-methylation of chalcone with TMSCF3. After screening a variety of inorganic bases (Table 1, entries 2–12), we found the use of KHF2, KOH, t-BuOK, or Cs2CO3 led to the formation of only product 2a in moderate yield (52–60%) without the formation of 1,4-adduct 3a. Interestingly, the trifluoro-methylated silyl ether 2a was obtained in high yield (94%) in the absence of cupric subcarbonate, which revealed Cs2CO3 was a highly active catalyst in this reaction (Entry 13). Under similar conditions, we found that fluorides and KOAc did not work well in term of the direct synthesis of trifluoromethylated silyl ethers (Entries 14–16 and 18). In addition, we found that K2CO3 gave an inferior yield (only 72% isolated yield of 2a) in this reaction, in comparison to that obtained with Cs2CO3 (Entry 17). Other catalysts, such as KOH and tBuOK, provided the desired product in 83% and 71% yield, respectively (Entries 19 and 20).

Table 1.
The optimization of reaction conditions for trifluoromethylation of chalcone 1a with TMSCF3.
In order to investigate or optimize the reactions, other solvents were examined. It seems that the trifluoromethylation of chalcone has a strong solvent effect. DCM was found to be suitable and the best solvent for this transformation in comparison with others (Table 2, entries 2–9). Notably, when THF was used, although the trifluoromethylation of chalcone occurs smoothly to give high conversion, the chemoselectivity is not good because of the low ratio of silyl ethers 2a and desilylated 4a (Table 2, entry 3, 2a/4a = 36/51). While DMF and DMA was used as solvent, the desilylated trifluoromethylcarbinol could be obtained as the solely product in good yields (Entries 10–11). Notably, the use of Cs2CO3 in CH2Cl2 gave the desired trifluoromethlyated silyl ether 2a without the formation of desilylated product 4a, which exhibited superior chemoselectivity than that in DMF/DMAc for this reaction.

Table 2.
The solvent effect on the Cs2CO3-catalyzed trifluoromethylation of chalcone 1a with TMSCF3.
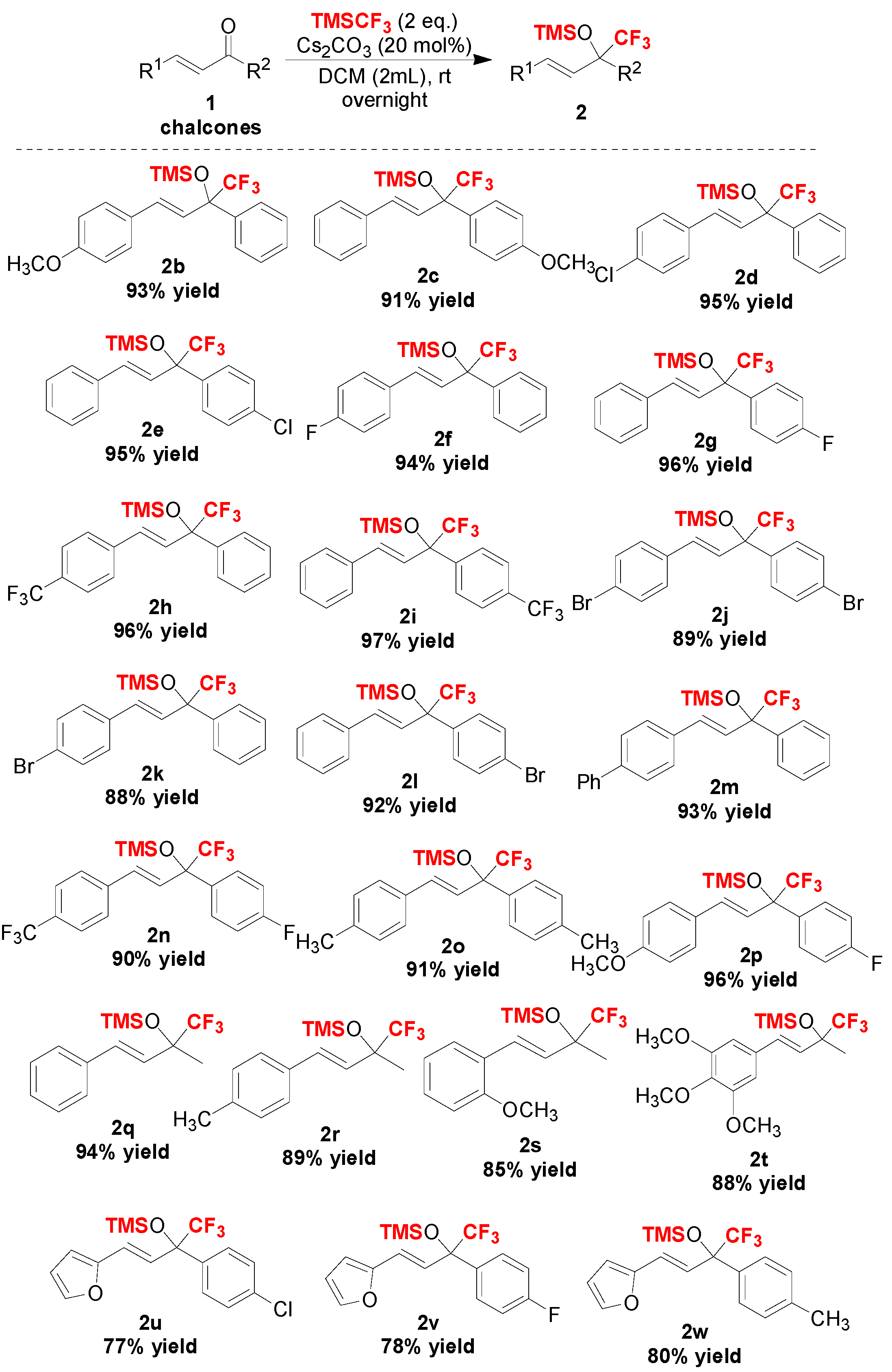
With the optimized reaction conditions in hand (Table 2, entry 1), we further examined the scope of the reaction, and the experimental results are summarized in Scheme 2 and Scheme 3, respectively. In the first part, we demonstrated the general applicability of the Cs2CO3 catalyst in the trifluoromethylation of more than 22 different chalcones (Scheme 2). The results showed that chalcones, including alkyl, aryl, halogen or other substituents on the phenyl moiety showed good to excellent conversion, affording the corresponding trifluoromethylated silyl ethers in high yields (Scheme 2). Moreover, heterocylic enones and alkyl enones were also suitable substrates in this reaction (Scheme 3).
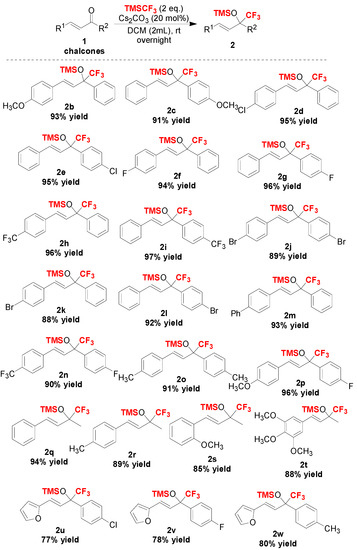
Scheme 2.
Scope of chalcones tested in this trifluoromethylation reaction.
