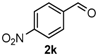Abstract
An efficient and practical protocol has been developed to synthesize dihydropyrimidinones and dihydropyrimidinethiones through FeCl3∙6H2O/TMSBr-catalyzed three-component cyclocondensation under microwave irradiation. This approach features high yields, broad substrate scope, short reaction time, mild reaction conditions, operational simplicity and easy work-up, thus affording a versatile method for the synthesis of dihydropyrimidinones and dihydropyrimidinethiones.
1. Introduction
Dihydropyrimidinones and dihydropyrimidinethiones have received great attention in synthetic organic chemistry because of their ubiquitous presence in a large number of natural products and pharmaceutical agents [1,2,3,4,5,6,7,8,9,10]. For example, they act as key components in natural marine alkaloids such as batzelladine A-I [11,12,13], ptilocaulin [14], and saxitoxin [15]. Moreover, they exhibit a broad spectrum of pharmacological activities such as antibacterial [16], antitumor [17,18,19,20], anti-inflammatory [20], antiviral [21], and antihypertensive activities [22,23]. They are also known as calcium channel blockers [24,25,26,27] and α1A-adrenergic receptor (α1A-AR) antagonists [28,29]. In addition, dihydropyrimidinones display as a key precursor in the synthesis of pyrimidine bases which constitute the basic skeleton of nucleic acids [30]. Therefore, an efficient access to these two structures is highly desirable for both organic synthesis and drug discovery.
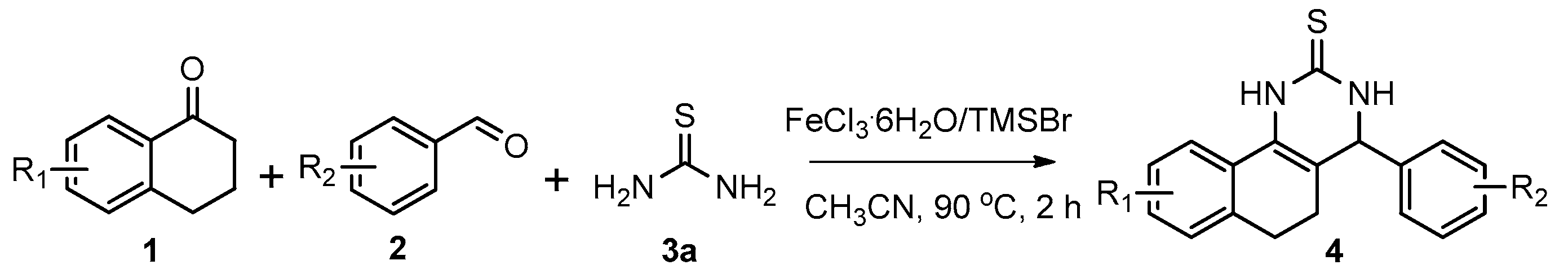
The first synthetic method for the preparation of dihydropyrimidinones and dihydropyrimidinethiones was reported by Biginelli in 1893 [31]. However, this method suffered from low yields and the usage of strong acids. Consequently, improved procedures, including the employment of Lewis acid catalysts [32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56], bases [57,58], ionic liquids [59,60,61,62], ultrasound irradiation [63], and nanocomposites [64,65,66] have been developed. Despite the remarkable achievements made, however, many of these methods still suffer from major or minor drawbacks, such as long reaction time, harsh reaction conditions, low yields, the stoichiometric requirements of the metal catalysts and the involvement of expensive or toxic reagents. Therefore, the development of a faster, milder, high-yielding and environmentally benign approach for the synthesis of dihydropyrimidinones and dihydropyrimidinethiones is still of great significance. Herein, we present our efforts towards FeCl3∙6H2O/TMSBr- (TMSBr = Bromotrimethylsilane) catalyzed three-component cyclocondensation under microwave irradiation to synthesize dihydropyrimidinones and dihydropyrimidinethiones. Our protocol features high yields, broad substrate scope, short reaction time, mild reaction conditions, operational simplicity and easy work-up, thus affording a rapid and convenient approach for the synthesis of dihydropyrimidinones and dihydropyrimidinethiones.
2. Results and Discussion
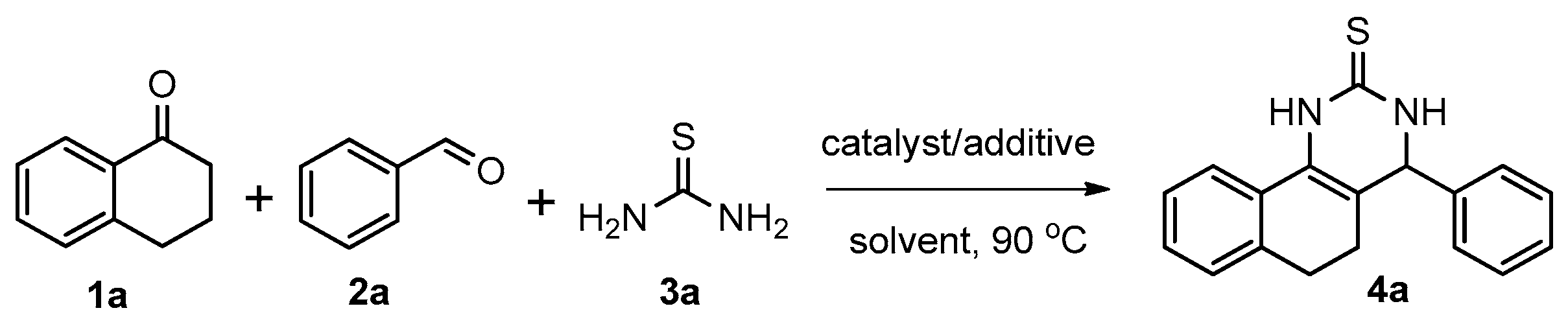
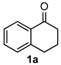
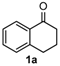
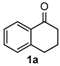
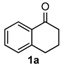
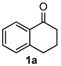
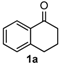
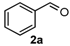
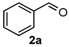
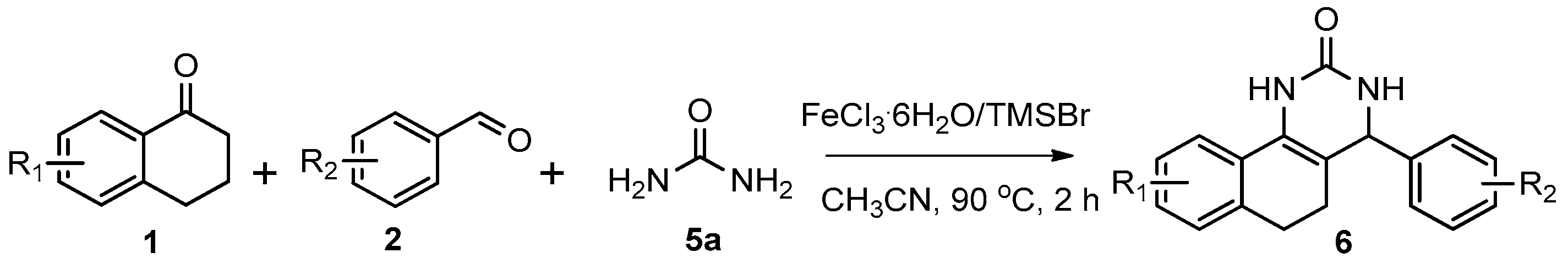
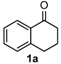
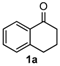
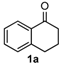
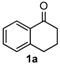
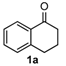
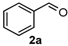
We began our study by investigating the reaction of 1-tetralone (1a), benzaldehyde (2a) and thiourea (3a) in CH3CN at 90 °C in an oil bath for 10 h employing FeCl3∙6H2O as the catalyst, considering the potential of FeCl3∙6H2O in Biginelli-like reactions [45,67,68] (Table 1, entry 1). Pleasingly, the desired product 4a was obtained, albeit with low yield. Considering that microwave-assisted organic synthesis (MAOS) is time and energy-saving [69,70,71] and its applications in Biginelli-like reactions [57,72,73,74,75,76,77,78,79,80,81], we then chose this technology to conduct the three-component condensation reaction. As a result, a similar yield was obtained under the same catalytic conditions when the reaction was carried out under microwave irradiation for just 2 h (Table 1, entry 2). Then, we tried to optimize the reaction conditions under microwave heating. At first, various catalysts such as ZnCl2, FeSO4∙7H2O, CuBr2, and AlCl3 were evaluated (Table 1, entries 3–6), and none of them exhibited a higher catalytic performance than FeCl3∙6H2O. Next, employing FeCl3∙6H2O as the catalyst, a series of additives were added and screened in order to improve the reaction yield (Table 1, entries 7–12). Both BF3∙OEt2 and BBr3 caused no obvious enhancement of the reaction yield (Table 1, entries 7 and 8). As TMS-X-type (X = Cl, I, OTf) compounds were proved to be efficient reagents which could significantly promote Biginelli-like reactions [44,45,56,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102], we then explored the effects of this kind of additive. We found that TMSOTf, TMSCl, TMSBr and TMSI could improve the yield to different degrees (Table 1, entries 9–12). To our delight, TMSBr was found to be the most efficient additive, with which product 4a was obtained in 88% yield by filtration (Table 1, entry 11). This might be because TMSBr could activate the carbonyl group of 1a to promote the reaction [103,104,105]. Subsequently, a further screening of the solvents revealed that increasing the polarity of the solvent generally had a positive effect on the reaction yield (Table 1, entry 13–16), and CH3CN displayed as the best choice to promote the transformation, although ethanol could be an alternative solvent with which a slight lower yield (84%) was observed (Table 1, entry 16). In addition, solvent-free conditions were also tested, and only a moderate yield (52%) was obtained because of the recovery of the materials (Table 1, entry 17). By contrast, a lower yield (80%) was observed when the reaction was heated with an oil bath for 8 h under the optimal reaction conditions because of the incomplete consumption of the substrates (Table 1, entry 18), and it took 10 hours to finish the reaction to obtain a comparable yield (87%) using an oil bath (Table 1, entry 19). These results highlighted the efficiency of microwave irradiation. In this way, FeCl3∙6H2O/TMSBr-catalyzed synthesis of dihydropyrimidinethiones through three-component cyclocondensation under microwave heating was developed.

