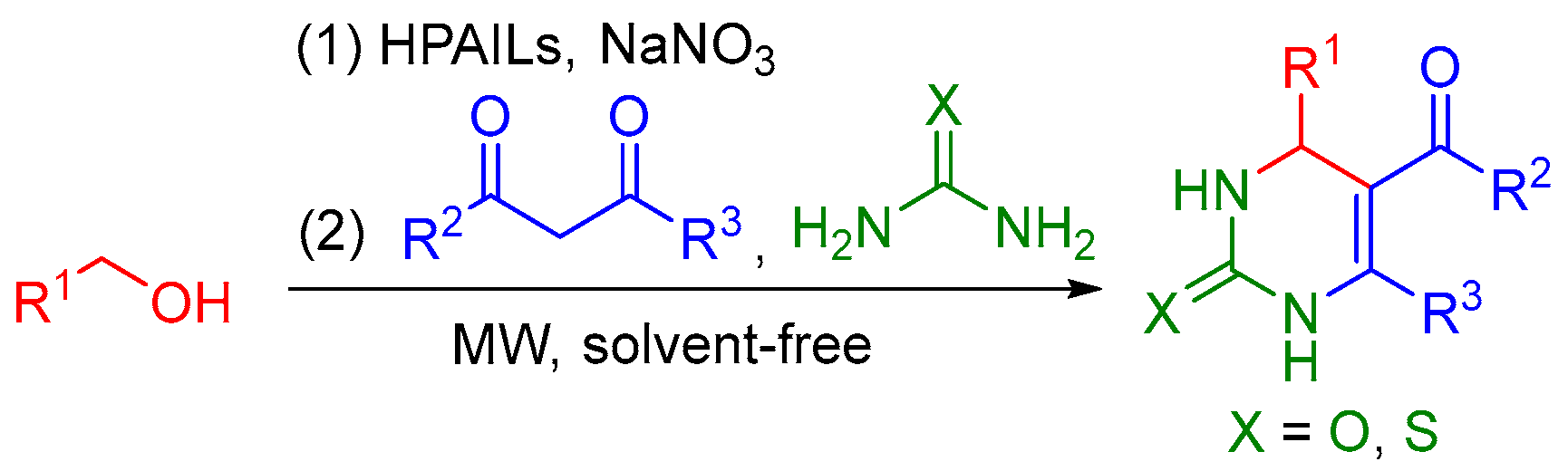
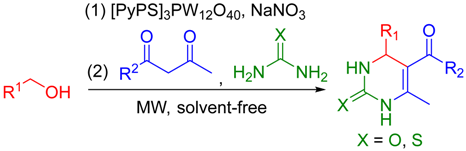
Abstract
Efficient, eco-friendly and sustainable access to 3,4-dihydropyrimidin-2(1H)-ones directly from alcohols under microwave and solvent-free conditions has been reported. The practical protocol involves heteropolyanion-based catalyzed oxidation of alcohols to aldehydes with NaNO3 as the oxidant followed by cyclocondensation with dicarbonyl compounds and urea or thiourea in a two-step, one-pot manner. Compatibility with different functional groups, good to excellent yields and reusable catalysts are the main highlights. The utilization of alcohols instead of aldehydes is a valid and green alternative to the classical Biginelli reaction.
1. Introduction
One-pot, sequential multi-step reactions have become an important area of research in organic chemistry, due to the improved atom economy, multiple-bond-forming efficiency, time and energy savings and avoiding waste and pollution [1,2,3,4,5]. Aldehydes are widely prevalent substrates in many efficient one-pot synthesis or multicomponent reactions (MCRs) [6,7,8]. However, they are generally very volatile, toxic and unstable, especially due to ease of aerial oxidation. In the practical process, aldehydes must be purified carefully just before use, otherwise the impurities will affect not only the concentration of the active aldehyde, but also the proceeding of the chemical reactions. It is apparent that these disadvantages have limited the application in industry. Recently, tandem oxidation processes (TOPs) in which oxidation of alcohols combined with the subsequent elaboration of the carbonyl intermediates (aldehydes) have gained considerable attention [9,10,11,12,13,14,15,16,17,18], while only a few reports described the combining alcohol oxidation with a MCR in a one-pot process [19,20]. Therefore, it is expected that the use of a single vessel oxidation-MCR protocol from alcohols would widen significantly the versatility and scope of aldehyde-based MCRs.
The century-old Biginelli reaction, which involves the one-pot condensation of an aldehyde, β-ketoester and urea or thiourea gives straightforward access to functionalized 3,4-dihydropyrimidin-2(1H)-ones (DHPMs) with a diverse range of biological properties [21,22,23,24,25,26], and is considered to be one of the most useful MCR [27,28,29,30,31]. Since the discovery of this reaction, a number of improved catalytic systems have been developed, such as Brønsted acids [32,33,34,35,36,37,38] or bases [39,40], metal Lewis acids [41,42,43,44,45,46,47,48,49,50], organocatalysts [51,52,53,54,55,56,57,58], and heterogeneous catalysts [59,60,61,62,63,64,65,66,67,68,69,70,71]. However, to the best of our knowledge, there have been only a few reports on the Biginelli reaction starting directly from alcohols [72,73,74,75].
With the common goal of being environmentally benign and allowing sustainable development in the chemical industry, the use of more eco-benign catalytic systems which are simple to recover helps to minimize waste production and maximize catalyst efficiency; this has been well studied in the past decades [76,77,78]. Among those, ionic liquids (ILs) have offered both economic and ecological benefits as ecofriendly and efficient alternatives to traditional organic solvents or catalysts [79,80]. In particular, a series of heteropolyanion-based ILs (HPAILs) [81,82,83], which were prepared by combining Keggin heteropolyanions with ‘task-specific’ IL cations containing special functional groups, are recently emerging as new species of hybrid materials [84,85]. In addition, reactions containing HPAILs are an attractive alternative for traditional acid-catalyzed [86,87,88,89,90,91] or oxidative [92,93,94,95,96,97,98,99] organic transformations because of their operational simplicity, lack of toxicity, ease of isolation and reusability. Therefore, HPAILs have proven to be a novel candidate for green and sustainable catalysts.
Recently, a large number of publications have clearly shown that the main benefit of microwave (MW) chemical processing is the significant rate enhancements, yield and selectivity improvements as well as less environmental pollution, matching with the goals of green chemistry [100,101,102]. Following our continued investigation into the development of useful and sustainable synthetic methodologies [103,104,105,106,107,108,109,110,111]—along with recent investigations involving oxidation of alcohols and the subsequent trapping of carbonyl intermediates with appropriate nucleophiles in a one-pot operation. [112]—herein, we report an efficient and environmentally benign TOPs for the Biginelli reaction, starting directly from alcohols, using HPAILs as catalysts and NaNO3 as an oxidant under MW and solvent-free conditions (Scheme 1). Compared with existing reports [72,73,74,75], short reaction times and reusable catalysts are the main highlights of this methodology.