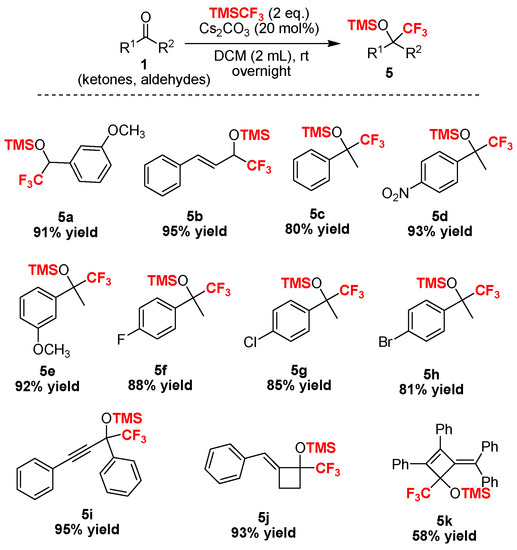
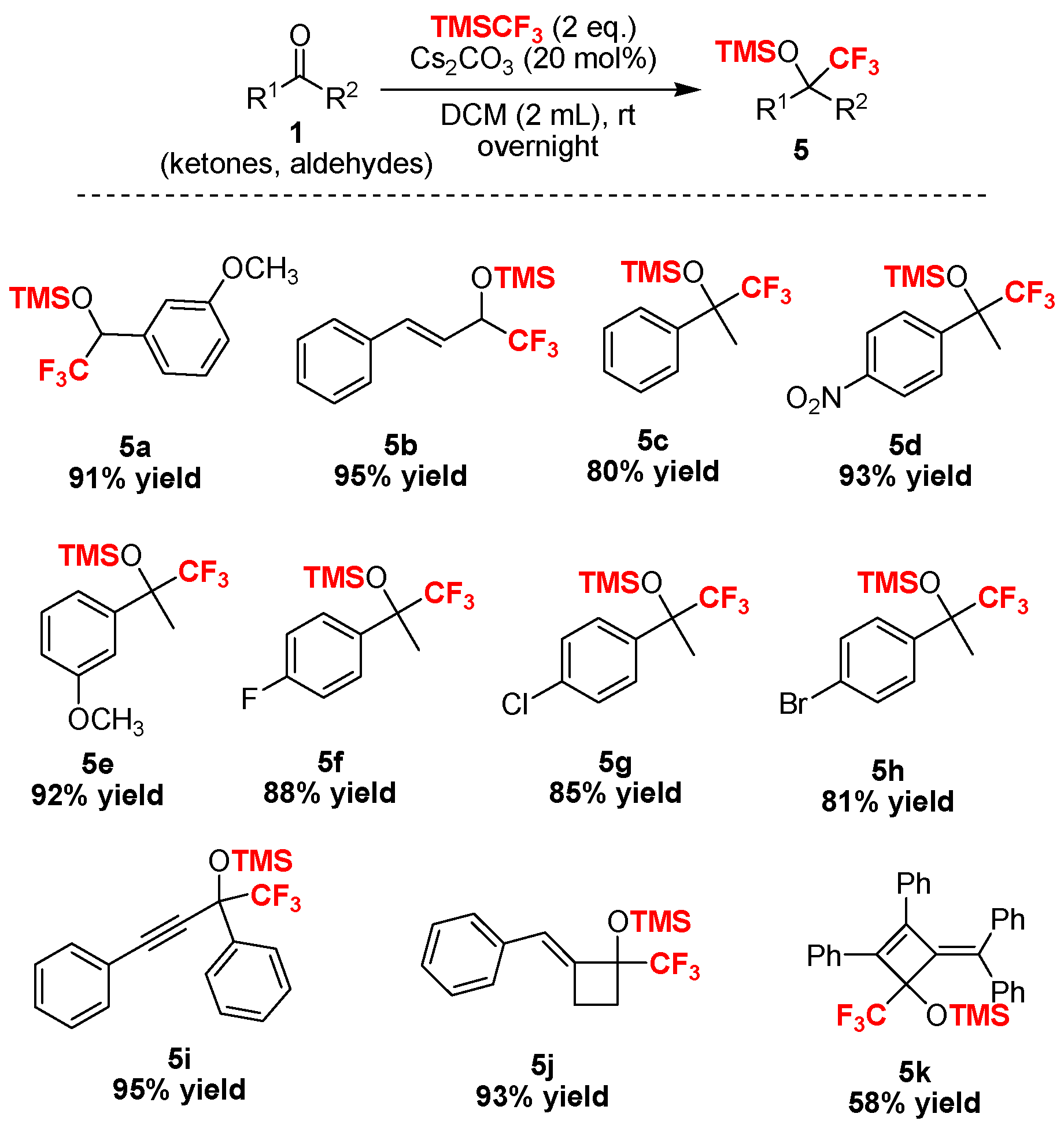
Scheme 3.
Substrate scope of ketones and aldehydes tested in this trifluoromethylation reaction.
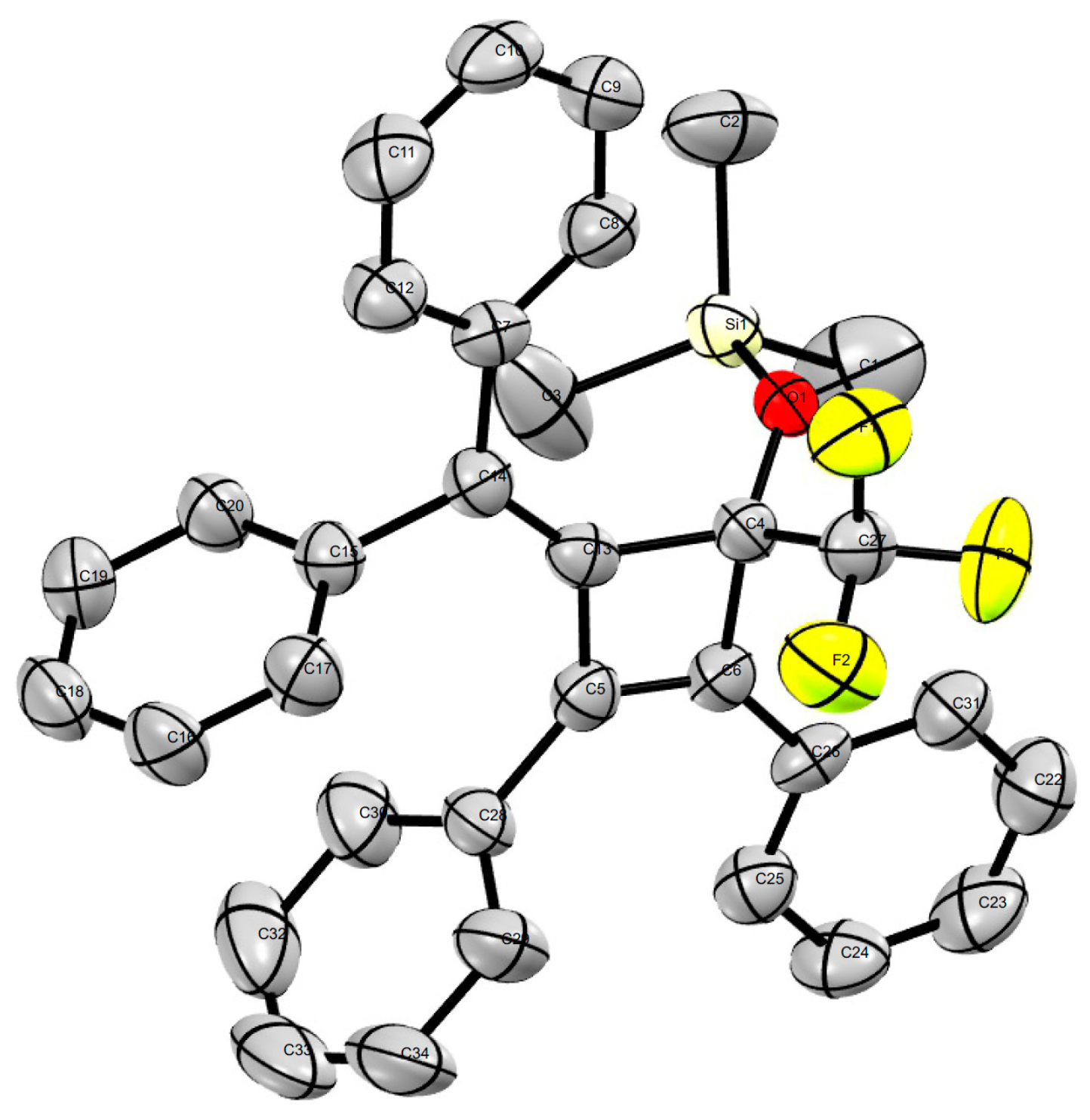
In addition, we found that a broad range of aldehydes and ketones bearing alkynyl and sterically demanding bulky substituents were fully converted into the trifluoromethylated silyl ethers without any evidence of the undesired desilylated byproducts, which illustrated the superior selectivity of this procedure in comparison with fluoride ion-promoted trifluoromethylation. Notably, we recently found that the alkylidenecyclobutenone 1k [54] was a highly reactive compound and could be easily used in ring-opening and ring expansion with Grignard reagents, organolithium species, primary amines, and water [55], in which the four-membered ring was easily broken by the nucleophilic reagent. Interestingly, we found that the four-membered ring was stable in the trifluoromethylation reaction [56] and the desired products 5j or 5k were isolated in promising yields. The structure of the novel compound 5k was unambiguously confirmed by X-ray diffraction analysis (Figure 1). Therefore, this Cs2CO3–initiated trifluoromethylation described can be performed successfully without the need for harsh reaction conditions.
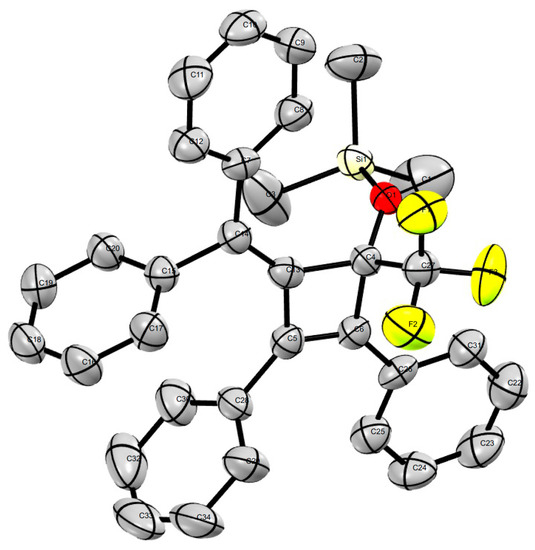
Figure 1.