Table 1.
Optimization of the reaction conditions a.
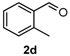
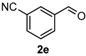
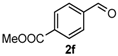
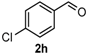
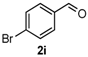
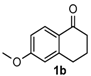
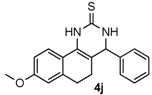
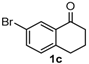
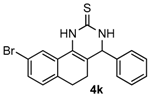
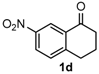
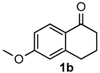
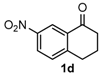
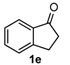
After determining the optimal reaction conditions, we then examined the general applicability of this process. In general, various substituted 3,4-dihydropyrimidin-2(1H)-thiones were easily prepared in good to high yields by the reaction of ketones, benzaldehydes and thiourea under the catalysis of FeCl3∙6H2O/TMSBr (Table 2). The reactions of benzaldehydes carrying electron-donating groups (Me, MeO) furnished the corresponding products 4b–4d in 80%–86% yields (Table 2, entries 2–4). The protocol was also compatible with benzaldehydes bearing electron-withdrawing groups (CN, COOMe) and afforded the desired products 4e–4f in good to high yields (Table 2, entries 5 and 6). Halogens (F, Cl, Br) were tolerated well and excellent yields (89%–90%) were obtained (Table 2, entries 7–9). Subsequently, the substituents on the 1-tetralones were investigated. As a result, the reactions of 1-tetralones with electron-donating group (MeO), halogen (Br) and electron-withdrawing group (NO2) on the benzene ring gave the corresponding products 4j–4l in high yields (Table 2, entries 10–12). In addition, a high yield (89%) was observed when 1-indanone was subjected to the optimal reaction conditions (Table 2, entries 13).

Table 2.
FeCl3∙6H2O/TMSBr catalyzed synthesis of 3,4-dihydropyrimidin-2(1H)-thiones a.
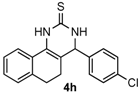
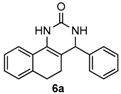
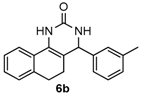
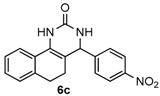
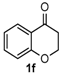
Next, a wide range of structurally diverse ketones, benzaldehydes and urea were subjected to the optimal reaction conditions to produce the corresponding 3,4-dihydropyrimidin-2(1H)-ones in high yields (Table 3). The reaction of benzaldehyde furnished the product 6a in 90% yield (Table 3, entry 1). Benzaldehydes with electron-donating group (Me), electron-withdrawing groups (NO2, CN) and halogens (F, Cl, Br) also reacted smoothly to achieve the desired products 6b–6g in high yields (Table 3, entries 2–7). In addition, the reactions of 1-tetralones carrying electron-donating group (MeO), halogen (Br) and electron-withdrawing group (NO2) on the benzene ring afforded the corresponding products 6h–6j in 81%–88% yields (Table 3, entries 8–10). Pleasingly, high yields were also obtained when 1-indanone and 4-chromanone were employed as substrates (Table 3, entries 11 and 12), these findings further broadened the substrate scope of this methodology. It should be noted that the structures of compounds 4 and 6 were confirmed by 1H-NMR (see Supplementary Files), 13C-NMR (see Supplementary Files), Low-resolution mass (LRMS) and high-resolution mass (HRMS).

Table 3.
FeCl3∙6H2O/TMSBr-catalyzed synthesis of 3,4-dihydropyrimidin-2(1H)-ones a.
3. Materials and Methods
3.1. General Information
The reagents were purchased from commercial suppliers and used without further purification. Analytical thin-layer chromatography (TLC) was performed on HSGF 254 (0.15–0.2 mm thickness), visualized by irradiation with UV light (254 nm). Column chromatography was performed using silica gel FCP 200–300. Melting points were measured with a micro melting point apparatus. Nuclear magnetic resonance spectra were recorded on a Brucker AMX-300 or 400 or 500 MHz instrument [TMS (Tetramethylsilane) as IS (Internal Standard)]. Chemical shifts were reported in parts per million (ppm, δ) downfield from tetramethylsilane. Proton coupling patterns were described as singlet (s), doublet (d), triplet (t), quartet (q), multiplet (m), and broad (br). Low and high-resolution mass (LRMS and HRMS) were measured by the EI (Electron Ionization) method with a Tsou-EI mass spectrometer. All the microwave-assisted reactions were performed in sealed tubes (capacity 10 mL) under a nitrogen atmosphere under a microwave heating system (CEM Discover) at the specified temperature. A feedback mechanism was involved in the temperature control system, and the reaction temperature which could be read from the temperature display screen was real-time monitored. It should be noted that a fixed power (30 W) was found to be appropriate to achieve the reaction temperature (90 °C) without overheating since a higher power led to the loss of efficacy of the temperature control system which resulted in overheating.
3.2. General Procedure for the Synthesis of 3,4-Dihydropyrimidin-2(1H)-thiones (4)
A high-pressure microwave vessel (capacity 10 mL) was loaded with ketones (0.5 mmol), benzaldehydes (0.5 mmol), thiourea (0.75 mmol), FeCl3∙6H2O (0.05 mmol) and TMSBr (0.5 mmol) in CH3CN (3.0 mL). The vessel was degassed, refilled with nitrogen, and sealed. Then the mixture was heated to 90 °C for 2 h under microwave irradiation using a CEM Discover (fixed power, 30 W). After cooling, the solids which had precipitated out were separated by filtration, and the solids obtained were washed with CH3CN to give the desired products 4.
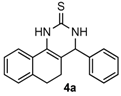
4-Phenyl-3,4,5,6-tetrahydrobenzo[h]quinazoline-2(1H)-thione (4a): White solid (128.7 mg, 88%), m.p. 246–248 °C. 1H-NMR (500 MHz, DMSO) δ 9.76 (s, 1H), 9.10 (s, 1H), 7.69 (d, J = 7.1 Hz, 1H), 7.39–7.35 (m, 2H), 7.34–7.28 (m, 3H), 7.24–7.16 (m, 2H), 7.15 (d, J = 6.6 Hz, 1H), 4.95 (s, 1H), 2.77–2.65 (m, 1H), 2.61–2.52 (m, 1H), 2.22–2.12 (m, 1H), 1.88–1.77 (m, 1H). 13C-NMR (126 MHz, DMSO) δ 174.28, 142.90, 135.45, 128.75, 127.98, 127.81, 127.75, 127.64, 127.02, 126.69, 126.38, 121.71, 111.23, 58.51, 27.37, 23.65. LRMS (EI): 292 (M+); HRMS (EI) calcd. for C18H16N2S (M+) 292.1034, found: 292.1031.
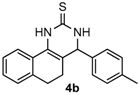
4-p-Tolyl-3,4,5,6-tetrahydrobenzo[h]quinazoline-2(1H)-thione (4b): White solid (131.3 mg, 86%), m.p. 230–232 °C. 1H-NMR (400 MHz, DMSO) δ 9.74 (s, 1H), 9.04 (s, 1H), 7.67 (d, J = 6.4 Hz, 1H), 7.23–7.15 (m, 7H), 4.90 (s, 1H), 2.71 (dt, J = 15.4, 7.6 Hz, 1H), 2.64–2.53 (m, 1H), 2.28 (s, 3H), 2.23–2.10 (m, 1H), 1.89–1.77 (m, 1H). 13C-NMR (126 MHz, DMSO) δ 174.15, 139.99, 137.20, 135.43, 129.25, 127.78, 127.75, 127.61, 126.96, 126.59, 126.35, 121.67, 111.37, 58.21, 27.36, 23.62, 20.75. LRMS (EI): 306 (M+); HRMS (EI) calcd. for C19H18N2S (M+) 306.1191, found: 306.1192.
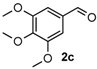
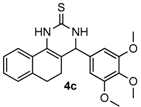
4-(3,4,5-Trimethoxyphenyl)-3,4,5,6-tetrahydrobenzo[h]quinazoline-2(1H)-thione (4c): White solid (158.6 mg, 83%), m.p. 224–226 °C. 1H-NMR (400 MHz, DMSO) δ 9.79 (s, 1H), 9.01 (s, 1H), 7.75–7.53 (m, 1H), 7.31–7.09 (m, 3H), 6.64 (s, 2H), 4.92 (s, 1H), 3.74 (s, 6H), 3.65 (s, 3H), 2.80–2.70 (m, 1H), 2.70–2.58 (m, 1H), 2.29–2.12 (m, 1H), 2.04–1.84 (m, 1H). 13C-NMR (126 MHz, DMSO) δ 174.21, 152.99, 138.30, 137.12, 135.51, 127.76, 127.73, 127.60, 126.92, 126.32, 121.65, 111.01, 104.22, 59.99, 58.43, 55.86, 27.39, 23.59. LRMS (EI): 382 (M+); HRMS (EI) calcd. for C21H22N2O3S (M+) 382.1351, found: 382.1349.
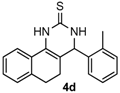
4-O-tolyl-3,4,5,6-tetrahydrobenzo[h]quinazoline-2(1H)-thione (4d): White solid (122.9 mg, 80%), m.p. 241–242 °C. 1H-NMR (400 MHz, DMSO) δ 9.72 (s, 1H), 8.97 (s, 1H), 7.70 (dd, J = 8.3, 6.4 Hz, 1H), 7.33–7.07 (m, 7H), 5.25 (d, J = 2.0 Hz, 1H), 2.76–2.64 (m, 1H), 2.60–2.52 (m, 1H), 2.41 (s, 3H), 2.18–1.99 (m, 1H), 1.77–1.59 (m, 1H). 13C-NMR (126 MHz, DMSO) δ 173.94, 140.83, 135.64, 135.41, 130.60, 128.42, 127.85, 127.71, 127.68, 127.56, 126.74, 126.59, 126.33, 121.62, 111.15, 55.55, 27.28, 23.31, 18.83. LRMS (EI): 306 (M+); HRMS (EI) calcd. for C19H18N2S (M+) 306.1191, found: 306.1193.
