Distinct Mechanisms of Biotic and Chemical Elicitors Enable Additive Elicitation of the Anticancer Phytoalexin Glyceollin I
Abstract
:1. Introduction
2. Results and Discussion
2.1. Imbibing Soybean Seeds Are the Most Abundant Source of Glyceollin I
2.2. Wall Glucan Elicitors from P. sojae and Pythium Elicit More Glyceollin I Than Rhizopus, Aspergillus, and Fusarium Microspores at Standard Treatment Concentrations
2.3. AgNO3 Elicits More Glyceollin I Than CuCl2, BTD, AVG, and SA at Equivalent Treatment Concentrations
2.4. AgNO3 and P. sojae WGE Elicit Glyceollin I with Different Dynamics
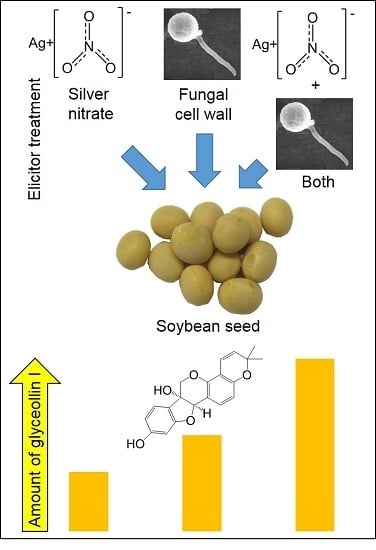
2.5. P. sojae WGE and AgNO3 Elicit the Accumulation of Glyceollin I Mainly by Distinct Mechanisms
2.6. P. sojae WGE and not AgNO3 Induces Major Accumulation of Glyceollin Gene Transcripts
2.7. AgNO3 Inhibits the Degradation of Glyceollin I and Enhances the Specific Hydrolysis of 6″-O-Malonyldaidzin
2.8. Discussion
3. Experimental Section
3.1. Chemicals
3.2. Plant Growth and Elicitation
3.3. Isoflavonoid Analyses
3.4. UPLC-PDA-MSn
3.5. qRT-PCR
3.6. Degradation of Isoflavonoids
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ahuja, I.; Kissen, R.; Bones, A.M. Phytoalexins in defense against pathogens. Trends Plant Sci. 2012, 17, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Großkinsky, D.K.; van der Graaff, E.; Roitsch, T. Phytoalexin transgenics in crop protection—Fairy tale with a happy end? Plant Sci. 2012, 195, 54–70. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P.; Hébrard, C.; Deville, M.-A.; Cordelier, S.; Dorey, S.; Aziz, A.; Crouzet, J. Deciphering the role of phytoalexins in plant-microorganism interactions and human health. Molecules 2014, 19, 18033–18056. [Google Scholar] [CrossRef] [PubMed]
- Ibraheem, F.; Gaffoor, I.; Tan, Q.; Shyu, C.-R.; Chopra, S. A sorghum MYB transcription factor induces 3-deoxyanthocyanidins and enhances resistance against leaf blights in maize. Molecules 2015, 20, 2388–2404. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-H.; Wang, J.-W.; Wang, S.; Wang, J.-Y.; Chen, X.-Y. Characterization of gawrky1, a cotton transcription factor that regulates the sesquiterpene synthase gene (+)-δ-cadinene synthase-a. Plant Physiol. 2004, 135, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, C.; Mizutani, E.; Okada, K.; Nakagawa, H.; Fukushima, S.; Tanaka, A.; Maeda, S.; Kamakura, T.; Yamane, H.; Takatsuji, H.; et al. Diterpenoid phytoalexin factor, a bHLH transcription factor, plays a central role in the biosynthesis of diterpenoid phytoalexins in rice. Plant J. 2015, 84, 1100–1113. [Google Scholar] [CrossRef] [PubMed]
- Akashi, T.; Sasaki, K.; Aoki, T.; Ayabe, S.-I.; Yazaki, K. Molecular cloning and characterization of a cdna for pterocarpan 4-dimethylallyltransferase catalyzing the key prenylation step in the biosynthesis of glyceollin, a soybean phytoalexin. Plant Physiol. 2009, 149, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Moy, P.; Qutob, D.; Chapman, B.P.; Atkinson, I.; Gijzen, M. Patterns of gene expression upon infection of soybean plants by Phytophthora sojae. Mol. Plant Microbe Interact. 2004, 17, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Beyer, E.M. A potent inhibitor of ethylene action in plants. Plant Physiol. 1976, 58, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, M.; Ward, E. Resistance, susceptibility and accumulation of glyceollins I–III in soybean organs inoculated with Phytophthora megasperma f. sp. glycinea. Physiol. Mol. Plant Path. 1986, 29, 227–237. [Google Scholar] [CrossRef]
- Yoshikawa, M. Diverse modes of action of biotic and abiotic phytoalexin elicitors. Nature 1978, 275, 546–547. [Google Scholar] [CrossRef]