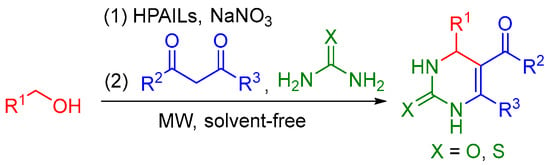
Scheme 1.
Tandem oxidation process (TOP) for the Biginelli reaction starting directly from alcohols.
2. Results and Discussion
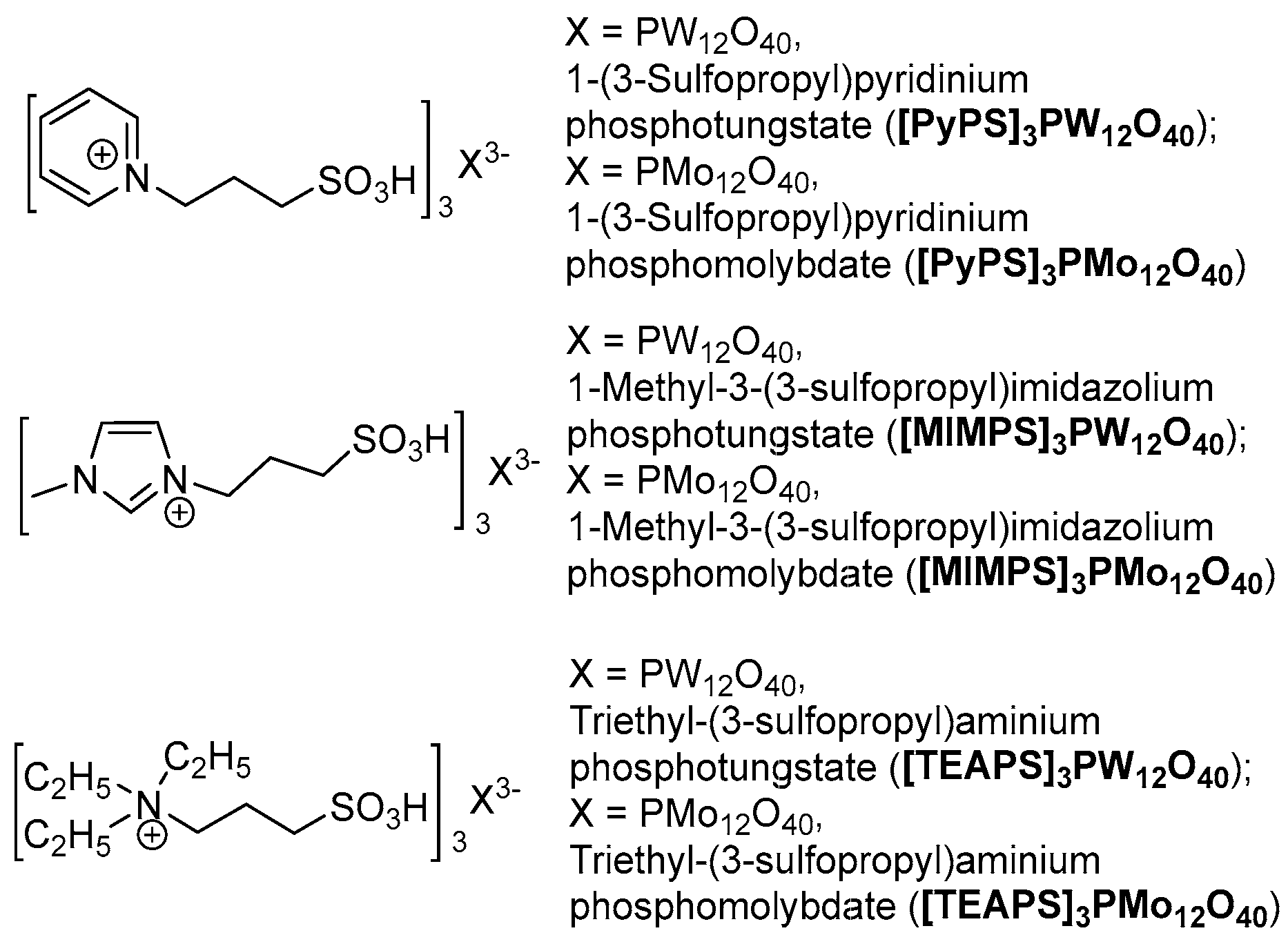
Based on our previous investigations into HPAILs catalyzed reactions [103,104,105,106,107,108,109,110,111,112], N-substituted imidazole, pyridine and triethylamine based HPAILs were chosen as potential catalysts for this tandem oxidative cyclocondensation (Figure 1).
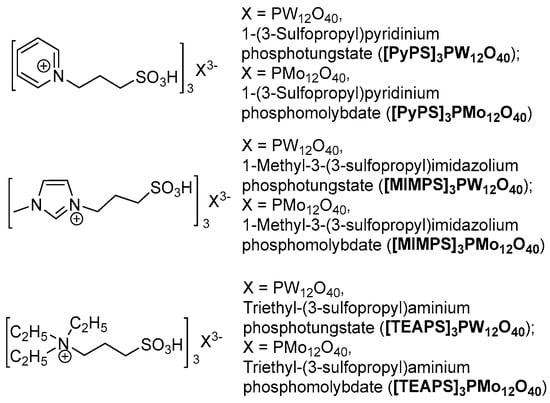
Figure 1.
N-substituted imidazole, pyrdine and triethylamine based heteropolyanion-based ionic liquids (HPAILs).
Initially, benzyl alcohol (3 mmol), ethylacetoacetate (3 mmol) and urea (4.5 mmol) were considered as standard model substrates to optimize the reaction conditions (Table 1). Firstly, we studied the tandem oxidative cyclocondensation using NaNO3 (3 mmol) as the oxidant in the presence of [PyPS]3PW12O40 (3 mol %) as catalyst at 80 °C under MW and solvent-free conditions in a one-step one-pot procedure, disappointingly the designed product was observed in only 13% yield even after long reaction times (Table 1, entry 1). However, to our delight, the DHPM was formed in 51% yield when ethyl acetoacetate and urea were added to the reaction mixture after the oxidation of alcohol to aldehyde was complete (Table 1, entry 2). Therefore, we aimed to accomplish the tandem oxidative cyclocondensation in a two-step one-pot manner. In order to improve the yield, some adjustments to the reaction conditions were made. It should be seen that higher temperature (100 °C) was harmful to the oxidation of alcohol due to the partial over-oxidation (Table 1, entry 3), whereas an obvious increased yield was obtained when the cyclocondensation was performed at higher temperature (100 °C and 120 °C) (Table 1, entries 4–5). In addition, more efficient results were observed by increasing the catalyst loading to 4 mol % and 5 mol % (Table 1, entries 6–7). Afterwards, the catalytic activities of other related catalysts prepared earlier were screened (Table 1, entries 8–12). It is expected that PyPS (1-(3-Sulfopropyl)pyridinium) species were found to be more efficient than MIMPS (1-Methyl-3-(3-sulfopropyl)imidazolium) and TEAPS (Triethyl-(3-sulfopropyl)aminium) species, while the results demonstrated that PW12O40 heteropolyanions were more active than PMo12O40 heteropolyanions. Although pure HPA (heteropolyacid) catalyst H3PW12O40 gave a moderate yield of 63%, its high solubility throughout organic solvents and water made its isolation from the reaction mixture difficult (Table 1, entry 13). Finally, an optimum result was obtained when the reaction was performed using 4 mol % of [PyPS]3PW12O40 at 80/120 °C under MW and solvent-free conditions affording DHPM in 92% yield (Table 1, entry 6).