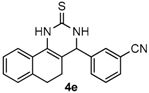
4-(3-Nitrilephenyl)-3,4,5,6-tetrahydrobenzo[h]quinazoline-2(1H)-thione (4e): White solid (144.1 mg, 91%), m.p. 243–244 °C. 1H-NMR (400 MHz, DMSO) δ 9.90 (s, 1H), 9.12 (s, 1H), 7.83–7.78 (m, 1H), 7.73 (s, 1H), 7.72–7.59 (m, 3H), 7.26–7.19 (m, 2H), 7.19–7.13 (m, 1H), 5.09 (s, 1H), 2.78–2.67 (m, 1H), 2.66–2.54 (m, 1H), 2.26–2.14 (m, 1H), 1.90–1.79 (m, 1H). 13C-NMR (126 MHz, DMSO) δ 174.51, 144.24, 135.50, 131.83, 131.76, 130.51, 130.21, 127.94, 127.61, 127.47, 127.21, 126.31, 121.81, 118.63, 111.50, 110.19, 57.53, 27.22, 23.34. LRMS (EI): 317 (M+); HRMS (EI) calcd. for C19H15N3S (M+) 317.0987, found: 317.0979.
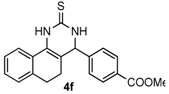
methyl 4-(2-Thioxo-1,2,3,4,5,6-hexahydrobenzo[h]quinazolin-4-yl)benzoate (4f): White solid (133.8 mg, 76%), m.p. 188–190 °C. 1H-NMR (400 MHz, DMSO) δ 9.86 (s, 1H), 9.17 (s, 1H), 7.98 (d, J = 8.1 Hz, 2H), 7.69 (d, J = 6.3 Hz, 1H), 7.47 (d, J = 8.1 Hz, 2H), 7.31–7.07 (m, 3H), 5.08 (s, 1H), 3.84 (s, 3H), 2.77–2.67 (m, 1H), 2.63–2.53 (m, 1H), 2.27–2.10 (m, 1H), 1.90–1.72 (m, 1H). 13C-NMR (126 MHz, DMSO) δ 174.49, 165.99, 147.92, 135.49, 129.73, 129.18, 127.95, 127.66, 127.61, 127.37, 126.99, 126.38, 121.79, 110.49, 58.13, 52.24, 27.31, 23.52. LRMS (EI): 350 (M+); HRMS (EI) calcd. for C20H18N2O2S (M+) 350.1089, found: 350.1085.
4-(4-Fluorophenyl)-3,4,5,6-tetrahydrobenzo[h]quinazoline-2(1H)-thione (4g): White solid (138.3 mg, 89%), m.p. 242–243 °C. 1H-NMR (400 MHz, DMSO) δ 9.80 (s, 1H), 9.10 (s, 1H), 7.68 (d, J = 6.4 Hz, 1H), 7.38–7.32 (m, 2H), 7.24–7.19 (m, 4H), 7.18–7.13 (m, 1H), 4.99 (s, 1H), 2.78–2.66 (m, 1H), 2.64–2.53 (m, 1H), 2.28–2.03 (m, 1H), 1.90–1.72 (m, 1H). 13C-NMR (126 MHz, DMSO) δ 174.23, 161.77 (d, JC–F = 243.9 Hz), 139.13, 135.48, 129.07 (d, JC–F = 8.3 Hz), 127.86, 127.68, 127.64, 126.82, 126.37, 121.76, 115.55 (d, JC–F = 21.4 Hz), 111.05, 57.66, 27.34, 23.55. LRMS (EI): 310 (M+); HRMS (EI) calcd. for C18H15FN2S (M+) 310.0940, found: 310.0933.
4-(4-Chlorophenyl)-3,4,5,6-tetrahydrobenzo[h]quinazoline-2(1H)-thione (4h): White solid (146.8 mg, 90%), m.p. 226–228 °C. 1H-NMR (400 MHz, DMSO) δ 9.82 (s, 1H), 9.11 (s, 1H), 7.72–7.64 (m, 1H), 7.46 (d, J = 8.4 Hz, 2H), 7.33 (d, J = 8.7 Hz, 2H), 7.23–7.18 (m, 2H), 7.07 (s, 1H), 4.99 (s, 1H), 2.78–2.66 (m, 1H), 2.67–2.54 (m, 1H), 2.24–2.12 (m, 1H), 1.88–1.75 (m, 1H). 13C-NMR (126 MHz, DMSO) δ 174.26, 141.74, 135.43, 132.45, 128.84, 128.70, 127.83, 127.58, 126.87, 126.31, 121.72, 110.69, 57.64, 27.26, 23.46. LRMS (EI): 326 (M+, Cl35), 328 (M+, Cl37); HRMS (EI) calcd. for C18H15ClN2S (M+) 326.0644, found: 326.0636.
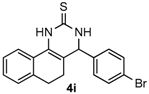
4-(4-Bromophenyl)-3,4,5,6-tetrahydrobenzo[h]quinazoline-2(1H)-thione (4i): White solid (166.5 mg, 90%), m.p. 229–230 °C. 1H-NMR (400 MHz, DMSO) δ 9.82 (s, 1H), 9.12 (s, 1H), 7.73–7.65 (m, 1H), 7.59 (d, J = 8.3 Hz, 2H), 7.27 (d, J = 8.3 Hz, 2H), 7.24–7.13 (m, 3H), 4.97 (s, 1H), 2.77–2.65 (m, 1H), 2.64–2.54 (m, 1H), 2.27–2.09 (m, 1H), 1.89–1.74 (m, 1H). 13C-NMR (126 MHz, DMSO) δ 174.27, 142.14, 135.44, 131.63, 129.20, 127.85, 127.59, 126.89, 126.32, 121.73, 121.05, 110.64, 57.72, 27.27, 23.46. LRMS (EI): 370 (M+, Br79), 372 (M+, Br81); HRMS (EI) calcd. for C18H15BrN2S (M+) 370.0139, found: 370.0134.
8-Methoxy-4-phenyl-3,4,5,6-tetrahydrobenzo[h]quinazoline-2(1H)-thione (4j): White solid (135.0 mg, 84%), m.p. 247–248 °C. 1H-NMR (400 MHz, DMSO) δ 9.71 (s, 1H), 9.06 (s, 1H), 7.73–7.55 (m, 1H), 7.38 (dd, J = 8.9, 5.7 Hz, 2H), 7.34–7.27 (m, 3H), 6.82–6.69 (m, 2H), 4.92 (s, 1H), 3.74 (s, 3H), 2.76–2.63 (m, 1H), 2.63–2.53 (m, 1H), 2.22–2.10 (m, 1H), 1.87–1.72 (m, 1H). 13C-NMR (101 MHz, DMSO) δ 174.20, 158.91, 143.10, 137.53, 128.74, 127.93, 127.01, 126.53, 123.12, 120.63, 113.97, 110.85, 108.56, 58.51, 55.18, 27.78, 23.62. LRMS (EI): 322 (M+); HRMS (EI) calcd. for C19H18N2OS (M+) 322.1140, found: 322.1139.
9-Bromo-4-phenyl-3,4,5,6-tetrahydrobenzo[h]quinazoline-2(1H)-thione (4k): White solid (159.1 mg, 86%), m.p. 225–226 °C. 1H-NMR (400 MHz, DMSO) δ 9.97 (s, 1H), 9.12 (s, 1H), 7.96 (s, 1H), 7.42–7.35 (m, 3H), 7.35–7.28 (m, 3H), 7.12 (d, J = 8.0 Hz, 1H), 4.96 (s, 1H), 2.74–2.63 (m, 1H), 2.60–2.52 (m, 1H), 2.28–2.09 (m, 1H), 1.93–1.74 (m, 1H). 13C-NMR (101 MHz, DMSO) δ 174.38, 142.67, 134.76, 130.27, 129.94, 129.58, 128.81, 128.07, 127.03, 125.93, 124.67, 119.60, 112.85, 58.44, 26.74, 23.51. LRMS (EI): 370 (M+, Br79), 372 (M+, Br81); HRMS (EI) calcd. for C18H15BrN2S (M+) 370.0139, found: 370.0145.
9-Nitro-4-phenyl-3,4,5,6-tetrahydrobenzo[h]quinazoline-2(1H)-thione (4l): White solid (154.8 mg, 92%), m.p. 228–229 °C. 1H-NMR (400 MHz, DMSO) δ 10.36 (s, 1H), 9.18 (s, 1H), 8.63 (d, J = 2.3 Hz, 1H), 8.08 (dd, J = 8.2, 2.3 Hz, 1H), 7.45 (d, J = 8.3 Hz, 1H), 7.43–7.37 (m, 2H), 7.36–7.31 (m, 3H), 5.00 (d, J = 2.4 Hz, 1H), 2.93–2.81 (m, 1H), 2.72 (ddd, J = 16.0, 9.0, 6.9 Hz, 1H), 2.37–2.15 (m, 1H), 2.02–1.78 (m, 1H). 13C-NMR (101 MHz, DMSO) δ 174.53, 146.49, 143.72, 142.50, 129.21, 128.81, 128.09, 127.06, 125.73, 122.63, 116.89, 113.98, 58.33, 27.34, 23.02. LRMS (EI): 337 (M+); HRMS (EI) calcd. for C18H15N3O2S (M+) 337.0885, found: 337.0889.