Crystal structure of 5k (CCDC 1487785).
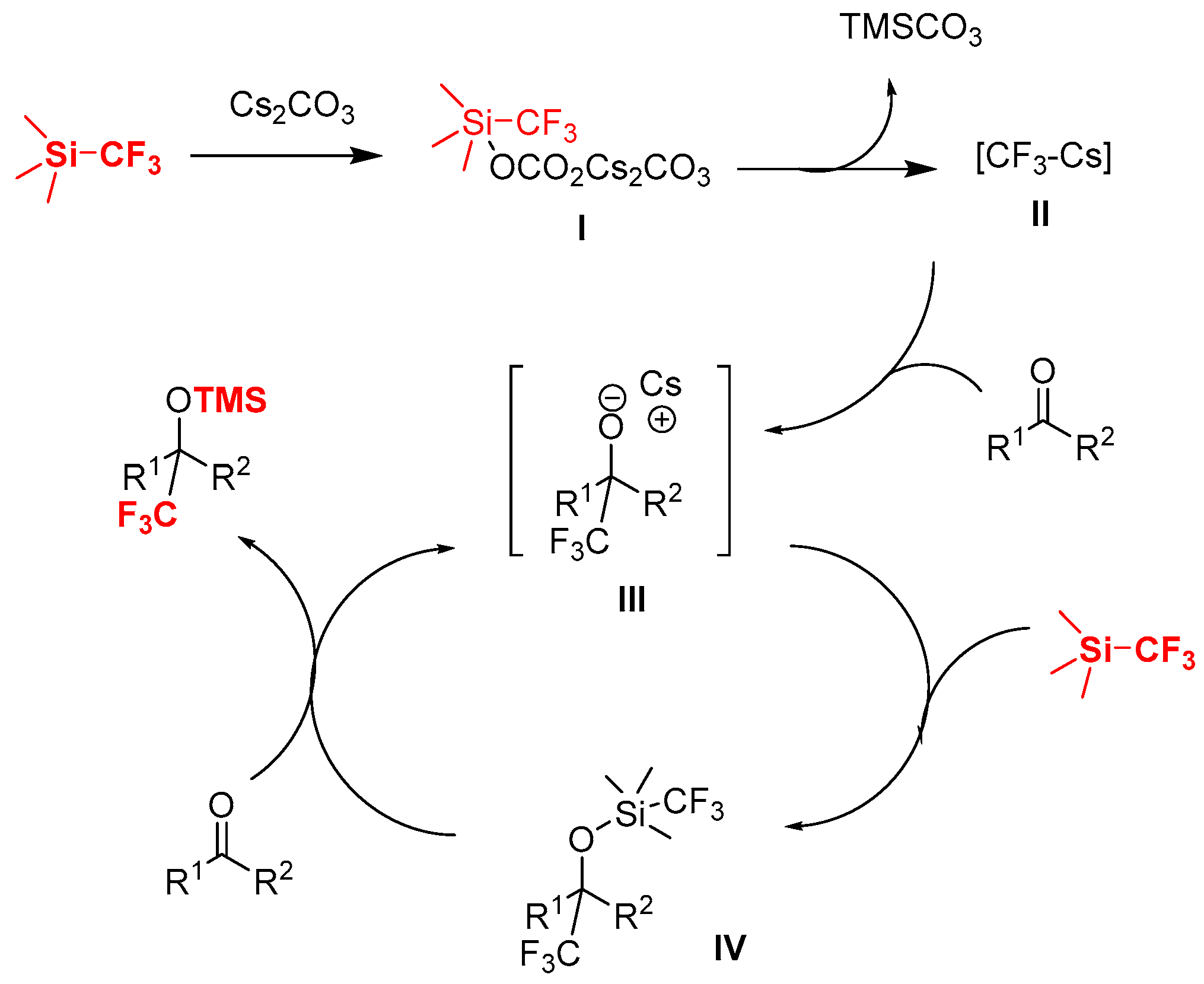
From a mechanistic standpoint, as shown in Scheme 4, it is reasonable to consider that the initiation by carbonate anion of Cs2CO3 leads to the formation of the possible hypervalent silicon complex [3,23,57,58,59,60,61,62] to give trifluoromethylated nucleophilic reagent II, which then reacted with the ketone to give the reactive, and more nucleophilic alkoxide III, and the reaction between III and TMSCF3 leads to the pentavalent complex IV, then IV readily gives the product through CF3 transfer and O-silylation in the presence of ketone with regeneration of the catalyst III.
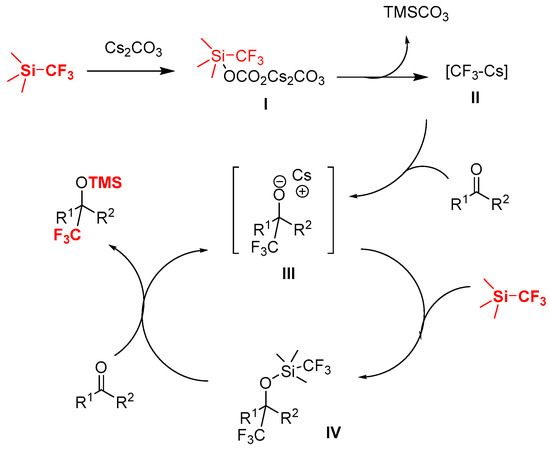
Scheme 4.
Possible mechanism for the Cs2CO3-catalyzed trifluoromethylation of ketones with TMSCF3.
3. Materials and Methods
3.1. General Information
All solvents were purified by standard method. All reagents were received from commercial sources (Aldrich, Shanghai, China; Alfa Aesar, Shanghai, China; TCI, Shanghai, China). NMR spectra were recorded on 500 MHz or 400 MHz spectrometers (Bruker, Shanghai, China). Chemical shifts (δ) are reported in ppm relative to the signal of an internal TMS standard (δ 0.0) Coupling constants (J) are in Hertz (Hz). The following abbreviations were used to explain the multiplicities: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, br = broad, Flash column chromatograph was carried out at medium pressure using 300–400 mesh silica gel (Qingdao Haiyang Chemical Co., Ltd., Qingdao, China). High-resolution mass spectrometry was performed on a JMS-700 MStation (FAB-MS and EI-MS, JEOL, Shanghai, China), 6520 Accurate Mass Q-TOFLC/MS (ESI-MS, Agilent, Shanghai, China) or EXACTIVE Plus (ESI-MS, Thermo Fisher Scientific, Shanghai, China) instrument.
3.2. Experimental Procedures
3.2.1. General Procedure for Cs2CO3-Catalyzed Addition of TMSCF3 and Chalcone 1a (Scheme 5)
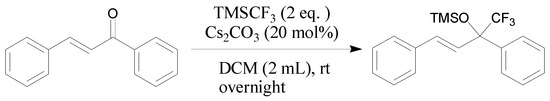
Scheme 5.
The reaction between TMSCF3 and chalcone 1a.
A solution of chalcone (1a, 0.1040 g, 0.5 mmol) and TMSCF3 (0.1420 g, 1.0 mmol) in CH2Cl2 (2.0 mL) and Cs2CO3 (0.0325 g, 0.1 mmol) in a reaction tube were mixed. After stirring at r.t. overnight, once starting material was consumed (monitored by TLC), the mixture was purified by column chromatography (silica gel, petroleum ether/EtOAc = 50:1).
3.2.2. Characterization of Compounds 2a–2w
(1,3-Diphenyl-1-trifluoromethyl-allyloxy)trimethylsilane (2a). 1H-NMR (400 MHz, CDCl3) δ = 7.67–7.56 (m, 2H), 7.44–7.23 (m, 8H), 6.70 (d, J = 16.3 Hz, 1H), 6.56 (d, J = 16.4 Hz, 1H), 0.15 (s, 9H). 13C-NMR (100 MHz, CDCl3) δ = 136.1, 133.7, 133.3, 126.9, 126.4, 125.9, 124.9, 121.7, 78.2 (q, JC-C-F = 28 Hz), 0.0. 19F-NMR (470 MHz, CDCl3) δ = −77.5 (s, 3F), HRMS (ESI) calcd for C19H21F3NaOSi [M + Na]+: 373.1206; found 373.1215.
[3-(4-Methoxyphenyl)-1-phenyl-1-trifluoromethyl-allyloxy]trimethylsilane (2b). 1H-NMR (400 MHz, CDCl3) δ = 7.60 (d, J = 7.3 Hz, 2H), 7.41–7.29 (m, 5H), 6.88 (d, J = 8.6 Hz, 2H), 6.60 (d, J = 16.3 Hz, 1H), 6.42 (d, J = 16.3 Hz, 1H), 3.81 (s, 3H), 0.11 (s, 9H). 13C-NMR (100 MHz, CDCl3) δ = 160.5, 138.1, 134.8, 128.0, 127.8, 124.6, 123.6, 114.2, 80.1 (q, JC-C-F = 29 Hz), 55.2, 1.9. 19F-NMR (470 MHz, CDCl3) δ = −77.6 (s, 3F), HRMS (ESI) calcd for C20H23F3NaO2Si [M + Na]+: 403.1312; found 403.1325.