- Selvam, C.; Jordan, B.C.; Prakash, S.; Mutisya, D.; Thilagavathi, R. Pterocarpan scaffold: A natural lead molecule with diverse pharmacological properties. Eur. J. Med. Chem. 2017, 128, 219–236. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.C.; Tilghman, S.L.; Boue, S.M.; Salvo, V.A.; Elliott, S.; Williams, K.Y.; Skripnikova, E.V.; Ashe, H.; Payton-Stewart, F.; Vanhoy-Rhodes, L.; et al. Glyceollin I, a novel antiestrogenic phytoalexin isolated from activated soy. J. Pharm. Exp. Ther. 2010, 332, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, L.V.; Tilghman, S.L.; Boue, S.M.; Wang, S.; Khalili, H.; Muir, S.E.; Bratton, M.R.; Zhang, Q.; Wang, G.; Burow, M.E. Glyceollins as novel targeted therapeutic for the treatment of triple-negative breast cancer. Oncol. Lett. 2012, 3, 163–171. [Google Scholar] [PubMed]
- Luniwal, A.; Khupse, R.; Reese, M.; Liu, J.; El-Dakdouki, M.; Malik, N.; Fang, L.; Erhardt, P. Multigram synthesis of glyceollin I. Org. Process Res. Dev. 2011, 15, 1149–1162. [Google Scholar] [CrossRef]
- Simons, R.; Vincken, J.P.; Roidos, N.; Bovee, T.F.H.; van Iersel, M.; Verbruggen, M.A.; Gruppen, H. Increasing soy isoflavonoid content and diversity by simultaneous malting and challenging by a fungus to modulate estrogenicity. J. Agric. Food Chem. 2011, 59, 6748–6758. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, M.; Ward, E. Expression of gene-specific and age-related resistance and the accumulation of glyceollin in soybean leaves infected with Phytophthora megasperma f. sp. glycinea. Physiol. Mol. Plant Path. 1986, 29, 105–113. [Google Scholar] [CrossRef]
- Graham, T.L.; Graham, M.Y. Signaling in soybean phenylpropanoid responses (dissection of primary, secondary, and conditioning effects of light, wounding, and elicitor treatments). Plant Physiol. 1996, 110, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Burow, M.E.; Boue, S.M.; Collins-Burow, B.M.; Melnik, L.I.; Duong, B.N.; Carter-Wientjes, C.H.; Li, S.F.; Wiese, T.E.; Cleveland, T.E.; McLachlan, J.A. Phytochemical glyceollins, isolated from soy, mediate antihormonal effects through estrogen receptor alpha and beta. J. Clin. Endocr. Metab. 2001, 86, 1750–1758. [Google Scholar] [PubMed]
- Cheng, Q.; Li, N.; Dong, L.; Zhang, D.; Fan, S.; Jiang, L.; Wang, X.; Xu, P.; Zhang, S. Overexpression of soybean isoflavone reductase (GmIFR) enhances resistance to Phytophthora sojae in soybean. Front. Plant Sci. 2015, 6, 1024–1035. [Google Scholar] [CrossRef] [PubMed]
- Lygin, A.V.; Zernova, O.V.; Hill, C.B.; Kholina, N.A.; Widholm, J.M.; Hartman, G.L.; Lozovaya, V.V. Glyceollin is an important component of soybean plant defense against Phytophthora sojae and macrophomina phaseolina. Phytopathology 2013, 103, 984–994. [Google Scholar] [CrossRef] [PubMed]
- Ayers, A.R.; Ebel, J.; Valent, B.; Albersheim, P. Host-pathogen interactions 10. Fractionation and biological-activity of an elicitor isolated from mycelial walls of Phytophthora-megasperma var. sojae. Plant Physiol. 1976, 57, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Van De Schans, M.G.; Vincken, J.-P.; De Waard, P.; Hamers, A.R.; Bovee, T.F.; Gruppen, H. Glyceollins and dehydroglyceollins isolated from soybean act as serms and er subtype-selective phytoestrogens. J. Steroid Biochem. Mol. Biol. 2016, 156, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Aisyah, S.; Gruppen, H.; Madzora, B.; Vincken, J.-P. Modulation of isoflavonoid composition of rhizopus oryzae elicited soybean (Glycine max) seedlings by light and wounding. J. Agric. Food Chem. 2013, 61, 8657–8667. [Google Scholar] [CrossRef] [PubMed]