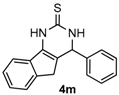
4-Phenyl-3,4-dihydro-1H-indeno[1,2-d]pyrimidine-2(5H)-thione (4m): White solid (123.4 mg, 89%), m.p. 199–201 °C. 1H-NMR (400 MHz, DMSO) δ 10.82 (s, 1H), 9.07 (s, 1H), 7.96–7.71 (m, 1H), 7.42–7.24 (m, 7H), 7.22–7.11 (m, 1H), 5.51 (s, 1H), 3.33 (d, J = 23.8 Hz, 1H), 2.88 (d, J = 23.2 Hz, 1H). 13C-NMR (126 MHz, DMSO) δ 174.24, 143.42, 142.26, 136.48, 132.95, 128.76, 127.75, 126.57, 126.40, 125.45, 124.09, 118.84, 115.34, 57.56, 34.92. LRMS (EI): 278 (M+); HRMS (EI) calcd. for C17H14N2S (M+) 278.0878, found: 278.0877.
3.3. General Procedure for the Synthesis of 3,4-Dihydropyrimidin-2(1H)-ones (6)
A high-pressure microwave vessel (capacity 10 mL) was loaded with ketones (0.5 mmol), benzaldehydes (0.5 mmol), urea (0.75 mmol), FeCl3∙6H2O (0.05 mmol) and TMSBr (0.5 mmol) in CH3CN (3.0 mL). The vessel was degassed, refilled with nitrogen, and sealed. Then the mixture was heated to 90 °C for 2 h under microwave irradiation using a CEM Discover (fixed power, 30 W). After cooling, the solids which had precipitated out were separated by filtration, and the solids obtained were washed with CH3CN to give the desired products 6.
4-Phenyl-3,4,5,6-tetrahydrobenzo[h]quinazolin-2(1H)-one (6a): White solid (124.1 mg, 90%), m.p. 270–272 °C. 1H-NMR (400 MHz, DMSO) δ 8.60 (s, 1H), 7.58 (dd, J = 13.4, 6.3 Hz, 1H), 7.39–7.30 (m, 5H), 7.30–7.24 (m, 1H), 7.25–7.12 (m, 3H), 4.94 (s, 1H), 2.75–2.65 (m, 1H), 2.62–2.52 (m, 1H), 2.19–2.03 (m, 1H), 1.84–1.65 (m, 1H). 13C-NMR (101 MHz, DMSO) δ 153.49, 144.20, 135.47, 128.85, 128.65, 127.72, 127.67, 127.54, 127.52, 126.95, 126.40, 121.27, 108.16, 59.16, 27.66, 23.58. LRMS (EI): 276 (M+); HRMS (EI) calcd. for C18H16N2O (M+) 276.1263, found: 276.1260.
4-M-tolyl-3,4,5,6-tetrahydrobenzo[h]quinazolin-2(1H)-one (6b): White solid (124.9 mg, 86%), m.p. 273–275 °C. 1H-NMR (400 MHz, DMSO) δ 8.54 (s, 1H), 7.57 (d, J = 6.8 Hz, 1H), 7.27–7.17 (m, 4H), 7.16–7.08 (m, 4H), 4.89 (s, 1H), 2.75–2.65 (m, 1H), 2.62–2.52 (m, 1H), 2.29 (s, 3H), 2.19–2.02 (m, 1H), 1.84–1.67 (m, 1H). 13C-NMR (101 MHz, DMSO) δ 153.41, 144.19, 137.71, 135.47, 128.87, 128.54, 128.31, 127.63, 127.54, 127.49, 127.47, 126.39, 124.14, 121.25, 108.17, 59.18, 27.66, 23.56, 21.16. LRMS (EI): 290 (M+); HRMS (EI) calcd. for C19H18N2O (M+) 290.1419, found: 276.1412.
4-(4-Nitrophenyl)-3,4,5,6-tetrahydrobenzo[h]quinazolin-2(1H)-one (6c): White solid (146.1 mg, 91%), m.p. 214–216 °C. 1H-NMR (400 MHz, DMSO) δ 8.71 (s, 1H), 8.24 (d, J = 8.7 Hz, 2H), 7.64–7.56 (m, 3H), 7.46 (s, 1H), 7.24–7.11 (m, 3H), 5.14 (s, 1H), 2.77–2.65 (m, 1H), 2.64–2.52 (m, 1H), 2.22–2.09 (m, 1H), 1.81–1.68 (m, 1H). 13C-NMR (126 MHz, DMSO) δ 153.18, 146.62, 143.71, 143.66, 130.27, 128.66, 127.74, 126.93, 126.78, 122.34, 116.19, 110.97, 58.94, 27.59, 22.92. LRMS (EI): 321 (M+); HRMS (EI) calcd. for C18H15N3O3 (M+) 321.1113, found: 321.1114.
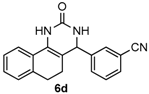
3-(2-Oxo-1,2,3,4,5,6-hexahydrobenzo[h]quinazolin-4-yl)benzonitrile (6d): White solid (127.1 mg, 84%), m.p. 286–287 °C. 1H-NMR (400 MHz, DMSO) δ 8.70 (s, 1H), 7.81–7.73 (m, 2H), 7.68 (s, 1H), 7.65–7.56 (m, 2H), 7.41 (s, 1H), 7.26–7.10 (m, 3H), 5.07 (s, 1H), 2.78–2.66 (m, 1H), 2.64–2.54 (m, 1H), 2.20–2.08 (m, 1H), 1.81–1.69 (m, 1H). 13C-NMR (126 MHz, DMSO) δ 153.33, 145.69, 135.58, 131.95, 131.62, 130.59, 130.22, 128.64, 128.38, 127.78, 127.64, 126.46, 121.47, 118.86, 111.46, 107.09, 58.35, 27.62, 23.34. LRMS (EI): 301 (M+); HRMS (EI) calcd. for C19H15N3O (M+) 301.1215, found: 301.1210.
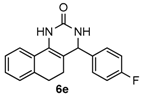
4-(4-Fluorophenyl)-3,4,5,6-tetrahydrobenzo[h]quinazolin-2(1H)-one (6e): White solid (120.9 mg, 82%), m.p. 209–210 °C. 1H-NMR (400 MHz, DMSO) δ 8.58 (s, 1H), 7.66–7.51 (m, 1H), 7.40–7.32 (m, 2H), 7.30 (s, 1H), 7.25–7.10 (m, 5H), 4.97 (s, 1H), 2.76–2.66 (m, 1H), 2.63–2.53 (m, 1H), 2.19–1.99 (m, 1H), 1.81–1.67 (m, 1H). 13C-NMR (101 MHz, DMSO) δ 161.58 (d, JC–F = 243.1 Hz), 153.27 (s), 140.43 (s), 135.45 (s), 128.90 (d, JC–F = 8.2 Hz), 128.75 (s), 127.82 (s), 127.51 (s), 126.35 (s), 121.29 (s), 115.35 (d, JC–F = 21.4 Hz), 107.89 (s), 58.30 (s), 27.61 (s), 23.45 (s). LRMS (EI): 294 (M+); HRMS (EI) calcd. for C18H15FN2O (M+) 294.1168, found: 294.1168.
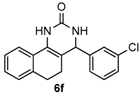
4-(3-Chlorophenyl)-3,4,5,6-tetrahydrobenzo[h]quinazolin-2(1H)-one (6f): White solid (140.1 mg, 90%), m.p. 279–280 °C. 1H-NMR (400 MHz, DMSO) δ 8.65 (s, 1H), 7.59 (d, J = 6.6 Hz, 1H), 7.42-7.33 (m, 4H), 7.30 (d, J = 7.4 Hz, 1H), 7.24–7.12 (m, 3H), 4.94 (s, 1H), 2.77–2.65 (m, 1H), 2.63–2.53 (m, 1H), 2.21–2.03 (m, 1H), 1.87–1.70 (m, 1H). 13C-NMR (101 MHz, DMSO) δ 153.33, 146.67, 135.50, 133.23, 130.68, 128.67, 128.11, 127.66, 127.62, 127.59, 126.77, 126.41, 125.61, 121.36, 107.46, 58.50, 27.62, 23.42. LRMS (EI): 310 (M+, Cl35), 312 (M+, Cl37); HRMS (EI) calcd. for C18H15ClN2O (M+) 310.0873, found: 310.0864.
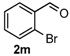
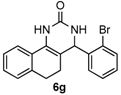
4-(2-Bromophenyl)-3,4,5,6-tetrahydrobenzo[h]quinazolin-2(1H)-one (6g): White solid (142.7 mg, 80%), m.p. 271–273 °C. 1H-NMR (400 MHz, DMSO) δ 8.67 (s, 1H), 7.63–7.57 (m, 2H), 7.49–7.39 (m, 2H), 7.33 (s, 1H), 7.27–7.16 (m, 3H), 7.16–7.11 (m, 1H), 5.54–5.38 (m, 1H), 2.75–2.64 (m, 1H), 2.59–2.52 (m, 1H), 2.22–2.01 (m, 1H), 1.81–1.58 (m, 1H). 13C-NMR (101 MHz, DMSO) δ 153.15, 142.77, 135.53, 132.70, 130.12, 129.79, 128.72, 128.64, 128.22, 127.66, 127.55, 126.42, 121.95, 121.38, 107.18, 58.35, 27.55, 22.97. LRMS (EI): 354 (M+, Br79), 356 (M+, Br81); HRMS (EI) calcd. for C18H15BrN2O (M+) 354.0368, found: 354.0366.
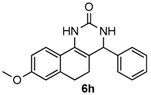
8-Methoxy-4-phenyl-3,4,5,6-tetrahydrobenzo[h]quinazolin-2(1H)-one (6h): White solid (124.4 mg, 81%), m.p. 247–249 °C. 1H-NMR (400 MHz, DMSO) δ 8.54 (s, 1H), 7.54 (d, J = 9.2 Hz, 1H), 7.39–7.30 (m, 4H), 7.30–7.23 (m, 2H), 6.82–6.67 (m, 2H), 4.90 (s, 1H), 3.74 (s, 3H), 2.75–2.63 (m, 1H), 2.60–2.52 (m, 1H), 2.15–2.03 (m, 1H), 1.80–1.68 (m, 1H). 13C-NMR (101 MHz, DMSO) δ 158.66, 153.45, 144.37, 137.44, 128.57, 127.55, 127.50, 126.91, 122.59, 121.71, 113.81, 110.81, 105.43, 59.11, 55.10, 28.03, 23.50. LRMS (EI): 306 (M+); HRMS (EI) calcd. for C19H18N2O2 (M+) 306.1368, found: 306.1367.