[1-(4-Methoxyphenyl)-3-phenyl-1-trifluoromethylallyloxy]trimethylsilane (2c). 1H-NMR (400 MHz, CDCl3) δ = 7.50 (d, J = 8.8 Hz, 2H), 7.44–7.27 (m, 5H), 6.95–6.85 (m, 2H), 6.69 (d, J = 16.3 Hz, 1H), 6.53 (d, J = 16.3 Hz, 1H), 3.83 (s, 3H), 0.13 (s, 9H). 13C-NMR (100 MHz, CDCl3) δ = 159.8, 135.8, 135.0, 130.0, 129.3, 128.8, 128.5, 127.1, 126.8, 126.5, 123.6, 113.3, 79.9 (q, JC-C-F = 29 Hz), 55.2, 2.0. 19F-NMR (470 MHz, CDCl3) δ = −77.5 (s, 3F), HRMS (ESI) calcd for C20H23F3NaO2Si [M + Na]+: 403.1312; found 403.1320.
[3-(4-Chlorophenyl)-1-phenyl-1-trifluoromethylallyloxy]trimethylsilane (2d). 1H-NMR (400 MHz, CDCl3) δ = 7.62–7.52 (m, 2H), 7.45–7.28 (m, 7H), 6.65 (d, J = 16.3 Hz, 1H), 6.51 (d, J = 16.3 Hz, 1H), 0.18 (s, 9H). 13C-NMR (100 MHz, CDCl3) δ = 137.8, 136.2, 134.3, 134.2, 134.0, 133.7, 127.9, 127.6, 127.2, 126.1, 119.5, 80.0 (q, JC-C-F = 28 Hz), 3.7, 3.4. 19F-NMR (470 MHz, CDCl3) δ = −77.2 (s, 3F), HRMS (ESI) calcd for C19H20ClF3NaOSi [M + Na]+: 407.0816; found 407.0832.
[1-(4-Chlorophenyl)-3-phenyl-1-trifluoromethylallyloxy]trimethylsilane (2e). 1H-NMR (400 MHz, CDCl3) δ = 7.53 (d, J = 8.6 Hz, 2H), 7.45–7.28 (m, 7H), 6.65 (d, J = 16.4 Hz, 1H), 6.53 (d, J = 16.4 Hz, 1H), 0.19 (s, 9H). 13C-NMR (100 MHz, CDCl3) δ = 136.8, 135.8, 135.7, 135.1, 134.8, 128.9, 128.6, 128.2, 127.7, 126.9, 126.7, 126.5, 126.4, 124.4, 79.9 (q, JC-C-F = 28 Hz), 2.1. 19F-NMR (470 MHz, CDCl3) δ = −77.7 (s, 3F), HRMS (ESI) calcd for C19H20ClF3NaOSi [M + Na]+: 407.0816; found 407.0820.
[3-(4-Fluorophenyl)-1-phenyl-1-trifluoromethylallyloxy]trimethylsilane (2f). 1H-NMR (400 MHz, CDCl3) δ = 7.64–7.54 (m, 2H), 7.44–7.33 (m, 5H), 7.04 (dd, J = 12.0, 5.2 Hz, 2H), 6.65 (d, J = 16.3 Hz, 1H), 6.46 (d, J = 16.3 Hz, 1H), 0.14 (s, 9H). 13C-NMR (100MHz, CDCl3) δ = 164.1, 162.0, 161.7, 137.9, 137.1, 133.9, 128.3, 127.8, 126.1, 115.8 (d, JC-F = 88 Hz), 80.1 (q, JC-C-F = 29 Hz), 1.9. 19F-NMR (470 MHz, CDCl3) δ = −77.3 (s, 3F), −112.7 (s, 1F), HRMS (ESI) calcd for C19H20F4NaOSi [M + Na]+: 391.1112; found 391.1121.
[1-(4-Fluorophenyl)-3-phenyl-1-trifluoromethylallyloxy]trimethylsilane (2g). 1H-NMR (400 MHz, CDCl3) δ = 7.57 (dd, J = 8.6, 5.5 Hz, 2H), 7.45–7.27 (m, 5H), 7.07 (t, J = 8.7 Hz, 2H), 6.67 (d, J = 16.4 Hz, 1H), 6.54 (d, J = 16.4 Hz, 1H), 0.15 (s, 9H). 13C-NMR (100 MHz, CDCl3) δ = 164.3, 163.6, 161.8, 135.7, 130.0, 128.9, 126.9, 115.0 (d, JC-F = 88 Hz), 79.9 (q, JC-C-F = 29 Hz), 2.1. 19F-NMR (470 MHz, CDCl3) δ = −77.8 (s, 3F), −113.7 (s, 1F), HRMS (ESI) calcd for C19H20F4NaOSi [M + Na]+: 391.1112; found 391.1131.
Trimethyl-[1-phenyl-1-trifluoromethyl-3-(4-trifluoromethylphenyl)allyloxy]silane (2h). 1H-NMR (400 MHz, CDCl3) δ = 7.65–7.47 (m, 6H), 7.39 (dt, J = 7.2, 2.2 Hz, 3H), 6.76 (d, J = 16.3 Hz, 1H), 6.63 (d, J = 16.3 Hz, 1H), 0.14 (s, 9H). 13C-NMR (100 MHz, CDCl3) δ = 139.0, 138.9, 137.5, 137.4, 133.0, 126.8, 126.7, 125.6, 125.4, 123.1 (q, JC-F = 312 Hz), 122.3, 79.9 (q, JC-C-F = 27 Hz), 1.7. 19F-NMR (470 MHz, CDCl3) δ = −62.6 (s, 3F), −76.9 (s, 3F), HRMS (ESI) calcd for C20H20F6NaOSi [M + Na]+: 441.1080; found 441.1088.
Trimethyl-[3-phenyl-1-trifluoromethyl-1-(4-trifluoromethylphenyl)allyloxy]silane (2i). 1H-NMR (400 MHz, CDCl3) δ = 7.74 (d, J = 8.3 Hz, 2H), 7.66 (d, J = 8.4 Hz, 2H), 7.43–7.29 (m, 5H), 6.64 (d, J = 16.4 Hz, 1H), 6.55 (d, J = 16.4 Hz, 1H), 0.17 (s, 9H). 13C-NMR (100MHz, CDCl3) δ = 142.1, 135.9, 135.1, 128.9, 128.6, 126.9, 126.42, 124.8, 124.1, 122.9, 80.0 (q, JC-C-F = 28 Hz), 2.0. 19F-NMR (470 MHz, CDCl3) δ = −62.6 (s, 3F), −77.6 (s, 3F), HRMS (ESI) calcd for C20H20F6NaOSi [M + Na]+: 441.1080; found 441.1069.
[1,3-Bis-(4-bromophenyl)-1-trifluoromethylallyloxy]trimethylsilane (2j). 1H-NMR (400 MHz, CDCl3) δ = 7.56–7.42 (m, 6H), 7.28–7.22 (m, 2H), 6.59 (d, J = 16.4 Hz, 1H), 6.50 (d, J = 16.4 Hz, 1H), 0.14 (s, 9H). 13C-NMR (100 MHz, CDCl3) δ = 137.0, 136.9, 134.4, 132.1, 131.2, 129.7, 128.3, 127.2, 123.1 (q, JC-F = 108 Hz), 79.9 (q, JC-C-F = 29 Hz), 2.0. 19F-NMR (470 MHz, CDCl3) δ = −77.6 (s, 3F), HRMS (ESI) calcd for C19H19Br2F3NaOSi [M + Na]+: 528.9416; found 528.9438.
[3-(4-Bromophenyl)-1-phenyl-1-trifluoromethylallyloxy]trimethylsilane (2k). 1H-NMR (400 MHz, CDCl3) δ = 7.62–7.54 (m, 2H), 7.51–7.45 (m, 2H), 7.43–7.35 (m, 3H), 7.29–7.24 (m, 2H), 6.64 (d, J = 16.3 Hz, 1H), 6.53 (d, J = 16.3 Hz, 1H), 0.13 (s, 9H). 13C-NMR (100 MHz, CDCl3) δ = 137.7, 133.7, 131.8, 130.0, 127.7, 126.3 126.1, 126.0, 125.7, 125.9, 120.6, 80.0 (q, JC-C-F = 31 Hz), 1.8. 19F-NMR (470 MHz, CDCl3) δ = −77.2 (s, 3F), HRMS (ESI) calcd for C19H20BrF3NaOSi [M + Na]+: 451.0311; found 451.0322.