Table 1.
Optimization of the reaction conditions for benzyl alcohol, ethylacetoacetate and urea. a
To explore the scope and generality of this reaction, we extended the procedure to aromatic, heterocyclic, and aliphatic alcohols. In all cases, the reaction proceeded smoothly to afford the corresponding DHPMs within short reaction times (10–18 min) in good to excellent yields (71–95%) (Table 2). It could be noticed that a wide range of aryl alcohols bearing electron-withdrawing groups or electron-donating groups afforded good yields of DHPMs (Table 2, entries 1–7 and 11–28). Another important feature of this procedure is the compatibility with a variety of functional groups such as alkyl, alkoxyl, halide, nitro and hydroxyl under the present reaction conditions. Meanwhile, besides β-ketoesters, β-diketones (Table 2, entries 23–28) could also be employed with similar success to provide the corresponding products. In addition, the reactions with both urea and thiourea resulted in corresponding Biginelli adducts with good yields; nevertheless, longer reaction times were required in the cases of thiourea. It is worth mentioning that good yields were achieved (71–84% yield) in the cases of heterocyclic and aliphatic aldehydes (Table 2, entries 8–10), which normally were less reactive or completely inert in the Biginelli reaction.

Table 2.
Scope of the Biginelli reaction starting directly from alcohols. a
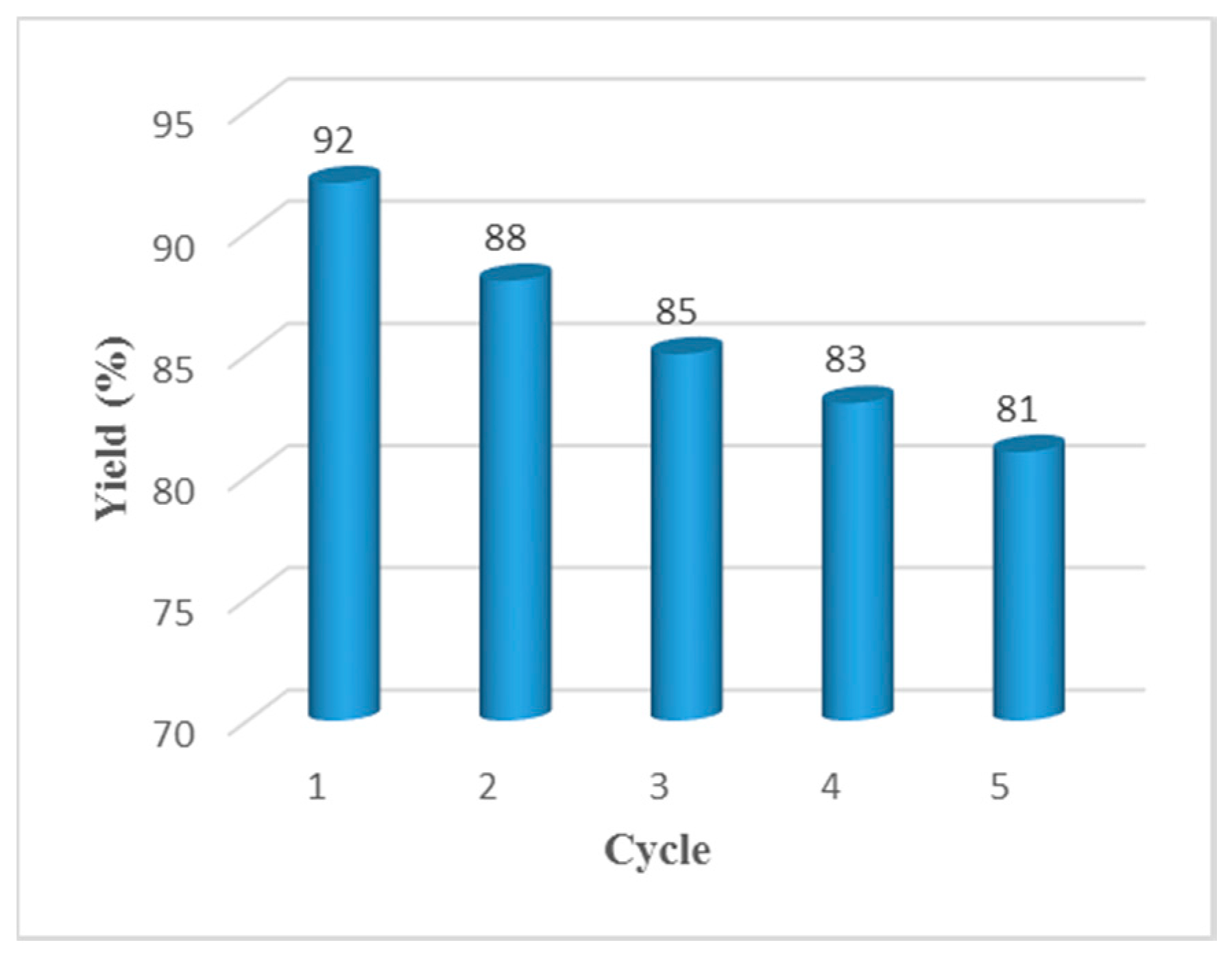
In consideration of sustainable chemistry, the potential recycling of HPAILs was investigated with the reaction of benzyl alcohol, ethylacetoacetate and urea. Upon completion of the reaction in the first run, hot EtOAc was added to the reaction mixture in order to dissolve the final DHPM product. After vigorous stirring, the solid catalyst can be easily retrieved by simple filtration as well as washing with ethyl acetate and then ice water to remove traces of the previous reaction mixture and inorganic salt. After concentration of the filtrate, the almost pure product was obtained and recrystallization could be used for further purification. The recovered catalyst was used for further runs of the same reaction. As is evident from Figure 2, the reaction was repeated for up to five consecutive runs with a little loss of catalytic efficiency. Thus, the robustness of the catalyst and its reusability were demonstrated.