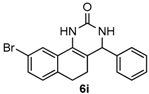
9-Bromo-4-phenyl-3,4,5,6-tetrahydrobenzo[h]quinazolin-2(1H)-one (6i): White solid (147.2 mg, 83%), m.p. 277–279 °C. 1H-NMR (400 MHz, DMSO) δ 8.67 (s, 1H), 7.78 (s, 1H), 7.40–7.27 (m, 7H), 7.10 (d, J = 7.9 Hz, 1H), 4.90 (s, 1H), 2.72–2.61 (m, 1H), 2.59–2.52 (m, 1H), 2.24–2.05 (m, 1H), 1.89–1.65 (m, 1H). 13C-NMR (101 MHz, DMSO) δ 153.25, 143.94, 134.73, 131.04, 129.98, 129.48, 128.91, 128.68, 127.74, 126.95, 124.17, 119.62, 109.87, 59.06, 27.02, 23.40. LRMS (EI): 354 (M+, Br79), 356 (M+, Br81); HRMS (EI) calcd. for C18H15BrN2O (M+) 354.0368, found: 354.0371.
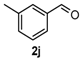
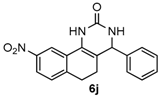
9-Nitro-4-phenyl-3,4,5,6-tetrahydrobenzo[h]quinazolin-2(1H)-one (6j): White solid (141.1 mg, 88%), m.p. 319–321 °C. 1H-NMR (400 MHz, DMSO) δ 8.99 (s, 1H), 8.48 (d, J = 2.2 Hz, 1H), 8.06 (dd, J = 8.2, 2.2 Hz, 1H), 7.45–7.40 (m, 1H), 7.40–7.31 (m, 5H), 7.32–7.26 (m, 1H), 4.97 (s, 1H), 2.84 (m, 1H), 2.77–2.64 (m, 1H), 2.27–2.14 (m, 1H), 1.81 (m, 1H). 13C-NMR (126 MHz, DMSO) δ 153.18, 146.62, 143.71, 143.66, 130.27, 128.66, 127.74, 126.93, 126.78, 122.34, 116.19, 110.97, 58.94, 27.59, 22.92. LRMS (EI): 321 (M+); HRMS (EI) calcd. for C18H15N3O3 (M+) 321.1113, found: 321.1108.
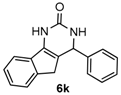
4-Phenyl-3,4-dihydro-1H-indeno[1,2-d]pyrimidin-2(5H)-one (6k): White solid (114.3 mg, 87%), m.p. 269–270 °C. 1H-NMR (400 MHz, DMSO) δ 9.45 (s, 1H), 7.62 (d, J = 7.5 Hz, 1H), 7.45–7.20 (m, 8H), 7.20–7.08 (m, 1H), 5.45 (s, 1H), 3.26 (d, J = 22.9 Hz, 1H), 2.78 (d, J = 22.8 Hz, 1H). 13C-NMR (101 MHz, DMSO) δ 153.38, 144.46, 142.76, 137.51, 134.82, 128.63, 127.42, 126.40, 126.23, 125.11, 123.95, 118.19, 112.24, 57.18, 34.66. LRMS (EI): 262 (M+); HRMS (EI) calcd. for C17H14N2O (M+) 262.1106, found: 262.1103.
4-Phenyl-3,4-dihydro-1H-chromeno[4,3-d]pyrimidin-2(5H)-one (6l): White solid (113.8 mg, 82%), m.p. 251–252 °C. 1H-NMR (400 MHz, DMSO) δ 8.91 (s, 1H), 7.63 (dd, J = 7.8, 1.3 Hz, 1H), 7.47 (s, 1H), 7.43–7.27 (m, 5H), 7.24–7.14 (m, 1H), 7.00–6.90 (m, 1H), 6.83–6.75 (m, 1H), 4.98 (s, 1H), 4.74–4.68 (m, 1H), 4.21 (d, J = 13.6 Hz, 1H). 13C-NMR (126 MHz, DMSO) δ 153.48, 152.99, 143.11, 129.61, 128.78, 127.90, 126.76, 125.17, 121.97, 121.22, 117.40, 115.83, 101.30, 64.80, 56.08. LRMS (EI): 278 (M+); HRMS (EI) calcd. for C17H14N2O2 (M+) 278.1055, found: 278.1049.
4. Conclusions
In conclusion, we have developed an efficient and practical approach to synthesize dihydropyrimidinones and dihydropyrimidinethiones through FeCl3∙6H2O/TMSBr-catalyzed three-component cyclocondensation under microwave heating. This protocol features high yields, broad substrate scope, short reaction time, mild reaction conditions, operational simplicity and easy work-up. These advantages demonstrate the great potential of this method for the synthesis of dihydropyrimidinones and dihydropyrimidinethiones. More importantly, our ongoing research has revealed that the simple derivatives of compounds 4 or 6 are found to be potential EV71 3C protein inhibitors, which are worthy of further investigation for the development of medical therapies for hand, foot and mouth disease (HFMD). We anticipate that these important heterocyclic compounds may find their potent pharmaceutical applications after further exploration.
Supplementary Materials
Supplementary Files (Copies of 1H and 13C-NMR spectra of compounds 4 and 6) are available online.
Acknowledgments
We gratefully acknowledge financial support from the National Natural Science Foundation of China (No. 21602022), the Open Project Program of Antibiotics Research and Re-evaluation Key Laboratory of Sichuan Province (No. ARRLKF15-01), Chengdu University New Faculty Start-up Funding (No. 2081915037) and the Open Project Program of Key Laboratory of Medicinal and Edible Plants Resources Development of Sichuan Education Department (No. 10Y201711).
Author Contributions
F.Z. conceived and designed the experiments; X.J., P.L., J.Z. and J.H. performed the experiments; F.Z. analyzed the data; H.L. and L.L. contributed reagents and materials; F.Z. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sari, O.; Roy, V.; Métifiot, M.; Marchand, C.; Pommier, Y.; Bourg, S.; Bonnet, P.; Schinazi, R.F.; Agrofoglio, L.A. Synthesis of dihydropyrimidine α,γ-diketobutanoic acid derivatives targeting HIV integrase. Eur. J. Med. Chem. 2015, 104, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Lacotte, P.; Puente, C.; Ambroise, Y. Synthesis and evaluation of 3,4-dihydropyrimidin-2(1H)-ones as sodium iodide symporter inhibitors. ChemMedChem 2013, 8, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, C.; Ok, T.; So, W.; Jo, M.; Seo, M.; Kim, Y.; Sohn, J.-H.; Park, Y.; Ju, M.K.; et al. Discovery of 3,4-dihydropyrimidin-2(1H)-ones with inhibitory activity against HIV-1 replication. Bioorg. Med. Chem. Lett. 2012, 22, 2119–2124. [Google Scholar] [CrossRef] [PubMed]
- Gijsen, H.J.M.; Berthelot, D.; Cleyn, M.A.J.D.; Geuens, I.; Brône, B.; Mercken, M. Tricyclic 3,4-dihydropyrimidine-2-thione derivatives as potent TRPA1 antagonists. Bioorg. Med. Chem. Lett. 2012, 22, 797–800. [Google Scholar] [CrossRef] [PubMed]
- Lokwani, D.; Azad, R.; Sarkate, A.; Reddanna, P.; Shinde, D. Structure based library design (SBLD) for new 1,4-dihydropyrimidine scaffold as simultaneous COX-1/COX-2 and 5-LOX inhibitors. Bioorg. Med. Chem. 2015, 23, 4533–4543. [Google Scholar] [CrossRef] [PubMed]
- Bhosle, M.R.; Deshmukh, A.R.; Pal, S.; Srivastava, A.K.; Mane, R.A. Synthesis of new thiazolylmethoxyphenyl pyrimidines and antihyperglycemic evaluation of the pyrimidines, analogues isoxazolines and pyrazolines. Bioorg. Med. Chem. Lett. 2015, 25, 2442–2446. [Google Scholar] [CrossRef] [PubMed]
- Mokale, S.N.; Shinde, S.S.; Elgire, R.D.; Sangshetti, J.N.; Shinde, D.B. Synthesis and anti-inflammatory activity of some 3-(4,6-disubtituted-2-thioxo-1,2,3,4-tetrahydropyrimidin-5-yl) propanoic acid derivatives. Bioorg. Med. Chem. Lett. 2010, 20, 4424–4426. [Google Scholar] [CrossRef] [PubMed]
- Bahekar, S.S.; Shinde, D.B. Synthesis and anti-inflammatory activity of some [4,6-(4-substituted aryl)-2-thioxo-1,2,3,4-tetrahydro-pyrimidin-5-yl]-acetic acid derivatives. Bioorg. Med. Chem. Lett. 2004, 14, 1733–1736. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Ding, X.; Chen, T.; Chen, L.; Liu, F.; Jia, X.; Lu, X.; Shen, X.; Che, K.; Jian, H.; et al. Design, synthesis, and interaction study of quinazoline-2(1H)-thione derivatives as novel potential Bcl-xL inhibitors. J. Med. Chem. 2010, 53, 3465–3479. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Chen, C.; Liu, H.; Zheng, L.; Tong, Y.; Qu, D.; Han, S. Biological evaluation of halogenated thiazolo [3,2-a] pyrimidin-3-one carboxylic acid derivatives targeting the YycG histidine kinase. Eur. J. Med. Chem. 2014, 87, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.A.; Day, K.A.; Durón, S.G.; Gin, D.Y. Total synthesis of (+)-Batzelladine A and (−)-Batzelladine D via [4+2]-annulation of vinyl carbodiimides with N-alkyl imines. J. Am. Chem. Soc. 2006, 128, 13255–13260. [Google Scholar] [CrossRef] [PubMed]
- Shimokawa, J.; Ishiwata, T.; Shirai, K.; Koshino, H.; Tanatani, A.; Nakata, T.; Hashimoto, Y.; Nagasawa, K. Total synthesis of (+)-batzelladine A and (−)-batzelladine D, and identification of their target protein. Chem. Eur. J. 2005, 11, 6878–6888. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.D.; Freyer, A.J.; Taylor, P.B.; Carté, B.; Zuber, G.; Johnson, R.K.; Faulkner, D.J. Batzelladines F−I, novel alkaloids from the sponge Batzella. sp.: Inducers of p56lck-CD4 dissociation. J. Org. Chem. 1997, 62, 1814–1819. [Google Scholar] [CrossRef]