[1-(4-Bromophenyl)-3-phenyl-1-trifluoromethylallyloxy]trimethylsilane (2l). 1H-NMR (400 MHz, CDCl3) δ = 7.58–7.44 (m, 4H), 7.36 (dt, J = 12.1, 7.5 Hz, 5H), 6.64 (d, J = 16.4 Hz, 1H), 6.52 (d, J = 16.4 Hz, 1H), 0.15 (s, 9H). 13C-NMR (100 MHz, CDCl3) δ = 137.1, 135.6, 133.4, 129.1, 127.7, 126.8, 124.8, 124.3, 122.8, 79.8 (q, JC-C-F = 27 Hz), 1.9. 19F-NMR (470 MHz, CDCl3) δ = −77.7 (s, 3F), HRMS (ESI) calcd for C19H20BrF3NaOSi [M + Na]+: 451.0311; found 451.0330.
(3-Biphenyl-4-yl-1-phenyl-1-trifluoromethylallyloxy)trimethylsilane (2m). 1H-NMR (400 MHz, CDCl3) δ = 7.65–7.56 (m, 6H), 7.51–7.33 (m, 8H), 6.73 (d, J = 16.3 Hz, 1H), 6.60 (d, J = 16.4 Hz, 1H), 0.17 (s, 9H). 13C-NMR (100 MHz, CDCl3) δ = 139.5, 138.4, 136.0, 132.7, 126.7, 126.5, 125.9, 125.5, 125.2, 124.9, 78.1 (q, JC-C-F = 22 Hz), 0.1. 19F-NMR (470 MHz, CDCl3) δ = −77.3 (s, 3F), HRMS (ESI) calcd for C25H25F3NaOSi [M + Na]+: 449.1519; found 449.1528.
[1-(4-Fluorophenyl)-1-trifluoromethyl-3-(4-trifluoromethylphenyl)allyloxy]trimethylsilane (2n). 1H-NMR (400 MHz, CDCl3) δ = 7.62 (d, J = 8.2 Hz, 2H), 7.58–7.48 (m, 4H), 7.13–7.04 (m, 2H), 6.73 (d, J = 16.4 Hz, 1H), 6.61 (d, J = 16.3 Hz, 1H), 0.14 (s, 9H). 13C-NMR (100 MHz, CDCl3) δ = 162.2, 159.7, 137.0, 137.0, 131.7, 128.8, 128.6, 128.0, 127.9, 125.0, 123.9 (q, JC-F = 16 Hz), 113.1, 113.0, 112.9, 77.7 (q, JC-C-F = 29 Hz), 0.0. 19F-NMR (470 MHz, CDCl3) δ = −62.7 (s, 3F), −77.4 (s, 3F), −113.3 (s, 1F), HRMS (ESI) calcd for C20H19F7NaOSi [M + Na]+: 459.0986; found 459.0989.
(1,3-Di-p-tolyl-1-trifluoromethylallyloxy)trimethylsilane (2o). 1H-NMR (400 MHz, CDCl3) δ = 7.47 (d, J = 8.1 Hz, 2H), 7.30 (d, J = 8.1 Hz, 2H), 7.17 (dd, J = 13.2, 8.1 Hz, 4H), 6.65 (d, J = 16.3 Hz, 1H), 6.49 (d, J = 16.3 Hz, 1H), 2.36 (d, J = 10.7 Hz, 6H), 0.13 (s, 9H). 13C-NMR (100 MHz, CDCl3) δ = 136.5, 136.2, 133.1, 131.0, 127.4, 126.5, 125.9, 124.7, 123.9, 78.0 (q, JC-C-F = 24 Hz), 19.1, 0.0. 19F-NMR (470 MHz, CDCl3) δ = −77.8 (s, 3F), HRMS (ESI) calcd for C21H25F3NaOSi [M + Na]+: 401.1519; found 401.1525.
[1-(4-Fluorophenyl)-3-(4-methoxyphenyl)-1-trifluoromethylallyloxy]trimethylsilane (2p). 1H-NMR (400 MHz, CDCl3) δ = 7.57 (dd, J = 8.5, 5.5 Hz, 2H), 7.34 (d, J = 8.7 Hz, 2H), 7.13–7.02 (m, 2H), 6.89 (d, J = 8.7 Hz, 2H), 6.57 (d, J = 16.4 Hz, 1H), 6.40 (d, J = 16.4 Hz, 1H), 3.82 (s, 3H), 0.14 (s, 9H). 13C-NMR (100 MHz, CDCl3) δ = 162.0, 159.6, 158.2, 133.2, 132.0 (d, J = 3.1 Hz), 127.9, 126.19, 122.3, 112.7 (d, J = 84 Hz), 112.6, 112.3, 77.8 (q, JC-C-F = 29 Hz), 53.3, 0.0. 19F-NMR (470 MHz, CDCl3) δ = −78.1 (s, 3F), −113.9 (s, 1F), HRMS (ESI) calcd for C20H22F4NaO2Si [M + Na]+: 421.1217; found 421.1227.
Trimethyl-(1-methyl-3-phenyl-1-trifluoromethylallyloxy)silane (2q) [23]. 1H-NMR (400 MHz, CDCl3) δ = 7.39–7.13 (m, 5H), 6.67(dd, J = 16.1, 2.1 Hz, 1H), 6.18 (d, J = 16.0 Hz, 1H), 1.51 (s, 3H), 0.12 (s, 9H). 13C-NMR (100 MHz, CDCl3) δ = 136.0, 132.6, 128.7, 128.3, 127.6, 126.8, 73.9 (q, JC-C-F = 29 Hz), 21.7, 2.1. 19F-NMR (470 MHz, CDCl3) δ = −82.1 (s, 3F), HRMS (ESI) calcd for C14H19F3NaOSi [M + Na]+: 311.1049; found 311.1040.
Trimethyl-(1-methyl-3-p-tolyl-1-trifluoromethylallyloxy)silane (2r). 1H-NMR (500 MHz, CDCl3) δ = 7.37–7.29 (m, 2H), 6.90–6.81 (m, 2H), 6.67 (dd, J = 16.0, 7.1 Hz, 1H), 6.11 (dd, J = 16.0, 6.9 Hz, 1H), 3.81 (d, J = 7.2 Hz, 3H), 1.58 (s, 3H), 0.18 (s, 9H). 13C-NMR (125 MHz, CDCl3) δ = 159.7, 131.9, 128.6, 128.0, 126.5, 125.3, 124.2, 114.1, 76.0 (q, JC-C-F = 24 Hz), 55.3, 21.6, 2.1. 19F-NMR (470 MHz, CDCl3) δ = −77.3 (s, 3F), HRMS (ESI) calcd for C15H21F3OSi [M + H]+: 303.1225; found 303.1229.
[3-(2-Methoxyphenyl)-1-methyl-1-trifluoromethylallyloxy]trimethylsilane (2s). 1H-NMR (500 MHz, CDCl3) δ= 7.43 (d, J = 7.6 Hz, 1H), 7.26 (d, J = 7.5 Hz, 1H), 7.09 (d, J = 16.3 Hz, 1H), 6.99–6.84 (m, 2H), 6.30 (d, J = 16.2 Hz, 1H), 3.84 (d, J = 2.9 Hz, 3H), 1.60 (s, 3H), 0.19 (s, 9H). 13C-NMR (125 MHz, CDCl3) δ = 157.1, 129.3, 127.7, 127.1, 126.5, 124.9, 124.3, 120.7, 111.0, 76.3 (q, JC-C-F = 24 Hz), 55.4, 21.6, 2.1. 19F-NMR (470 MHz, CDCl3) δ = −82.2 (s, 3F), HRMS (ESI) calcd for C15H21F3NaO2Si [M + Na]+: 341.1155; found 341.1158.
Trimethyl-[1-methyl-1-trifluoromethyl-3-(3,4,5-trimethoxyphenyl)allyloxy]silane (2t). 1H-NMR (500 MHz, CDCl3) δ = 6.68 (d, J = 15.9 Hz, 1H), 6.64 (s, 2H), 6.16 (d, J = 15.9 Hz, 1H), 3.90 (s, 9H), 1.60 (s, 3H), 0.21 (s, 9H). 13C-NMR (125 MHz, CDCl3) δ = 153.4, 138.4, 132.5, 131.5, 127.0, 126.4, 124.1, 105.4, 103.9, 75.9 (q, JC-C-F = 23 Hz), 60.8, 56.1, 21.6, 2.1. 19F-NMR (470 MHz, CDCl3) δ = −82.2 (s, 3F), HRMS (ESI) calcd for C17H26F3O4Si [M + H]+: 379.1547; found 379.1556.