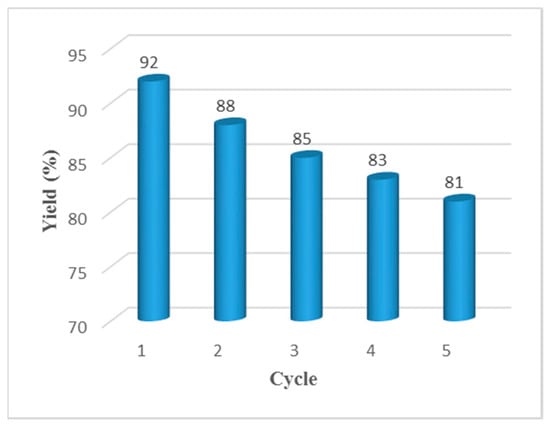
Figure 2.
Reusability studies of the catalyst for the Biginelli reaction starting directly from alcohols.
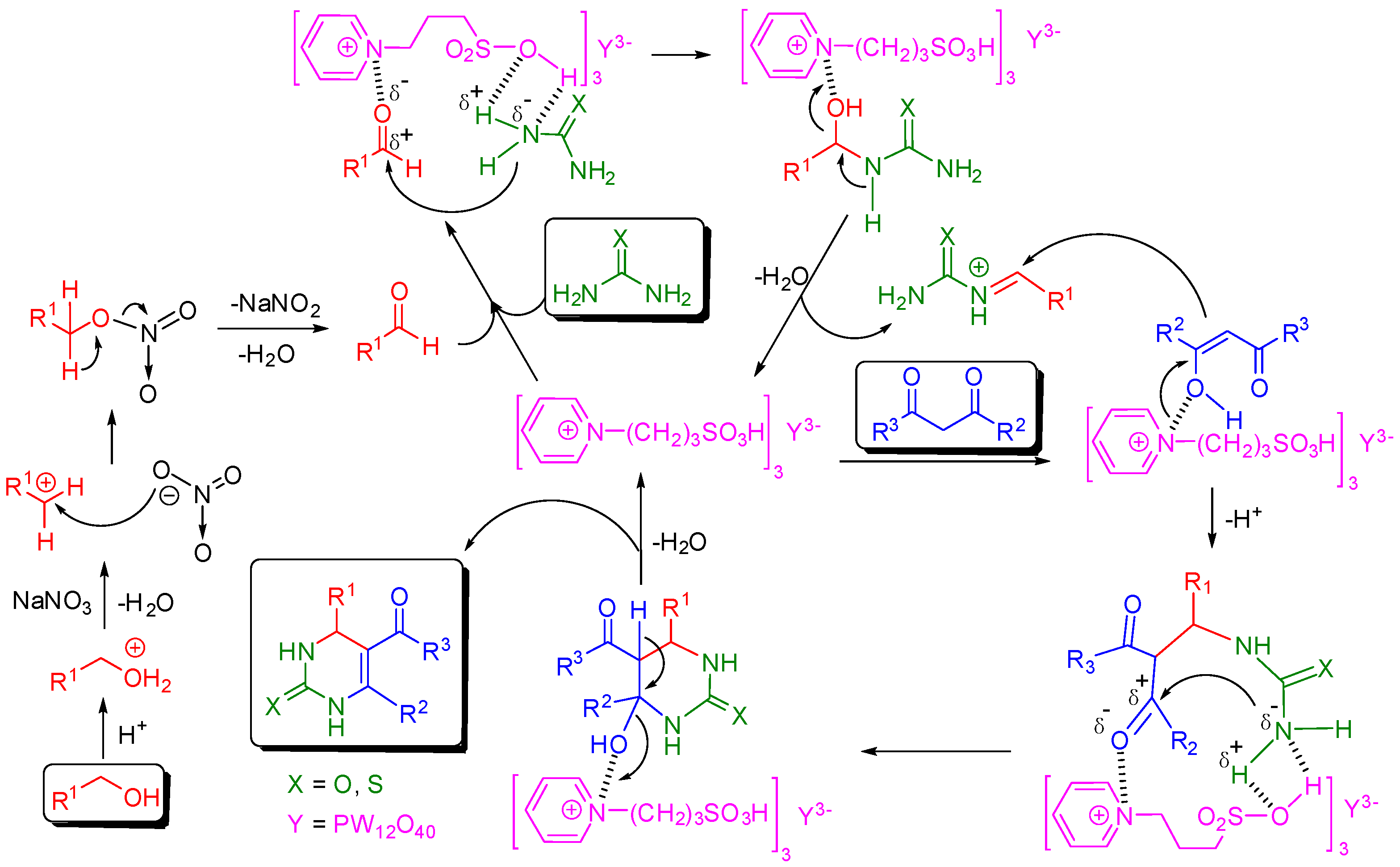
Although the mechanism of Biginelli reaction has been a topic of much debate [27,28,29,30,31], the acidic proton and the cation of HPAIL appeared to play important roles in this tandem oxidative cyclocondensation based on the above discussions. Thus, according to the iminium route mechanism suggested by Folkers, Johnson and Kappe [113,114,115], a plausible mechanism for the reaction is depicted in Figure 3. Initially, the alcohol substrate was easily oxidized to aldehyde by NaNO3 with assistance of the acidic HPAIL catalyst. The catalytic cycle starts with the activation of the carbonyl of aldehyde by the coordination with an aminium cation and N–H activation of urea by the sulfonic group in the HPAIL cation. The adsorbed aldehyde-amine species undergoes an addition of the amine to the carbonyl carbon atom to obtain the dipolar adduct. After proton-exchange, desorption and dehydration, an imine intermediate is formed with a regenerated HPAIL catalyst. Meanwhile, the HPAIL cation stabilizes the enolization of 1,3-dicarbonyl compounds to form an enolate intermediate, which undergoes the Mannich-like addition with the imine intermediate followed by intramolecular cyclocondensation. Then, the final Biginelli product is obtained, resuming the HPAIL catalyst by proton-exchange, desorption and dehydration.
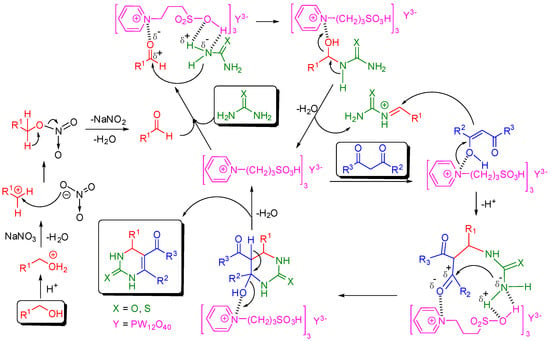
Figure 3.
Plausible mechanism of HPAIL catalyzed Biginelli reaction.