- Patil, A.D.; Kumar, N.V.; Kokke, W.C.; Bean, M.F.; Freyer, A.J.; Brosse, C.D.; Mai, S.; Truneh, A.; Faulkner, D.J.; Carte, B.; et al. Novel alkaloids from the sponge Batzella. sp.: Inhibitors of HIV gp120-human CD4 binding. J. Org. Chem. 1995, 60, 1182–1188. [Google Scholar] [CrossRef]
- Bordner, J.; Thiessen, W.E.; Bates, H.A.; Rapoport, H. Structure of a crystalline derivative of saxitoxin. Structure of saxitoxin. J. Am. Chem. Soc. 1975, 97, 6008–6012. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Singh, K.; Wan, B.; Franzblau, S.; Chibale, K.; Balzarini, J. Facile transformation of Biginelli pyrimidin-2(1H)-ones to pyrimidines. In vitro evaluation as inhibitors of Mycobacterium tuberculosis and modulators of cytostatic activity. Eur. J. Med. Chem. 2011, 46, 2290–2294. [Google Scholar] [CrossRef] [PubMed]
- Amr, A.G.; Mohamed, A.M.; Mohamed, S.F.; Abdel-Hafez, N.A.; Hammam, A.-F. Anticancer activities of some newly synthesized pyridine, pyrane, and pyrimidine derivatives. Bioorg. Med. Chem. 2006, 14, 5481–5488. [Google Scholar] [CrossRef] [PubMed]
- Kappe, C.O. Biologically active dihydropyrimidones of the Biginelli-type—A literature survey. Eur. J. Med. Chem. 2000, 35, 1043–1052. [Google Scholar] [CrossRef]
- Kaan, H.Y.; Ulaganathan, V.; Rath, O.; Prokopcová, H.; Dallinger, D.; Kappe, C.O.; Kozielski, F. Structural basis for inhibition of Eg5 by dihydropyrimidines: Stereoselectivity of antimitotic inhibitors enastron, dimethylenastron and fluorastrol. J. Med. Chem. 2010, 53, 5676–5683. [Google Scholar] [CrossRef] [PubMed]
- Sadanandam, Y.S.; Shetty, M.M.; Diwan, P.V. Synthesis and biological evaluation of new 3,4-dihydro-6-methyl-5-N-methyl-carbamoyl-4-(substituted phenyl)-2(1H)pyrimidinones and pyrimidinethiones. Eur. J. Med. Chem. 1992, 27, 87–92. [Google Scholar] [CrossRef]
- Hurst, E.W.; Hull, R. Two new synthetic substances active against viruses of the psittacosis-lymphogranuloma-trachoma group. J. Med. Chem. 1960, 3, 215–229. [Google Scholar] [CrossRef]
- Rovnyak, G.C.; Atwal, K.S.; Hedberg, A.; Kimball, S.D.; Moreland, S.; Gougoutas, J.Z.; O’Reilly, B.C.; Schwartz, J.; Malley, M.F. Dihydropyrimidine calcium channel blockers. 4. Basic 3-substituted-4-aryl-1,4-dihydropyrimidine-5-carboxylic acid esters. Potent antihypertensive agents. J. Med. Chem. 1992, 35, 3254–3263. [Google Scholar] [CrossRef] [PubMed]
- Atwal, K.S.; Swanson, B.N.; Unger, S.E.; Floyd, D.M.; Moreland, S.; Hedberg, A.; O’Reilly, B.C. Dihydropyrimidine calcium channel blockers. 3. 3-Carbamoyl-4-aryl-1,2,3,4-tetrahydro-6-methyl-5-pyrimidinecarboxylic acid esters as orally effective antihypertensive agents. J. Med. Chem. 1991, 34, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Rovnyak, G.C.; Kimball, S.D.; Beyer, B.; Cucinotta, G.; DiMarco, J.D.; Gougoutas, J.Z.; Hedberg, A.; Malley, M.F.; McCarthy, J.P.; Zhang, R.; et al. Calcium entry blockers and activators: Conformational and structural determinants of dihydropyrimidine calcium channel modulators. J. Med. Chem. 1995, 38, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Kappe, C.O. 4-Aryldihydropyrimidines via the Biginelli condensation: Aza-analogs of nifedipine-type calcium channel modulators. Molecules 1998, 3, 1–9. [Google Scholar] [CrossRef]
- Atwal, K.S.; Rovnyak, G.C.; Kimball, S.D.; Floyd, D.M.; Moreland, S.; Swanson, B.N.; Gougoutas, J.Z.; Schwartz, J.; Smillie, K.M.; Malley, M.F. Dihydropyrimidine calcium channel blockers. II. 3-Substituted-4-aryl-1,4-dihydro-6-methyl-5-pyrimidinecarboxylic acid esters as potent mimics of dihydropyridines. J. Med. Chem. 1990, 33, 2629–2635. [Google Scholar] [CrossRef] [PubMed]
- Jauk, B.; Pernat, T.; Kappe, C.O. Design and synthesis of a conformationally rigid mimic of the dihydropyrimidine calcium channel modulator SQ 32,926. Molecules 2000, 5, 227–239. [Google Scholar] [CrossRef]
- Lagu, B.; Tian, D.; Nagarathnam, D.; Marzabadi, M.R.; Wong, W.C.; Miao, S.W.; Zhang, F.-Q.; Sun, W.-Y.; Chiu, G.; Fang, J.; et al. Design and synthesis of novel α1a adrenoceptor-selective antagonists. 3. Approaches to eliminate opioid agonist metabolites by using substituted phenylpiperazine side chains. J. Med. Chem. 1999, 42, 4794–4803. [Google Scholar] [CrossRef] [PubMed]
- Nagarathnam, D.; Miao, S.-W.; Lagu, B.; Chiu, G.; Fang, J.; Dhar, T.G.M.; Zhang, J.; Tyagarajan, S.; Marzabadi, M.R.; Zhang, F.-Q.; et al. Design and synthesis of novel α1a adrenoceptor-selective antagonists. 1. Structure−activity relationship in dihydropyrimidinones. J. Med. Chem. 1999, 42, 4764–4777. [Google Scholar] [CrossRef] [PubMed]
- Cech, D.; Hein, L.; Wattke, R.; Janta-Lipinski, M.V.; Otto, A.; Langen, P. Synthesis of substituted 5-fluoro-5, 6-dihydropyrimidines. Nucleic Acid. Res. 1975, 2, 2177–2182. [Google Scholar] [CrossRef] [PubMed]
- Biginelli, P. The urea-aldehyde derivatives of acetoacetic esters. Gazz. Chim. Ital. 1893, 23, 360–416. [Google Scholar]
- Lu, J.; Bai, Y.-J.; Wang, Z.-J.; Yang, B.-Q.; Ma, H.-R. One-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones using lanthanum chloride as a catalyst. Tetrahedron Lett. 2000, 41, 9075–9078. [Google Scholar] [CrossRef]
- Ma, Y.; Qian, C.-T.; Wang, L.-M.; Yang, M. Lanthanide triflate catalyzed Biginelli reaction. One-pot synthesis of dihydropyrimidinones under solvent-free conditions. J. Org. Chem. 2000, 65, 3864–3868. [Google Scholar] [CrossRef] [PubMed]
- Reddy, C.V.; Mahesh, M.; Raju, P.V.K.; Babu, T.R.; Reddy, V.V.N. Zirconium(IV) chloride catalyzed one-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones. Tetrahedron Lett. 2002, 43, 2657–2659. [Google Scholar] [CrossRef]
- Fu, N.-Y.; Yuan, Y.-F.; Cao, Z.; Wang, S.-W.; Wang, J.-T.; Peppe, C. Indium(III) bromide-catalyzed preparation of dihydropyrimidinones: Improved protocol conditions for the Biginelli reaction. Tetrahedron 2002, 58, 4801–4807. [Google Scholar] [CrossRef]
- Varala, R.; Alam, M.M.; Adapa, S.R. Bismuth triflate catalyzed one-pot synthesis of 3,4-dihydropyrimidin-2 (1H)-ones: An improved protocol for the Biginelli reaction. Synlett 2003, 67–70. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reedy, B.V.S.; Srinivas, R.; Venugopal, C.; Ramalingam, T. LiClO4-catalyzed one-pot synthesis of dihydropyrimidinones: An improved protocol for Biginelli reaction. Synthesis 2001, 1341–1345. [Google Scholar] [CrossRef]
- Dondoni, A.; Massi, A.; Minghini, E.; Sabbatini, S.; Bertolasi, V. Model studies toward the synthesis of dihydropyrimidinyl and pyridyl α-amino acids via three-component Biginelli and Hantzsch cyclocondensations. J. Org. Chem. 2003, 68, 6172–6183. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Bai, Y.-J.; Guo, Y.-H.; Wang, Z.-J.; Ma, H.-R. CoCl2·6H2O or LaCl3·7H2O catalyzed Biginelli reaction. one-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones. Chin. J. Chem. 2002, 20, 681–687. [Google Scholar] [CrossRef]
- Labrie, P. NiCl2 and NiCl2·6H2O: A very useful mild lewis acid in organic synthesis. Synlett 2003, 279–280. [Google Scholar] [CrossRef]
- Russowsky, D.; Lopes, F.A.; Silva, V.S.S.; Canto, K.F.S.; D’Oca, M.G.M.; Godoi, M.N. Multicomponent Biginelli’s synthesis of 3,4-dihydropyrimidin-2(1H)-ones promoted by SnCl2·2H2O. J. Braz. Chem. Soc. 2004, 15, 165–169. [Google Scholar] [CrossRef]