[1-(4-Chlorophenyl)-3-furan-2-yl-1-trifluoromethylallyloxy]trimethylsilane (2u). 1H-NMR (400 MHz, CDCl3) δ = 7.51 (d, J = 8.6 Hz, 2H), 7.41 (d, J = 1.5 Hz, 1H), 7.39–7.33 (m, 2H), 6.45 (d, J = 7.1 Hz, 2H), 6.41 (dd, J = 3.3, 1.8 Hz, 1H), 6.33 (d, J = 3.3 Hz, 1H), 0.15 (s, 9H). 13C-NMR (100 MHz, CDCl3) δ = 149.2, 141.2, 134.5, 132.7, 127.4, 126.2, 122.7, 121.5, 109.6, 108.6, 77.5 (q, JC-C-F = 29 Hz), 0.0. 19F-NMR (470 MHz, CDCl3) δ = −77.7 (s, 3F), HRMS (ESI) calcd for C17H18ClF3NaO2Si [M + Na]+: 397.0609; found 397.0627.
[1-(4-Fluorophenyl)-3-furan-2-yl-1-trifluoromethylallyloxy]trimethylsilane (2v). 1H-NMR (400 MHz, CDCl3) δ = 7.56 (dd, J = 8.5, 5.4 Hz, 2H), 7.39 (d, J = 1.6 Hz, 1H), 7.09–7.01 (m, 2H), 6.48 (d, J = 5.9 Hz, 2H), 6.39 (dd, J = 3.3, 1.8 Hz, 1H), 6.32 (d, J = 3.3 Hz, 1H), 0.15 (s, 9H). 13C-NMR (100 MHz, CDCl3) δ = 162.2, 159.7, 149.4, 141.2, 131.7, 127.9, 123.0, 121.5, 112.9 (d, JC-F = 22 Hz), 109.6, 108.5, 77.6 (q, JC-C-F = 29 Hz), 0.0. 19F-NMR (470 MHz, CDCl3) δ = −77.9 (s, 3F), −113.8 (s, 1F), HRMS (ESI) calcd for C17H18F4NaO2Si [M + Na]+: 381.0904; found 381.0915.
(3-Furan-2-yl-1-p-tolyl-1-trifluoromethylallyloxy)trimethylsilane (2w). 1H-NMR (400 MHz, CDCl3) δ = 7.46 (d, J = 8.1 Hz, 2H), 7.38 (d, J = 1.5 Hz, 1H), 7.18 (d, J = 8.1 Hz, 2H), 6.49 (d, J = 2.7 Hz, 2H), 6.38 (dd, J = 3.3, 1.8 Hz, 1H), 6.30 (d, J = 3.3 Hz, 1H), 2.36 (s, 3H), 0.13 (s, 9H). 13C-NMR (100 MHz, CDCl3) δ = 149.6, 141.0, 136.4, 132.8, 126.7, 125.89, 123.5, 121.1, 109.5, 108.1, 77.9 (q, JC-C-F = 29Hz), 19.0, 0.0. 19F-NMR (470 MHz, CDCl3) δ = −77.5 (s, 3F), HRMS (ESI) calcd for C18H21F3NaO2Si [M + Na]+: 377.1155; found 377.1169.
3.2.3. Characterization of Compounds 5a–5k
The spectral data of compounds 5a, 5b, 5c, 5d, 5e, 5g, 5h and 5i matched the reported data in all respects [42,44,45,63].
Trimethyl-[2,2,2-trifluoro-1-(4-fluorophenyl)-1-methylethoxy]silane (5f). 1H-NMR (500 MHz, CDCl3) δ = 7.56 (dd, J = 8.3, 5.5 Hz, 2H), 7.13–7.02 (m, 2H), 1.85 (s, 3H), 0.19 (s, 9H). 13C-NMR (125 MHz, CDCl3) δ = 163.7, 161.8, 135.9, 128.6, 126.3, 124.0, 114.8, 114.7, 76.0 (q, JC-C-F = 24 Hz), 22.6, 1.8. 19F-NMR (470 MHz, CDCl3) δ = −81.9 (s, 3F), −114.2 (s, 1F), HRMS (ESI) calcd for C12H17F4OSi [M + H]+: 281.0979; found 281.0983.
(2-Benzylidene-1-trifluoromethylcyclobutoxy)trimethylsilane (5j). 1H-NMR (500 MHz, CDCl3) δ = 7.30 (ddd, J = 26.9, 16.5, 6.9 Hz, 5H), 6.59 (d, J = 2.4 Hz, 1H), 2.89 (dt, J = 14.7, 5.9 Hz, 2H), 2.63 (ddd, J = 7.1, 5.8, 4.4 Hz, 1H), 2.37 (d, J = 10.5 Hz, 1H), 0.20 (s, 9H). 13C-NMR (125 MHz, CDCl3) δ = 140.0, 136.1, 128.6, 128.2, 127.5, 126.2, 126.0, 123.7, 79.5 (q, JC-C-F = 25 Hz), 30.73, 29.7, 25.9, 1.6. 19F-NMR (470 MHz, CDCl3) δ = −82.9 (s, 3F), HRMS (ESI) calcd for C15H20F3OSi [M + H]+: 301.1230; found 301.1233.
(4-Benzhydrylidene-2,3-diphenyl-1-trifluoromethylcyclobut-2-enyloxy)trimethylsilane (5k). 1H-NMR (400 MHz, CDCl3) δ = 7.47–7.39 (m, 4H), 7.28–7.18 (m, 7H), 7.08 (d, J = 7.3 Hz, 1H), 7.01 (t, J = 7.4 Hz, 2H), 6.85 (dd, J = 8.1, 5.2 Hz, 6H), 0.16 (s, 9H). 13C-NMR (100 MHz, CDCl3) δ = 145.3, 139.5, 133.0, 131.0, 130.4, 130.2, 128.6, 128.1, 127.9, 127.5, 126.9, 126.7, 1.6. 19F-NMR (470 MHz, CDCl3) δ = −73.2 (s, 3F), HRMS (ESI) calcd for C33H30F3OSi [M + H]+: 527.2013; found 527.2017.
All the 1H-NMR and 13C-NMR spectra of compounds 2a–2w and 5a–5k can be found in Supplementary Materials. CCDC 1487785 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44 1223 336033; E-mail: deposit@ccdc.cam.ac.uk).
4. Conclusions
In summary, we have developed a simple and very efficient method for the synthesis of trifluoromethylated silyl ethers via the direct nucleophilic trifluoromethylation of ketones, enones, and aldehydes with TMS-CF3 in the presence of catalytic amounts of Cs2CO3. The reaction features an experimentally convenient and atom-economic process for this anionic 1,2-trifluoromethylation. This work also provides a good example of carbonate ion as an active anion that can interact with silicon to promote the chemoselective cleavage of the silicon-carbon bond in TMSCF3, but is not effective in the desilylation of trifluoromethylated silyl ethers.
Supplementary Materials
Supplementary Materials are available online.
Acknowledgments
The authors gratefully thank the financial support of the National Natural Science Foundation of China (21472031, and 21503060), Zhejiang Provincial Natural Science Foundation of China (LY17E030003, LR14B030001), Science and Technology Department of Zhejiang Province (2014C31131, 2015C31138), and Hangzhou Science and Technology Bureau of China (20140432B04, 20160432B08).