3. Materials and Methods
3.1. General Methods
Reagent grade solvents were used for extraction, recrystallization and flash chromatography. All other commercial reagents were used as received without additional purification. The progress of reactions was checked by analytical thin-layer chromatography (TLC, silica gel 60 F-254 plates) (Qingdao Haiyang Chemical Co., Ltd., Qingdao, Shandong, China). The plates were visualized first with UV illumination followed by iodine or phosphomolybdic acid hydrate. Column chromatography was performed using silica gel (200–300 mesh) (Qingdao Haiyang Chemical Co., Ltd., Qingdao, Shandong, China). NMR spectra were obtained using BRUKER AVANCE III instrument (Bruker Co., Ltd., Switzerland). 1H-NMR spectra were recorded at 300 MHz or 400 MHz and are reported in parts per million (ppm) on the δ scale relative to tetramethylsilane (TMS) as an internal standard. 13C-NMR spectra were recorded at 75 MHz or 100 MHz and are reported in parts per million (ppm) on the δ scale relative to CDCl3 (δ 77.16) and DMSO-d6 (δ 39.52). Multiplicities are indicated as the following: s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet; dd, doubled doublet; td, tripled doublet; br, broad. Coupling constants (J values) where noted are quoted in hertz. Mass spectra were obtained using Agilent 1260-6120 (ESI) instrument (Agilent Technologies Co., Ltd., Santa Clara, CA, USA). MW-promoted heating was obtained using MAS-II instrument manufactured (Sineo Microwave Chemistry Technology Co., Ltd., Shanghai, China). The melting point was uncorrected.
3.2. General Procedure for the Synthesis of HPAILs
To a well-stirred mixture of 12.2 g 1,3-propane sulfone (0.10 mol) in 30 mL toluene was added 8.9 mL pyridine (0.11 mol). The reaction mixture was stirred for 24 h at 50 °C under a nitrogen atmosphere resulting in a white precipitate (PyPS). After the completion of reaction, it was cooled to room temperature. PyPS was obtained after filtration, washed with diethyl ether and dried in a vacuum. Then, 18.1 g PyPS (0.09 mol) was added to an aqueous solution of 86.4 g H3PW12O40 (0.03 mol). After stirring at room temperature for 24 h, the solution was removed in a vacuum to give the HPAIL product [PyPS]3PW12O40 as a solid. Thus, [PyPS]3PMo12O40, [MIMPS]3PW12O40, [MIMPS]3PMo12O40, [TEAPS]3PW12O40 and [TEAPS]3PMo12O40 were prepared using according starting materials. Characterization data and copies of NMR spectra of all products can be found in the supplementary materials.
3.3. General Procedure for the Synthesis of 3,4-Dihydropyrimidin-2(1H)-ones
A mixture of alcohol (3 mmol), NaNO3 (0.255 g, 3 mmol), and [PyPS]3PW12O40 (0.42 g, 0.12 mmol) was added to a 30 mL glass pressure tube. After the pressure tube was closed, the reaction mixture was stirred at 80 °C under MW for 5–8 min (700 W). After the oxidation of alcohol (monitored by TLC), 1,3-dicarbonyl compound (3 mmol) and urea or thiourea (4.5 mmol) were added to the reaction mixture. The mixture was heated with stirring at 120 °C under MW for 5–10 min. On completion of the reaction (monitored by TLC), the mixture was diluted with hot ethyl acetate (20 mL) with stirring for 30 min. The insoluble catalyst was recovered by filtration as well as washing with ethyl acetate and subsequent ice water to remove traces of the previous organic reaction mixture and inorganic salt. The filtrate was evaporated and the residue was obtained in near pure form. Recrystallization or column chromatography could be used for further purification. Characterization data and copies of NMR spectra of all products can be found in the supplementary materials.
4. Conclusions
In conclusion, all the above results demonstrate that an efficient, eco-friendly and sustainable approach for the Biginelli reaction starting directly from alcohols using NaNO3-HPAIL system was achieved. The protocol provides compatibility with various functional groups and moderate to excellent yields to afford DHPMs in a two-step one-pot process. Moreover, the HPAILs are recyclable and reused for more than five cycles. The present work complements the well-known Biginelli reaction. Thus, the expansion as a valid and green alternative to other aldehyde-based MCRs is currently underway.
Supplementary Materials
Supplementary materials are available online.
Acknowledgments
Financial Support from the Suzhou Science and Technology Project (Grant No. SNG201620), Changshu Science and Technology Project (Grant No. CN201714), Natural Science Foundation of Jiangsu Province (Grant No. BK20161267 and BK20160405), National Natural Science Foundation of China (Grant No. 21302013 and 21602017) and Qinglan Project of Jiangsu Province.
Author Contributions
Y.Y. and J.Y. conceived and designed the experiments; R.F., X.M., Y.S. and J.L. performed the experiments; H.G., H.H. and X.Z. checked and analyzed the data; R.F. wrote the paper; X.Z., Y.Y. and J.Y. revised the manuscript; all authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tietze, L.F. Domino reactions in organic synthesis. Chem. Rev. 1996, 96, 115–136. [Google Scholar] [CrossRef] [PubMed]
- Climent, M.J.; Corma, A.; Iborra, S. Heterogeneous catalysts for the one-pot synthesis of chemicals and fine chemicals. Chem. Rev. 2011, 111, 1072–1133. [Google Scholar] [CrossRef] [PubMed]
- Pellissier, H. Stereocontrolled domino reactions. Chem. Rev. 2013, 113, 442–524. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, X.; Xue, W.; Chen, G.; Zhang, W.; Zhu, X. Initiator and photocatalyst-free visible light induced one-pot reaction: Concurrent RAFT polymerization and CuAAC Click reaction. Macromol. Rapid Commun. 2016, 37, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Jiang, L.; Zuo, J.; Hor, T.S.A. Hybrid NS ligands supported Cu(I)/(II) complexes for azide-alkyne cycloaddition reactions. Dalton Trans. 2013, 42, 11319–11326. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.G.; Wang, Q.F.; Song, X.K.; Sun, J. One-step synthesis of pyrido[1,2-a]benzimidazole derivatives by a novel multicomponent reaction of chloroacetonitrile, malononitrile, aromatic aldehyde, and pyridine. J. Org. Chem. 2009, 74, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Ermolat’ev, D.S.; Song, G.; Eycken, E.V.V. Synthesis of symmetric 1,4-diamino-2-butynes via a Cu(I)-catalyzed one-pot A3-coupling/decarboxylative coupling of a propiolic acid, an aldehyde, and an amine. J. Org. Chem. 2012, 77, 5149–5154. [Google Scholar] [CrossRef] [PubMed]