- Bose, D.S.; Fatima, L.; Mereyala, H.B. Green chemistry approaches to the synthesis of 5-alkoxycarbonyl-4-aryl-3,4-dihydropyrimidin-2(1H)-ones by a three-component coupling of one-pot condensation reaction: Comparison of ethanol, water, and solvent-free conditions. J. Org. Chem. 2003, 68, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.A.; Kasthuraiah, M.; Reddy, C.S.; Reddy, C.D. Mn(OAc)3·2H2O-mediated three-component, one-pot, condensation reaction: An efficient synthesis of 4-aryl-substituted 3,4-dihydropyrimidin-2-ones. Tetrahedron Lett. 2001, 42, 7873–7875. [Google Scholar] [CrossRef]
- Mirza-Aghayan, M.; Moradi, A.; Bolourtchian, M. A novel and efficient one-pot method to Biginelli-like scaffolds. J. Iran. Chem. Soc. 2010, 7, 269–274. [Google Scholar] [CrossRef]
- Wang, Z.-T.; Xu, L.-W.; Xia, C.-G.; Wang, H.-Q. Novel Biginelli-like three-component cyclocondensation reaction: Efficient synthesis of 5-unsubstituted 3,4-dihydropyrimidin-2(1H)-ones. Tetrahedron Lett. 2004, 45, 7951–7953. [Google Scholar] [CrossRef]
- Wang, M.; Song, J.; Lu, Q.; Wang, Q. Green Biginelli-type reaction: Solvent-free synthesis of 5-unsubstituted 3,4-dihydropyrimdin-2(1H)-ones. J. Heterocycl. Chem. 2015, 52, 1907–1910. [Google Scholar] [CrossRef]
- Gore, S.; Baskaran, S.; Koenig, B. Efficient synthesis of 3,4-dihydropyrimidin-2-ones in low melting tartaric acid–urea mixtures. Green. Chem. 2011, 13, 1009–1013. [Google Scholar] [CrossRef]
- Murata, H.; Ishitani, H.; Iwamoto, M. Synthesis of Biginelli dihydropyrimidinone derivatives with various substituents on aluminium-planted mesoporous silica catalyst. Org. Biomol. Chem. 2010, 8, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Hu, E.H.; Sidler, D.R.; Dolling, U.H. Unprecedented catalytic three component one-pot condensation reaction: An efficient synthesis of 5-alkoxycarbonyl-4-aryl-3,4-dihydropyrimidin-2(1H)-ones. J. Org. Chem. 1998, 63, 3454–3457. [Google Scholar] [CrossRef]
- Lu, J.; Bai, Y. Catalysis of the Biginelli reaction by ferric and nickel chloride hexahydrates. One-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones. Synthesis 2002, 12, 466–470. [Google Scholar] [CrossRef]
- Ranu, B.C.; Hajra, A.; Jana, U. Indium(III) chloride-catalyzed one-pot synthesis of dihydropyrimidinones by a three-component coupling of 1,3-dicarbonyl compounds, aldehydes, and urea: An improved procedure for the Biginelli reaction. J. Org. Chem. 2000, 65, 6270–6272. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, Y.-G. A rapid and efficient Biginelli reaction catalyzed by zinc triflate. Chin. J. Chem. 2003, 21, 327–331. [Google Scholar]
- Paraskar, A.S.; Dewkar, G.K.; Sudalai, A. Cu(OTf)2: A reusable catalyst for high-yield synthesis of 3,4-dihydropyrimidin-2(1H)-ones. Tetrahedron Lett. 2003, 44, 3305–3308. [Google Scholar] [CrossRef]
- Dondoni, A.; Massi, A. Parallel synthesis of dihydropyrimidinones using Yb(III)-resin and polymer-supported scavengers under solvent-free conditions. A green chemistry approach to the Biginelli reaction. Tetrahedron Lett. 2001, 42, 7975–7978. [Google Scholar] [CrossRef]
- Chen, R.-F.; Qian, C.-T. One-pot syntheses of 3,4-dihydropyrimidine-2(1H)-thiones catalyzed by La(OTf)3. Chin. J. Chem. 2002, 20, 427–430. [Google Scholar] [CrossRef]
- Sabitha, G.; Reddy, G.S.K.K.; Reddy, C.S.; Yadav, J.S. One-pot synthesis of dihydropyrimidinones using iodotrimethylsilane. Facile and new improved protocol for the Biginelli reaction at room temperature. Synlett 2003, 858–860. [Google Scholar] [CrossRef]
- Kawade, D.S.; Chaudhari, M.A.; Gujar, J.B.; Shingare, M.S. DBU: An efficient catalyst for the synthesis of 5-unsubstituted-3,4-dihydropyrimidin-2(1H)-one derivatives under microwave irradiation. Heterocycl. Lett. 2015, 5, 637–643. [Google Scholar]
- Shen, Z.-L.; Xu, X.-P.; Ji, S.-J. Brønsted base-catalyzed one-pot three-component Biginelli-type reaction: An efficient synthesis of 4,5,6-triaryl-3,4-dihydropyrimidin-2(1H)-one and mechanistic study. J. Org. Chem. 2010, 75, 1162–1167. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.-J.; Deng, Y.-Q. Ionic liquids catalyzed Biginelli reaction under solvent-free conditions. Tetrahedron Lett. 2001, 42, 5917–5919. [Google Scholar] [CrossRef]
- Janardhan, B.; Laxmi, S.V.; Rajitha, B. One-pot synthesis of fused 3,4-dihydropyrimidin-2(1H)-ones and thiones using a novel ionic liquid as an efficient and reusable catalyst: Improved protocol conditions for the Biginelli-like scaffolds. Heterocycl. Commun. 2012, 18, 93–97. [Google Scholar] [CrossRef]
- Gupta, R.; Chaudhary, R.P. Ionic liquid-mediated facile synthesis and antimicrobial study of thiazolo[2,3-b]benzo[h]quinazolines and thiazino[2,3-b]benzo-[h]quinazolines. Phosphorus Sulfur 2012, 187, 735–742. [Google Scholar] [CrossRef]
- Legeay, J.-C.; Eynde, J.J.V.; Bazureau, J.P. Ionic liquid phase technology supported the three component synthesis of Hantzsch 1,4-dihydropyridines and Biginelli 3,4-dihydropyrimidin-2(1H)-ones under microwave dielectric heating. Tetrahedron. 2005, 61, 12386–12397. [Google Scholar] [CrossRef]
- Dutta, M.; Gogoi, J.; Shekarrao, K.; Goswami, J.; Gogoi, S.; Boruah, R.C. Simple ultrasound-assisted synthesis of 3,4-dihydropyrimidin-2(1H)-one and 3,4-dihydropyrimidine-2(1H)-thione-fused steroidal derivatives by a three-component reaction. Synthesis 2012, 2614–2622. [Google Scholar] [CrossRef]
- Heirati, S.Z.D.; Shirini, F.; Shojaei, A.F. PEG-SANM nanocomposite: A new catalytic application towards clean and highly efficient Biginelli-like reaction under solvent-free conditions. RSC Adv. 2016, 6, 67072–67085. [Google Scholar] [CrossRef]
- Azarifar, D.; Abbasi, Y.; Badalkhani, O. Sulfonic acid-functionalized titanomagnetite nanoparticles as recyclable heterogeneous acid catalyst for one-pot solvent-free synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones. J. Iran. Chem. Soc. 2016, 13, 2029–2038. [Google Scholar] [CrossRef]
- Safari, J.; Gandomi-Ravandi, S. Fe3O4–CNTs nanocomposites: A novel and excellent catalyst in the synthesis of diarylpyrimidinones using grindstone chemistry. RSC Adv. 2014, 4, 11486–11492. [Google Scholar] [CrossRef]
- Lu, J.; Ma, H. Iron(III)-catalyzed synthesis of dihydropyrimidinones. Improved conditions for the Biginelli reaction. Synlett 2000, 63–64. [Google Scholar]
- Sedova, V.F.; Krivopalov, V.P.; Shkurko, O.P. Synthesis of 5-nitro-3,4-dihydropyrimidin-2(1H)-ones catalyzed by metal salts. Retro-Henry reaction with formation of N,N'-disubstituted ureas. Russ. J. Org. Chem. 2007, 43, 90–95. [Google Scholar] [CrossRef]
- Kappe, C.O. Controlled microwave heating in modern organic synthesis. Angew. Chem. Int. Edit. 2004, 43, 6250–6284. [Google Scholar] [CrossRef] [PubMed]
- Kappe, C.O.; Dallinger, D. The impact of microwave synthesis on drug discovery. Nat. Rev. Drug Discov. 2006, 5, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Caddick, S.; Fitzmaurice, R. Microwave enhanced synthesis. Tetrahedron 2009, 65, 3325–3355. [Google Scholar] [CrossRef]
- Stadler, A.; Kappe, C.O. Microwave-mediated Biginelli reactions revisited. On the nature of rate and yield enhancements. J. Chem. Soc. Perk. Trans 2 2000, 1363–1368. [Google Scholar] [CrossRef]
- Mirza-Aghayan, M.; Bolourtchian, M.; Hosseini, M. Microwave-assisted efficient synthesis of dihydropyrimidines in solvent-free condition. Synth. Commun. 2004, 34, 3335–3341. [Google Scholar] [CrossRef]
- Jetti, S.R.; Upadhyaya, A.; Jain, S. 3,4-Hydropyrimidin-2-(1H)one derivatives: Solid silica-based sulfonic acid catalyzed microwave-assisted synthesis and their biological evaluation as antihypertensive and calcium channel blocking agents. Med. Chem. Res. 2014, 23, 4356–4366. [Google Scholar] [CrossRef]