Author Contributions
L.-W.X. and Z.-J.Z. conceived and designed the experiments; C.D. performed the experiments; J.-Y.L., Y.-M.C. and J.C. analyzed the data; L.-W.X. and Z.-J.Z. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References and Notes
- Nie, J.; Guo, H.C.; Cahard, D.; Ma, J.A. Asymmetric Construction of Stereogenic Carbon Centers Featuring a Trifluoromethyl Group from Prochiral Trifluoromethylated Substrates. Chem. Rev. 2011, 111, 455–529. [Google Scholar] [CrossRef] [PubMed]
- Dolfen, J.; de Kimpe, N.; D’hooghe, M. Deployment of Small-Ring Azaheterocycles as Building Blocks for the Synthesis of Organofluorine Compounds. Synlett 2016, 27, 1486–1510. [Google Scholar] [CrossRef]
- Prakash, G.K.S.; Yudin, A.K. Perfluoroalkylation with Organosilicon Reagents. Chem. Rev. 1997, 97, 757–786. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.A.; Cahard, D. Asymmetric Fluorination, Trifluoromethylation, and Perfluoroalkylation Reactions. Chem. Rev. 2004, 104, 6119–6146. [Google Scholar] [CrossRef]
- Caron, S.; Do, N.M.; Sieser, J.E.; Arpin, P.; Vazquez, E. Process Research and Development of an NK-1 Receptor Antagonist. Enantioselective Trifluoromethyl Addition to a Ketone in the Preparation of a Chiral Isochroman. Org. Proc. Res. Dev. 2007, 11, 1015–1024. [Google Scholar] [CrossRef]
- Boechat, N.; Bastos, M.M. Trifluoromethylation of Carbonyl Compounds. Curr. Org. Chem. 2010, 7, 403–410. [Google Scholar] [CrossRef]
- Tomashenko, O.A.; Grushin, V.V. Aromatic trifluoromethylation with metal complexes. Chem. Rev. 2011, 111, 4475–4521. [Google Scholar] [CrossRef] [PubMed]
- Konno, T. Trifluoromethylated internal alkynes: Versatile building blocks for the preparation of various fluorine-containing molecules. Synlett 2014, 25, 1350–1370. [Google Scholar] [CrossRef]
- Ma, J.A.; Cahard, D. Strategies for nucleophilic, electrophilic, and radical trifluoromethylations. J. Fluor. Chem. 2007, 128, 975–996. [Google Scholar] [CrossRef]
- Shibata, N.; Mizuta, S.; Kawai, H. Recent advances in enantioselective trifluoromethylation reactions. Tetrahedron Asymmetry 2008, 19, 2633–2644. [Google Scholar] [CrossRef]
- Nagib, D.A.; Scott, M.E.; MacMillan, D.W.C. Enantioselective α-Trifluoromethylation of Aldehydes via Photoredox Organocatalysis. J. Am. Chem. Soc. 2009, 131, 10875–10877. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.E.; MacMillan, D.W.C. The Productive Merger of Iodonium Salts and Organocatalysis: A Non-photolytic Approach to the Enantioselective α-Trifluoromethylation of Aldehydes. J. Am. Chem. Soc. 2010, 132, 4986–4987. [Google Scholar] [CrossRef] [PubMed]
- Pham, P.V.; Nagib, D.A.; MacMillan, D.W.C. Photoredox Catalysis: A Mild, Operationally Simple Approach to the Synthesis of α-Trifluoromethyl Carbonyl Compounds. Angew. Chem. Int. Ed. 2011, 50, 6119–6122. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, T.; Nishimine, T.; Tokunaga, E.; Hasegawa, K.; Shiro, M.; Shibata, N. Organocatalyzed Regio- and Enantioselective Allylic Trifluoromethylation of Morita-Baylis-Hillman Adducts Using Ruppert-Prakash Reagent. Org. Lett. 2011, 13, 3972–3975. [Google Scholar] [CrossRef] [PubMed]
- Matousek, V.; Togni, A.; Bizet, V.; Cahard, D. Synthesis of α-CF3-substituted carbonyl compounds with relative and absolute stereocontrol using electrophilic CF3-transfer reagents. Org. Lett. 2011, 13, 5762–5765. [Google Scholar] [CrossRef] [PubMed]
- Prakash, G.K.S.; Jog, P.V.; Batamack, P.T.D.; Olah, G.A. Taming of Fluoroform: Direct Nucleophilic Trifluoromethylation of Si, B, S, and C Centers. Science 2012, 338, 1324–1327. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, R.R.; Jia, Y.X. Asymmetric friedel-crafts alkylation reaction in the construction of trifluoromethylated all-carbon quaternary stereocenters. Synlett 2014, 25, 457–460. [Google Scholar] [CrossRef]
- Liu, H.Q.; Zhang, Z.P.; Dong, W.; Luo, X.Z. A practical method for metal-free radical trifluoromethylation of styrenes with NaSO2CF3. Synlett 2014, 25, 1307–1311. [Google Scholar]
- Zhang, Z.K.; Feng, J.J.; Xu, Y.; Zhang, S.N.; Ye, X.X.; Li, T.J.; Wang, X.; Chen, J.; Zhang, Y.; Wang, J.B. Synthesis of trifluoromethylated cycloheptatrienes from N-tosylhydrazones: Transition-metal-free Buchner ring expansion. Synlett 2015, 26, 59–62. [Google Scholar] [CrossRef]
- Zhu, C.L.; Zhang, Y.Q.; Yuan, Y.A.; Xu, H. Copper-Catalyzed Aerobic C-H Trifluoromethylation of Phenanthrolines. Synlett 2015, 26, 345–349. [Google Scholar] [PubMed]
- Prakash, G.K.S.; Krishnamurti, R.; Olah, G.A. Synthetic methods and reactions. 141. Fluoride-induced trifluoromethylation of carbonyl compounds with trifluoromethyltrimethylsilane (TMS-CF3). A trifluoromethide equivalent. J. Am. Chem. Soc. 1989, 111, 393–395. [Google Scholar] [CrossRef]
- Singh, R.P.; Kirchmeier, R.L.; Shreeve, J.M. CsF-Catalyzed Nucleophilic Trifluoromethylation of trans-Enones with Trimethyl(trifluoromethyl)silane: A Facile Synthesis of trans-α-Trifluoromethyl Allylic Alcohols. Org. Lett. 1999, 1, 1047–1049. [Google Scholar] [CrossRef]
- Motherwell, W.B.; Storey, L.J. Trifluoromethylacetophenone-N,N-dimethyltrimethylsilylamine Adduct—A New Shelf Stable Reagent for Nucleophilic Trifluoromethylation. Synlett 2002, 4, 646–648. [Google Scholar] [CrossRef]
- Notte, G.T.; Sammakia, T.; Steel, P.J. Kinetic Resolution of α-Acetoxy N-Acyl Oxazolidinethiones by a Chiral O-Nucleophilic Acyl Transfer Catalyst. J. Am. Chem. Soc. 2005, 127, 13502–13503. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, R.; Sayalero, S.; Vicente, M.; Maestro, A. An Efficient Synthesis of Enantiomerically Enriched Trifluoromethylated 1,2-Diols and 1,2-Amino Alcohols with Quaternary Stereocenters by Diastereoselective Addition of TMSCF3 to Chiral 2-Acyl-1,3-perhydrobenzoxazines. J. Org. Chem. 2006, 71, 2177–2180. [Google Scholar] [CrossRef] [PubMed]
- Mizuta, S.; Shibata, N.; Akiti, S.; Fujimoto, H.; Nakamura, S.; Toru, T. Cinchona alkaloids/TMAF combination-catalyzed nucleophilic enantioselective trifluoromethylation of aryl ketones. Org. Lett. 2007, 9, 3707–3710. [Google Scholar] [CrossRef] [PubMed]
- Mizuta, S.; Shibata, N.; Hibino, M.; Nagano, S.; Nakamura, S.; Toru, T. Ammonium bromides/KF catalyzed trifluoromethylation of carbonyl compounds with (trifluoromethyl)trimethylsilane and its application in the enantioselective trifluoromethylation reaction. Tetrahedron 2007, 63, 8521–8528. [Google Scholar] [CrossRef]
- Nonnenmacher, J.; Massicot, F.; Grellepois, F.; Portella, C. Enantiopure Quaternary α-Trifluoromethyl-α-alkoxyaldehydes from L-Tartaric Acid Derived Ketoamides. J. Org. Chem. 2008, 73, 7990–7995. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, J.; Li, W.; Lin, L.; Liu, X.; Feng, X. Cinchona alkaloid-derived quaternary ammonium salt combined with NaH: A facile catalyst system for the asymmetric trifluoromethylation of ketones. Tetrahedron Lett. 2009, 50, 4378–4380. [Google Scholar] [CrossRef]
- Kawai, H.; Tachi, K.; Tokunaga, E.; Shiro, M.; Shibata, N. Cinchona Alkaloid-Catalyzed Asymmetric Trifluoromethylation of Alkynyl Ketones with Trimethylsilyl Trifluoromethane. Org. Lett. 2010, 12, 5104–5107. [Google Scholar] [CrossRef] [PubMed]
- Kawai, H.; Kitayama, T.; Tokunaga, E.; Shibata, N. A New Synthetic Approach to Efavirenz through Enantioselective Trifluoromethylation by Using the Ruppert-Prakash Reagent. Eur. J. Org. Chem. 2011, 2011, 5959–5961. [Google Scholar] [CrossRef]
- Wu, S.; Zeng, W.; Wang, Q.; Chen, F.X. Asymmetric trifluoromethylation of aromatic aldehydes by cooperative catalysis with (IPr)CuF and quinidine-derived quaternary ammonium salt. Org. Biomol. Chem. 2012, 10, 9334–9337. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Shreeve, J.M. Synthesis and characterization of novel trifluoromethyl-containing alcohols with Ruppert’s reagent. J. Fluor. Chem. 2012, 133, 20–26. [Google Scholar] [CrossRef]