- Guchhait, S.K.; Chandgude, A.L.; Priyadarshani, G. CuSO4–glucose for in situ generation of controlled Cu(I)–Cu(II) bicatalysts: Multicomponent reaction of heterocyclic azine and aldehyde with alkyne, and cycloisomerization toward synthesis of N-fused imidazoles. J. Org. Chem. 2012, 77, 4438–4444. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.; Rowe, D.J.; Taylor, R.J.K. Two carbon homologated α,β-unsaturated aldehydes from alcohols using the in situ oxidation–Wittig reaction. Chem. Commun. 2003, 2284–2285. [Google Scholar] [CrossRef]
- Taylor, R.J.K.; Reid, M.; Foot, J.; Raw, S.A. Tandem oxidation processes using manganese dioxide: discovery, applications, and current studies. Acc. Chem. Res. 2005, 38, 851–869. [Google Scholar] [CrossRef] [PubMed]
- Quesada, E.; Taylor, R.J.K. One-pot conversion of activated alcohols into terminal alkynes using manganese dioxide in combination with the Bestmann–Ohira reagent. Tetrahedron Lett. 2005, 46, 6473–6476. [Google Scholar] [CrossRef]
- Phillips, D.J.; Pillinger, K.S.; Wei, L.; Taylor, A.E.; Graham, A.E. Desymmetrization of diols by a tandem oxidation/Wittig olefination reaction. Chem. Commun. 2006, 2280–2282. [Google Scholar] [CrossRef] [PubMed]
- McAllister, G.D.; Oswald, M.F.; Paxton, R.J.; Raw, S.A.; Taylor, R.J.K. The direct preparation of functionalised cyclopropanes from allylic alcohols or α-hydroxyketones using tandem oxidation processes. Tetrahedron 2006, 62, 6681–6694. [Google Scholar] [CrossRef]
- Donald, J.R.; Edwards, M.G.; Taylor, R.J.K. Tandem oxime formation—Epoxide ring opening sequences for the preparation of oxazines related to the trichodermamides. Tetrahedron Lett. 2007, 48, 5201–5204. [Google Scholar] [CrossRef]
- Bromley, W.J.; Gibson, M.; Lang, S.; Raw, S.A.; Whitwood, A.C.; Taylor, R.J.K. Tandem inverse electron demand Diels–Alder, retro-Diels–Alder and intramolecular Diels–Alder sequences: One-pot synthesis of diaza-polycycles. Tetrahedron 2007, 63, 6004–6014. [Google Scholar] [CrossRef]
- Ekoue-Kovi, K.; Wolf, C. One-pot oxidative esterification and amidation of aldehydes. Chem. Eur. J. 2008, 14, 6302–6315. [Google Scholar] [CrossRef] [PubMed]
- Davi, M.; Lebel, H. Copper-Catalyzed Tandem Oxidation-Olefination Process. Org. Lett. 2009, 11, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Maki, B.E.; Scheidt, K.A. Single-flask synthesis of N-acylated indoles by catalytic dehydrogenative coupling with primary alcohols. Org. Lett. 2009, 11, 1651–1654. [Google Scholar] [CrossRef] [PubMed]
- Ngouansavanh, T.; Zhu, J. Alcohols in isonitrile-based multicomponent reaction: passerini reaction of alcohols in the presence of O-iodoxybenzoic acid. Angew. Chem. Int. Ed. 2006, 45, 3495–3497. [Google Scholar] [CrossRef] [PubMed]
- Brioche, J.; Masson, G.; Zhu, J. Passerini three-component reaction of alcohols under catalytic aerobic oxidative conditions. Org. Lett. 2010, 12, 1432–1435. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, M.B.; Salunkhe, S.M.; Patil, D.R.; Anbhule, P.V. A novel and efficient one step synthesis of 2-amino-5-cyano-6-hydroxy-4-aryl pyrimidines and their anti-bacterial activity. Eur. J. Med. Chem. 2009, 44, 2651–2654. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Arora, D.; Poremsk, E.; Lowery, J.; Moreland, R.S. N1-Alkylated 3,4-dihydropyrimidine-2(1H)-ones: Convenient one-pot selective synthesis and evaluation of their calcium channel blocking activity. Eur. J. Med. Chem. 2009, 44, 1997–2001. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.R.P.; Sankar, G.; Baig, R.B.N.; Chandrashekaran, S. Novel Biginelli dihydropyrimidines with potential anticancer activity: A parallel synthesis and CoMSIA study. Eur. J. Med. Chem. 2009, 44, 4192–4198. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, D.L.; Reis, F.S.; Muniz, D.R.; Ruiz, A.L.T.G.; de Carvalho, J.E.; Sabino, A.A.; Modolo, L.V.; de Fatima, A. Free radical scavenging and antiproliferative properties of Biginelli adducts. Bioorg. Med. Chem. 2012, 20, 2645–2650. [Google Scholar] [CrossRef] [PubMed]
- Bagal, S.K.; Brown, A.D.; Cox, P.J.; Omoto, K.; Owen, R.M.; Pryde, D.C.; Sidders, B.; Skerratt, S.E.; Stevens, E.B.; Storer, R.I.; et al. Ion channels as therapeutic targets: A drug discovery perspective. J. Med. Chem. 2013, 56, 593–624. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Chaudhary, S.; Kumar, K.; Gupta, M.K.; Rawal, R.K. Recent synthetic and medicinal perspectives of dihydropyrimidinones: A review. Eur. J. Med. Chem. 2017, 132, 108–134. [Google Scholar] [CrossRef] [PubMed]
- Kappe, C.O. Recent advances in the Biginelli dihydropyrimidine synthesis. New tricks from an old dog. Acc. Chem. Res. 2000, 33, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Dömling, A. Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem. Rev. 2006, 106, 17–89. [Google Scholar] [CrossRef] [PubMed]
- Dondoni, A.; Massi, A. Design and synthesis of new classes of heterocyclic c-glycoconjugates and carbon-linked sugar and heterocyclic amino acids by asymmetric multicomponent reactions (AMCRs). Acc. Chem. Res. 2006, 39, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Nagarajaiah, H.; Mukhopadhyay, A.; Moorthy, J.N. Biginelli reaction: An overview. Tetrahedron Lett. 2016, 57, 5135–5149. [Google Scholar] [CrossRef]
- Xue, H.; Zhao, Y.; Wu, H.; Wang, Z.; Yang, B.; Wei, Y.; Wang, Z.; Tao, L. Multicomponent combinatorial polymerization via the Biginelli reaction. J. Am. Chem. Soc. 2016, 138, 8690–8693. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Liu, C.; Luo, G. One-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones using chloroacetic acid as catalyst. Bioorg. Med. Chem. Lett. 2007, 17, 3508–3510. [Google Scholar] [CrossRef] [PubMed]