- Boue, S.M.; Carter, C.H.; Ehrlich, K.C.; Cleveland, T.E. Induction of the soybean phytoalexins coumestrol and glyceollin by aspergillus. J. Agric. Food Chem. 2000, 48, 2167–2172. [Google Scholar] [CrossRef] [PubMed]
- Simons, R.; Vincken, J.P.; Bohin, M.C.; Kuijpers, T.F.M.; Verbruggen, M.A.; Gruppen, H. Identification of prenylated pterocarpans and other isoflavonoids in Rhizopus spp. Elicited soya bean seedlings by electrospray ionisation mass spectrometry. Rapid Commun. Mass Spectrom. 2011, 25, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Bektas, Y.; Eulgem, T. Synthetic plant defense elicitors. Front. Plant Sci. 2014, 5, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Paradies, I.; Konze, J.R.; Elstner, E.F.; Paxton, J. Ethylene: Indicator but not inducer of phytoalexin synthesis in soybean. Plant Physiol. 1980, 66, 1106–1109. [Google Scholar] [CrossRef] [PubMed]
- Ward, E.W.; Cahill, D.M.; Bhattacharyya, M.K. Abscisic acid suppression of phenylalanine ammonia-lyase activity and mrna, and resistance of soybeans to Phytophthora megasperma f. sp. glycinea. Plant Physiol. 1989, 91, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-J.; Huhman, D.; Sumner, L.W.; Dixon, R.A. Regiospecific hydroxylation of isoflavones by cytochrome p450 81e enzymes from medicago truncatula. Plant J. 2003, 36, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Naoumkina, M.; Farag, M.A.; Sumner, L.W.; Tang, Y.; Liu, C.-J.; Dixon, R.A. Different mechanisms for phytoalexin induction by pathogen and wound signals in medicago truncatula. Proc. Natl. Acad. Sci. USA 2007, 104, 17909–17915. [Google Scholar] [CrossRef] [PubMed]
- Graham, T.L. Flavonoid and isoflavonoid distribution in developing soybean seedling tissues and in seed and root exudates. Plant Physiol. 1991, 95, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, T.B.; LaFayette, P.R.; Schmitz, R.J.; Parrott, W.A. Targeted genome modifications in soybean with crispr/cas9. BMC Biotechnol. 2015, 15, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Kovinich, N.; Saleem, A.; Arnason, J.T.; Miki, B. Combined analysis of transcriptome and metabolite data reveals extensive differences between black and brown nearly-isogenic soybean (Glycine max) seed coats enabling the identification of pigment isogenes. BMC Genom. 2011, 12, 381–400. [Google Scholar] [CrossRef] [PubMed]
- Ayers, A.R.; Ebel, J.; Finelli, F.; Berger, N.; Albersheim, P. Host-pathogen interactions 9. Quantitative assays of elicitor activity and characterization of elicitor present in extracellular medium of cultures of Phytophthora-mega-sperma var. sojae. Plant Physiol. 1976, 57, 751–759. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds used in this study are not available from the authors. |






© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farrell, K.; Jahan, M.A.; Kovinich, N. Distinct Mechanisms of Biotic and Chemical Elicitors Enable Additive Elicitation of the Anticancer Phytoalexin Glyceollin I. Molecules 2017, 22, 1261. https://doi.org/10.3390/molecules22081261
Farrell K, Jahan MA, Kovinich N. Distinct Mechanisms of Biotic and Chemical Elicitors Enable Additive Elicitation of the Anticancer Phytoalexin Glyceollin I. Molecules. 2017; 22(8):1261. https://doi.org/10.3390/molecules22081261
Chicago/Turabian StyleFarrell, Kelli, Md Asraful Jahan, and Nik Kovinich. 2017. "Distinct Mechanisms of Biotic and Chemical Elicitors Enable Additive Elicitation of the Anticancer Phytoalexin Glyceollin I" Molecules 22, no. 8: 1261. https://doi.org/10.3390/molecules22081261
APA StyleFarrell, K., Jahan, M. A., & Kovinich, N. (2017). Distinct Mechanisms of Biotic and Chemical Elicitors Enable Additive Elicitation of the Anticancer Phytoalexin Glyceollin I. Molecules, 22(8), 1261. https://doi.org/10.3390/molecules22081261