- Kawai, H.; Yuan, Z.; Tokunaga, E.; Shibata, N. A sterically demanding organo-superbase avoids decomposition of a naked trifluoromethyl carbanion directly generated from fluoroform. Org. Biomol. Chem. 2013, 11, 1446–1450. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Guo, J.; Sohail, M.; Cao, C.; Chen, F.X. The enantioselective trifluoromethylation of aromatic aldehydes by quaternary ammonium bromide and (IPr)CuF at low catalyst loading. J. Fluor. Chem. 2013, 148, 19–29. [Google Scholar] [CrossRef]
- Mukaiyama, T.; Kawano, Y.; Fujisawa, H. Lithium acetate–catalyzed trifluoromethylation of carbonyl compounds with (trifluoromethyl)trimethylsilane. Chem. Lett. 2005, 34, 88–89. [Google Scholar] [CrossRef]
- Kawano, Y.; Kaneko, N.; Mukaiyama, T. Lewis Base-catalyzed perfluoroalkylation of carbonyl compounds and imines with (perfluoroalkyl)trimethylsilane. Bull. Chem. Soc. Jpn. 2006, 79, 1133–1145. [Google Scholar] [CrossRef]
- Matsukawa, S.; Saijo, M. TTMPP-catalyzed trifluoromethylation of carbonyl compounds and imines with trifluoromethylsilane. Tetrahedron Lett. 2008, 49, 4655–4657. [Google Scholar] [CrossRef]
- Liu, X.; Xu, X.X.; Pan, L.; Zhang, Q.; Liu, Q. Efficient synthesis of trifluoromethylated cyclopentadienes/fulvenes/norbornenes from divinyl ketones. Org. Biomol. Chem. 2013, 11, 6703–6706. [Google Scholar] [CrossRef] [PubMed]
- Iakovenko, R.O.; Kazakova, A.N.; Muzalevskiy, V.M.; Ivanov, A.Y.; Boyarskaya, I.A.; Chicca, A.; Petrucci, V.; Gertsch, J.; Krasavin, M.; Starova, G.L.; et al. Reactions of CF3-enones with arenes under superelectrophilic activation: A pathway to trans-1,3-diaryl-1-CF3-indanes, new cannabinoid receptor ligands. Org. Biomol. Chem. 2015, 13, 8827–8842. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, C.; Wang, M.; Liu, Q. Trifluoromethyltrimethylsilane: Nucleophilic Trifluoromethylation and Beyond. Chem. Rev. 2015, 115, 683–730. [Google Scholar] [CrossRef] [PubMed]
- Matsukawa, S.; Takahashi, S.; Takahashi, H. TBD-Catalyzed Trifluoromethylation of Carbonyl Compounds with (Trifluoromethyl)-Trimethylsilane. Synth. Commun. 2013, 43, 1523–1529. [Google Scholar] [CrossRef]
- Iwanami, K.; Oriyama, T. A New and Efficient Method for the Trifluoromethylation of Carbonyl Compounds with Trifluoromethyltrimethylsilane in DMSO. Synlett 2006, 1, 112–114. [Google Scholar] [CrossRef]
- Kusuda, A.; Kawai, H.; Nakamura, S.C.; Shibata, N. Solkane R 365mfc is an environmentally benign alternative solvent for trifluoromethylation reactions. Green Chem. 2009, 11, 1733–1735. [Google Scholar] [CrossRef]
- Nagao, H.; Yamane, Y.; Mukaiyama, T. Asymmetric Trifluoromethylation of Ketones with (Trifluoromethyl)trimethylsilane Catalyzed by Chiral Quaternary Ammonium Phenoxides. Chem. Lett. 2007, 36, 666–667. [Google Scholar] [CrossRef]
- Surya Prakash, G.K.; Panja, C.; Vaghoo, H.; Surampudi, V.; Kultyshev, R.; Mandal, M.; Rasul, G.; Mathew, T.; Olah, G.A. Facile Synthesis of TMS-Protected Trifluoromethylated Alcohols Using Trifluoromethyltrimethylsilane (TMSCF3) and Various Nucleophilic Catalysts in DMF. J. Org. Chem. 2006, 71, 6806–6813. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Yue, Z.; Zhang, J.L. A highly efficient one-pot trifluoromethylation/ cyclization reaction of electron-deficient 1,3-conjugated enynes: Modular access to trifluoromethylated furans and 2,3-dihydrofurans. Org. Chem. Front. 2016, 3, 1416–1419. [Google Scholar] [CrossRef]
- Yang, W.; Cui, Y.M.; Zhou, W.; Li, L.; Yang, K.F.; Zheng, Z.J.; Lu, Y.X.; Xu, L.W. Enantioselective primary amine catalyzed aldol-type construction of trifluoromethylated tertiary alcohols. Synlett 2014, 25, 1461–1465. [Google Scholar] [CrossRef]
- Shang, J.Y.; Li, L.; Lu, Y.X.; Yang, K.F.; Xu, L.W. Enantioselective Fluorination Reaction of β-Ketoester-Catalyzed Chiral Primary Amine-Based Multifunctional Catalyst Systems. Synth. Commun. 2014, 44, 101–114. [Google Scholar] [CrossRef]
- Wang, H.; Yang, K.F.; Li, L.; Bai, Y.; Zheng, Z.J.; Zhang, W.Q.; Gao, Z.W.; Xu, L.W. Modulation of Silver-Titania Nanoparticles on Polymethylhydrosiloxane-based Semi-Interpenetrating Networks for Catalytic Alkynylation of Trifluoromethyl Ketones and Aromatic Aldehydes in Water. ChemCatChem 2014, 6, 580–591. [Google Scholar] [CrossRef]
- Zheng, L.S.; Wei, Y.L.; Jiang, K.Z.; Deng, Y.; Zheng, Z.J.; Xu, L.W. Enantioselective Fluorination of β-Ketoamides Catalyzed by Ar-BINMOL-derived Salan-Copper Complex. Adv. Synth. Catal. 2014, 356, 3769–3776. [Google Scholar] [CrossRef]
- Okusu, S.; Sugita, Y.; Tokunaga, E.; Shibata, N. Regioselective 1,4-trifluoromethylation of α,β-unsaturated ketones via a S-(trifluoromethyl)diphenylsulfonium salts/copper system. Beilstein J. Org. Chem. 2013, 9, 2189–2193. [Google Scholar] [CrossRef] [PubMed]
- Sosnovskikh, V.Y.; Sevenard, D.V.; Usachev, B.I.; Röschenthaler, G.V. The first example of a preparative 1,4-perfluoroalkylation using (perfluoroalkyl)trimethylsilanes. Tetrahedron Lett. 2003, 44, 2097–2099. [Google Scholar] [CrossRef]
- The polysubstituted alkylidenecyclobutenone 1k was easily achieved in good yield by a formal [2 + 2] cycloaddition reaction of ynamide with propargyl silyl ether. See [55].
- Chen, L.; Cao, J.; Xu, Z.; Zheng, Z.J.; Cui, Y.M.; Xu, L.W. Lewis acid catalyzed [2+2] cycloaddition of ynamides and propargyl silyl ethers: Synthesis of alkylidenecyclobutenones and their reactivity in ring-opening and ring expansion. Chem. Commun. 2016, 52, 9574–9577. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Kurohara, T.; Shibuya, M. CF3-Substituted semisquarate: A pluripotent building block for the divergent synthesis of trifluoromethylated functional molecules. Chem. Commun. 2015, 51, 16357–16360. [Google Scholar] [CrossRef] [PubMed]
- Chuit, C.; Corriu, R.J.P.; Reye, C.; Young, J.C. Reactivity of penta- and hexacoordinate silicon compounds and their role as reaction intermediates. Chem. Rev. 1993, 93, 1371–1448. [Google Scholar] [CrossRef]
- Holmes, R.R. Comparison of Phosphorus and Silicon: Hypervalency, Stereochemistry, and Reactivity. Chem. Rev. 1996, 96, 927–950. [Google Scholar] [CrossRef] [PubMed]
- Dilman, A.D.; Loffe, S.L. Carbon-carbon bond forming reactions mediated by silicon Lewis acids. Chem. Rev. 2003, 103, 733–772. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.M.; Li, L.; Li, F.; Jiang, K.Z.; Shang, J.Y.; Lai, G.Q.; Xu, L.W. Silicon-based Lewis acid assisted cinchona alkaloid catalysis: Highly enantioselective aza-Michael reaction under solvent-free conditions. Org. Lett. 2011, 13, 6508–6511. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.H.; Deng, Y.; Jiang, K.Z.; Lai, G.Q.; Ni, Y.; Yang, K.F.; Jiang, J.X.; Xu, L.W. Neighboring Acetal-Assisted Brønsted-Acid-Catalyzed Si–H Bond Activation: Divergent Synthesis of Functional Siloxanes through Silylation and Hydrolytic Oxidation of Organosilanes. Eur. J. Org. Chem. 2011, 2011, 1736–1742. [Google Scholar] [CrossRef]
- Rubiales, G.; Alonso, C.; Martínez de Marigorta, E.; Palacios, F. Nucleophilic trifluoromethylation of carbonyl compounds and derivatives. Arkivoc 2014, 2, 362–405. [Google Scholar]
- Rew, Y.; DeGraffenreid, M.; He, X.; Jaen, J.C.; McMinn, D.L.; Sun, D.; Tu, H.; Ursu, S.; Powers, J.P. Discovery and optimization of benzenesulfonanilide derivatives as a novel class of 11b-HSD1 inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 3786–3790. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 2a–2w and 5a–5k are available from the authors. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).