- Polshettiwar, V.; Varma, R.S. Biginelli reaction in aqueous medium: A greener and sustainable approach to substituted 3,4-dihydropyrimidin-2(1H)-ones. Tetrahedron Lett. 2007, 48, 7343–7346. [Google Scholar] [CrossRef]
- Shobha, D.; Chari, M.A.; Ahn, K.H. An efficient Biginelli one-pot synthesis of new benzoxazole-substituted dihydropyrimidinones and thiones catalysed by trifluoro acetic acid under solvent-free conditions. Chin. Chem. Lett. 2009, 20, 1059–1061. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, J.; Yang, H.; Miao, Z.; Chen, R. Solvent-free Biginelli reaction: A green method for the synthesis of 3,4-dihydropyrimidin-2-ones catalyzed by protic acids in large-scale. Lett. Org. Chem. 2011, 8, 264–267. [Google Scholar] [CrossRef]
- Gore, S.; Baskaran, S.; Koenig, B. Efficient synthesis of 3,4-dihydropyrimidin-2-ones in low melting tartaric acid–urea mixtures. Green Chem. 2011, 13, 1009–1013. [Google Scholar] [CrossRef]
- Ren, Y.; Cai, C.; Yang, R. Molecular iodine-catalyzed multicomponent reactions: An efficient catalyst for organic synthesis. RSC Adv. 2013, 3, 7182–7204. [Google Scholar] [CrossRef]
- Barbero, M.; Cadamuro, S.; Dughera, S. A Brønsted acid catalysed enantioselective Biginelli reaction. Green Chem. 2017, 19, 1529–1535. [Google Scholar] [CrossRef]
- Shen, Z.; Xu, X.; Ji, S. Brønsted base-catalyzed one-pot three-component Biginelli-type reaction: An efficient synthesis of 4,5,6-triaryl-3,4-dihydropyrimidin-2(1H)-one and mechanistic study. J. Org. Chem. 2010, 75, 1162–1167. [Google Scholar] [CrossRef] [PubMed]
- Tamaddon, F.; Razmi, Z.; Jafari, A.A. Synthesis of 3,4-dihydropyrimidin-2(1H)-ones and 1,4-dihydropyridines using ammonium carbonate in water. Tetrahedron Lett. 2010, 51, 1187–1189. [Google Scholar] [CrossRef]
- Lannou, M.; Hélion, F.; Namy, J. Applications of lanthanide trichloride hydrates, prepared from mischmetall, in the Biginelli reaction. Synlett 2008, 2008, 105–107. [Google Scholar]
- Prodius, D.; Macaev, F.; Mereacre, V.; Shova, S.; Lutsenco, Y.; Styngach, E.; Ruiz, P.; Muraviev, D.; Lipkowski, J.; Simonov, Y.A.; et al. Synthesis and characterization of {Fe2CuO} clusters as precursors for nanosized catalytic system for Biginelli reaction. Inorg. Chem. Commun. 2009, 12, 642–645. [Google Scholar] [CrossRef]
- Chitra, S.; Pandiarajan, K. Calcium fluoride: an efficient and reusable catalyst for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones and their corresponding 2(1H)thione: An improved high yielding protocol for the Biginelli reaction. Tetrahedron Lett. 2009, 50, 2222–2224. [Google Scholar] [CrossRef]
- Litvić, M.; Večenaj, I.; Ladišić, Z. M.; Lovrić, M.; Vinković, V.; Litvić, M.F. First application of hexaaquaaluminium(III) tetrafluoroborate as a mild, recyclable, non-hygroscopic acid catalyst in organic synthesis: A simple and efficient protocol for the multigram scale synthesis of 3,4-dihydropyrimidinones by Biginelli reaction. Tetrahedron 2010, 66, 3463–3471. [Google Scholar] [CrossRef]
- Sadek, K.U.; Al-Qalaf, F.; Abdelkhalik, M.M.; Elnagdi, M.H.J. Cerium (IV) ammonium nitrate as an efficient Lewis acid for the one-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones and their corresponding 2-(1H) thiones. Heterocycl. Chem. 2010, 47, 284–286. [Google Scholar]
- Narsaiah, A.V.; Reddy, A.R.; Yadav, J.S. Samarium triflate–catalyzed Biginelli condensation: An improved method for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones. Synth. Commun. 2011, 41, 2794–2799. [Google Scholar] [CrossRef]
- Lei, M.; Ma, L.; Hu, L. Cu(ClO4)2∙6H2O as an efficient catalyst for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones under solvent-free conditions. Synth. Commun. 2011, 41, 3071–3077. [Google Scholar] [CrossRef]
- Pasunooti, K.K.; Chai, H.; Jensen, C.N.; Gorityala, B.K.; Wang, S.; Liu, X. A microwave-assisted, copper-catalyzed three-component synthesis of dihydropyrimidinones under mild conditions. Tetrahedron Lett. 2011, 52, 80–84. [Google Scholar] [CrossRef]
- Starcevich, J.T.; Laughlin, T.J.; Mohan, R.S. Iron(III) tosylate catalyzed synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones via the Biginelli reaction. Tetrahedron Lett. 2013, 54, 983–985. [Google Scholar] [CrossRef]
- Nguyen, N.H.T.; Nguyen, P.P.T.; Nguyen, T.T.; Tran, M.T.; Huynh, T.T.H.; Tran, P.H. Au nanorod: An efficient catalyst for one-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones via the multicomponent Biginelli reaction. Chemistryselect 2017, 2, 3932–3936. [Google Scholar] [CrossRef]
- Mandhane, P.G.; Joshi, R.S.; Nagargoje, D.R.; Gill, C.H. An efficient synthesis of 3,4-dihydropyrimidin-2(1H)-ones catalyzed by thiamine hydrochloride in water under ultrasound irradiation. Tetrahedron Lett. 2010, 51, 3138–3140. [Google Scholar] [CrossRef]
- Rao, G.B.D.; Acharya, B.N.; Verma, S.K.; Kaushik, M.P. N,N′-Dichlorobis(2,4,6-trichlorophenyl)urea (CC-2) as a new reagent for the synthesis of pyrimidone and pyrimidine derivatives via Biginelli reaction. Tetrahedron Lett. 2011, 52, 809–812. [Google Scholar] [CrossRef]
- Silva, D.L.; Fernandes, S.A.; Sabino, A.A.; Fátima, Â. p-Sulfonic acid calixarenes as efficient and reusable organocatalysts for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/-thiones. Tetrahedron Lett. 2011, 52, 6328–6330. [Google Scholar] [CrossRef]
- Verma, S.; Jain, S.L. Thiourea dioxide in water as a recyclable catalyst for the synthesis of structurally diverse dihydropyrido[2,3-d]pyrimidine-2,4-diones. Tetrahedron Lett. 2012, 53, 2595–2600. [Google Scholar] [CrossRef]
- Xu, F.; Huang, D.; Lin, X.; Wang, Y. Highly enantioselective Biginelli reaction catalyzed by SPINOL-phosphoric acids. Org. Biomol. Chem. 2012, 10, 4467–4470. [Google Scholar] [CrossRef] [PubMed]