- Rezaei, R.; Mohammadi, M.K.; Khaledi, A. Microwave-assisted solvent-free one-pot Biginelli synthesis of dihydropyrimidinone compounds on melamine-formaldehyde as a solid support. Asian J. Chem. 2013, 25, 4588–4590. [Google Scholar]
- Bigdeli, M.A.; Jafari, S.; Mahdavinia, G.H.; Hazarkhani, H. Trichloroisocyanuric acid, a new and efficient catalyst for the synthesis of dihydropyrimidinones and dihydropyrimidinethiones. Catal. Commun. 2007, 8, 1641–1644. [Google Scholar] [CrossRef]
- Liang, B.; Wang, X.; Wang, J.-X.; Du, Z. New three-component cyclocondensation reaction: Microwave-assisted one-pot synthesis of 5-unsubstituted-3,4-dihydropyrimidin-2(1H)-ones under solvent-free conditions. Tetrahedron 2007, 63, 1981–1986. [Google Scholar] [CrossRef]
- Desai, B.; Dallinger, D.; Kappe, C.O. Microwave-assisted solution phase synthesis of dihydropyrimidine C5 amides and esters. Tetrahedron 2006, 62, 4651–4664. [Google Scholar] [CrossRef]
- Shanmugam, P.; Annie, G.; Perumal, P.T. Synthesis of novel 3,4-dihydropyrimidinones on water soluble solid support catalyzed by indium triflate. J. Heterocycl. Chem. 2003, 40, 879–883. [Google Scholar] [CrossRef]
- Stadler, A.; Kappe, C.O. Automated library generation using sequential microwave-assisted chemistry. Application toward the Biginelli multicomponent condensation. J. Comb. Chem. 2001, 3, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Zhan, H.W.; Wang, J.X.; Wang, X.T. Solvent- and catalyst-free synthesis of dihydropyrimidinethiones in one-pot under focused microwave irradiation conditions. Chin. Chem. Lett. 2008, 19, 1183–1185. [Google Scholar] [CrossRef]
- Zhu, Y.; Pan, Y.; Huang, S. Trimethylsilyl chloride: A facile and efficient reagent for one-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones. Synth. Commun. 2004, 34, 3167–3174. [Google Scholar] [CrossRef]
- Kefayati, H.; Fakhriyannejad, M.; Mohammadi, A.A. An efficient synthesis of new 3,4-dihydropyrimidin-2(1H)-ones incorporating a phenyl moiety at C-5 and C-6 catalyzed by TMSCl and Co(OAc)2∙4H2O. Phosphorus Sulfur 2009, 184, 1796–1804. [Google Scholar] [CrossRef]
- Heravi, M.M.; Derikvand, F.; Ranjbar, L.; Bamoharram, F.F. H6P2W18O62∙18H2O, a green and reusable catalyst for the three-component, one-pot synthesis of 4,6-diarylpyrimidin-2(1H)-ones under solvent-free conditions. Synth. Commun. 2010, 40, 1256–1263. [Google Scholar] [CrossRef]
- An, L.; Zhang, L.; Zheng, Y.; Xue, Y.; Mou, J.; Liu, L.; Liu, Y. Microwave irradiation assisted selective synthesis of 4,6-diaryl-3,4-dihydropyrimidin-2(1H)-ones and pyrimidin-2(1H)-ones. Chin. J. Org. Chem. 2012, 32, 1108–1111. [Google Scholar] [CrossRef]
- Kefayati, H.; Asghari, F.; Khanjanian, R. 1-Methylimidazolium hydrogen sulfate/chlorotrimethylsilane, an effective catalytic system for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones and hydroquinazoline-2,5-diones. J. Mol. Liq. 2012, 172, 147–151. [Google Scholar] [CrossRef]
- Zavyalov, S.I.; Kulikova, L.B. Trimethylchlorosilane-dimethylformamide, a new system for the Biginelli reaction. Khim. Farm. Zh. 1992, 26, 116–117. [Google Scholar]
- Zhu, Y.; Pan, Y.; Huang, S. Chemoselective multicomponent condensation of 1,3-cyclohexanedione, urea or thiourea with aldehydes: One-pot synthesis of two families of fused heterocyclic and spiro-fused heterocyclic aliphatic rings. Heterocycles 2005, 65, 133–142. [Google Scholar] [CrossRef]
- Kantevari, S.; Bantu, R.; Nagarapu, L. TMSCl mediated highly efficient one-pot synthesis of octahydroquinazolinone and 1,8-dioxo-octahydroxanthene derivatives. Arkivoc 2006, 16, 136–148. [Google Scholar]
- Ryabukhin, S.V.; Plaskon, A.S.; Ostapchuk, E.N.; Volochnyuk, D.M.; Tolmachev, A.A. N-Substituted ureas and thioureas in Biginelli reaction promoted by chlorotrimethylsilane: Convenient synthesis of N1-alkyl-, N1-aryl-, and N1,N3-dialkyl-3,4-dihydropyrimidin-2(1H)-(thi)ones. Synthesis 2007, 417–427. [Google Scholar] [CrossRef]
- Prokopcová, H.; Pisani, L.; Kappe, C.O. Synthesis of 5-aroyldihydropyrimidinones via Liebeskind-Srogl thiol ester-boronic acid cross-couplings. Synlett 2006, 43–46. [Google Scholar] [CrossRef]
- Matloobi, M.; Kappe, C.O. Microwave-assisted solution- and solid-phase synthesis of 2-amino-4-arylpyrimidine derivatives. J. Comb. Chem. 2007, 9, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Kremsner, J.M.; Stadler, A.; Kappe, C.O. High-throughput microwave assisted organic synthesis: Moving from automated sequential to parallel library-generation formats in silicon carbide microtiter plates. J. Comb. Chem. 2007, 9, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Pisani, L.; Prokopcová, H.; Kremsner, J.M.; Kappe, C.O. 5-Aroyl-3,4-dihydropyrim idin-2-one library generation via automated sequential and parallel microwave-assisted synthesis techniques. J. Comb. Chem. 2007, 9, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Bose, D.S.; Kumar, R.K.; Fatima, L. A remarkable rate acceleration of the one-pot three-component cyclocondensation reaction at room temperature: An expedient synthesis of mitotic kinesin Eg5 inhibitor monastrol. Synlett 2004, 279–282. [Google Scholar] [CrossRef]
- Chebanov, V.A.; Saraev, V.E.; Desenko, S.M.; Chernenko, V.N.; Knyazeva, I.V.; Groth, U.; Glasnov, T.N.; Kappe, C.O. Tuning of chemoand regioselectivities in multicomponent condensations of 5-aminopyrazoles, dimedone, and aldehydes. J. Org. Chem. 2008, 73, 5110–5118. [Google Scholar] [CrossRef] [PubMed]
- Ryabukhin, S.V.; Plaskon, A.S.; Ostapchuk, E.N.; Volochnyuk, D.M.; Shishkin, O.V.; Tolmachev, A.A. CF3-substituted 1,3-dicarbonyl compounds in the Biginelli reaction promoted by chlorotrimethylsilane. J. Fluorine Chem. 2008, 129, 625–631. [Google Scholar] [CrossRef]
- Nagarapu, L.; Bantu, R.; Mereyala, H.B. TMSCl-mediated one-pot, threecomponent synthesis of 2H-Indazolo[2,1-b]phthalazine-triones. J. Heterocyl. Chem. 2009, 46, 728–731. [Google Scholar] [CrossRef]
- Azizian, J.; Mirza, B.; Mohtahedi, M.M.; Abaee, M.S.; Sargordan, M. Biginelli reaction for synthesis of novel trifluoromethyl derivatives of bis(tetrahydropyrimidinone)benzenes. J. Fluorine Chem. 2008, 129, 1083–1089. [Google Scholar] [CrossRef]
- Sabitha, G.; Reddy, G.S.K.K.; Reddy, C.S.; Yadav, J.S. A novel TMSI-mediated synthesis of Hantzsch 1,4-dihydropyridines at ambient temperature. Tetrahedron Lett. 2003, 44, 4129–4131. [Google Scholar] [CrossRef]
- Sabitha, G.; Reddy, K.B.; Srinivas, R.; Yadav, J.S. Iodotrimethylsilane-accelerated one-pot synthesis of 5-unsubstituted 3,4-dihydropyrimidin-2(1H)-ones: A novel procedure for the Biginelli-like cyclocondensation reaction at room temperature. Helv. Chim. Acta 2005, 88, 2996–2999. [Google Scholar] [CrossRef]
- Wan, J.-P.; Liu, Y. Multicomponent reactions promoted by organosilicon reagents. Curr. Org. Chem. 2011, 15, 2758–2773. [Google Scholar] [CrossRef]
- Bandini, M.; Fagioli, M.; Melloni, A.; Umani-Ronchi, A. A general procedure for the synthesis of 1,3-bis(indolyl) compounds via Michael addition catalyzed by InBr3/TMSCl. Synthesis 2003, 397–402. [Google Scholar] [CrossRef]
- Ito, T.; Ishino, Y.; Mizuno, T.; Iswkawa, A.; Kobayashi, J. Zinc metal-promoted cross-coupling reaction of non-activated alkyl halides with aldehydes in the presence of chlorotrimethylsilane. Synlett 2002, 2116–2118. [Google Scholar] [CrossRef]
- Lee, P.H.; Ahn, H.; Lee, K.; Sung, S.; Kim, S. Studies on the reactions of α,β-enones with allyl indium reagent: Effects of TMSCl as promoter on regioselectivity. Tetrahedron Lett. 2001, 42, 37–39. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 4 and 6 are available from the authors. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).