- Nagarapu, L.; Gaikwad, H.K.; Palem, J.D.; Venkatesh, R.; Bantu, R.; Sridhar, B. Convenient approach for the one-pot, three-component synthesis of triheterocyclic 4H-pyrimido[2,1-b]benzothiazole derivatives using TBAHS. Synth. Commun. 2013, 43, 93–104. [Google Scholar] [CrossRef]
- Puripat, M.; Ramozzi, R.; Hatanaka, M.; Parasuk, W.; Parasuk, V. The Biginelli reaction is a urea-catalyzed organocatalytic multicomponent reaction. J. Org. Chem. 2015, 80, 6959–6967. [Google Scholar] [CrossRef] [PubMed]
- Khatri, C.K.; Rekunge, D.S.; Chaturbhuj, G.U. Sulfated polyborate: A new and eco-friendly catalyst for one-pot multicomponent synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones via Biginelli reaction. New J. Chem. 2016, 40, 10412–10417. [Google Scholar] [CrossRef]
- Cheng, Q.; Wang, Q.; Xu, X.; Ruan, M.; Yao, H.; Yang, X. Solvent-free synthesis of monastrol derivatives catalyzed by NaHSO4. J. Heterocycl. Chem. 2010, 47, 624–628. [Google Scholar] [CrossRef]
- Shobha, D.; Chari, M.A.; Mano, A.; Selvan, S.T.; Mukkanti, K.; Vinu, A. Synthesis of 3,4-dihydropyrimidin-2-ones (DHPMs) using mesoporous aluminosilicate (AlKIT-5) catalyst with cage type pore structure. Tetrahedron 2009, 65, 10608–10611. [Google Scholar] [CrossRef]
- Verma, S.; Jain, S.L.; Sain, B. PEG-embedded thiourea dioxide (PEG.TUD) as a novel organocatalyst for the highly efficient synthesis of 3,4-dihydropyrimidinones. Tetrahedron Lett. 2010, 51, 6897–6900. [Google Scholar] [CrossRef]
- Murata, H.; Ishitani, H.; Iwamoto, M. Synthesis of Biginelli dihydropyrimidinone derivatives with various substituents on aluminium-planted mesoporous silica catalyst. Org. Biomol. Chem. 2010, 8, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Regati, S.; Butcher, R.J.; Arman, H.D.; Chen, Z.; Xiang, S.; Chen, B.; Zhao, C. Hydrogen-bonding 2D metal–organic solids as highly robust and efficient heterogeneous green catalysts for Biginelli reaction. Tetrahedron Lett. 2011, 52, 6220–6222. [Google Scholar] [CrossRef] [PubMed]
- Konkala, K.; Sabbavarapu, N.M.; Katla, R.; Durga, N.Y.V.; Reddy, T.V.K.; Bethala, L.A.P.D.; Rachapudi, B.N.P. Revisit to the Biginelli reaction: a novel and recyclable bioglycerol-based sulfonic acid functionalized carbon catalyst for one-pot synthesis of substituted 3,4-dihydropyrimidin-2-(1H)-ones. Tetrahedron Lett. 2012, 53, 1968–1973. [Google Scholar] [CrossRef]
- Narahari, S.R.; Reguri, B.R.; Gudaparthi, O.; Mukkanti, K. Synthesis of dihydropyrimidinones via Biginelli multicomponent reaction. Tetrahedron Lett. 2012, 53, 1543–1545. [Google Scholar] [CrossRef]
- Sahu, P.K.; Sahu, P.K.; Agarwal, D.D. Efficient and facile synthesis of heterocycles and their mechanistic consideration using kaolin. RSC Adv. 2013, 3, 9854–9864. [Google Scholar] [CrossRef]
- Shi, X.; Yang, H.; Tao, M.; Zhang, W. Sulfonic acid-functionalized polypropylene fiber: highly efficient and recyclable heterogeneous Brønsted acid catalyst. RSC Adv. 2013, 3, 3939–3945. [Google Scholar] [CrossRef]
- Shen, P.; Xu, M.; Yin, D.; Xie, S.; Zhou, C.; Li, F. Halogenated macroporous sulfonic resins as efficient catalysts for the Biginelli reaction. Catal. Commun. 2016, 77, 18–21. [Google Scholar] [CrossRef]
- Sheykhan, M.; Yahyazadeh, A.; Ramezani, L. A novel cooperative Lewis acid/Brønsted base catalyst Fe3O4@SiO2-APTMS-Fe(OH)2: An efficient catalyst for the Biginelli reaction. Mol. Catal. 2017, 435, 166–173. [Google Scholar] [CrossRef]
- Zolfagharinia, S.; Kolvari, E.; Koukabi, N. A new type of magnetically-recoverable heteropolyacid nanocatalyst supported on zirconia-encapsulated Fe3O4 nanoparticles as a stable and strong solid acid for multicomponent reactions. Catal. Lett. 2017, 147, 1551–1566. [Google Scholar] [CrossRef]
- Ghosh, B. K.; Moitra, D.; Chandel, M.; Patra, M.K.; Vadera, S.R.; Ghosh, N. N. CuO nanoparticle immobilised mesoporous TiO2–cobalt ferrite nanocatalyst: A versatile, magnetically separable and reusable catalyst. Catal. Lett. 2017, 147, 1061–1076. [Google Scholar] [CrossRef]
- Khosropour, A.R.; Khodaei, M.M.; Beygzadeh, M.; Jokar, M. A one-pot synthesis of 3,4-dihydropyrimidin-2-(1H)-ones from primary alcohols promoted by Bi(NO3)3·5H2O in two different media: organic solvent and ionic liquid. Heterocycles 2005, 65, 767–773. [Google Scholar] [CrossRef]
- Garima; Srivastava, V.P.; Yadav, L.D.S. Biginelli reaction starting directly from alcohols. Tetrahedron Lett. 2010, 51, 6436–6438. [Google Scholar] [CrossRef]
- Kolvari, E.; Zolfigol, M.A.; Mirzaeean, M. Aluminium nitrate nonahydrate (Al(NO3)3∙9H2O): An efficient oxidant catalyst for the one-pot synthesis of Biginelli compounds from benzyl alcohols. Helv. Chim. Acta 2012, 95, 115–119. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Bose, A.; Mal, P. Solvent-free ball-milling Biginelli reaction by subcomponent synthesis. Eur. J. Org. Chem. 2015, 2015, 6994–6998. [Google Scholar] [CrossRef]
- Simon, M.; Li, C. Green chemistry oriented organic synthesis in water. Chem. Soc. Rev. 2012, 41, 1415–1427. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Poliakoff, M. Continuous reactions in supercritical carbon dioxide: Problems, solutions and possible ways forward. Chem. Soc. Rev. 2012, 41, 1428–1436. [Google Scholar] [CrossRef] [PubMed]
- Baig, R.B.N.; Varma, R. S. Alternative energy input: Mechanochemical, microwave and ultrasound-assisted organic synthesis. Chem. Soc. Rev. 2012, 41, 1559–1584. [Google Scholar] [CrossRef] [PubMed]
- Xin, B.W.; Hao, J.C. Imidazolium-based ionic liquids grafted on solid surfaces. Chem. Soc. Rev. 2014, 43, 7171–7187. [Google Scholar] [CrossRef] [PubMed]
- Wagh, K.V.; Bhanage, B.M. Synthesis of 2-phenylnaphthalenes from styrene oxides using a recyclable Brønsted acidic [HNMP]+HSO4− ionic liquid. Green Chem. 2015, 17, 4446–4451. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Raman, K.; Herrera, R.; Zhang, Q.; Archer, L.A.; Giannelis, E.P. A liquid derivative of 12-tungstophosphoric acid with unusually high conductivity. J. Am. Chem. Soc. 2004, 126, 15358–15359. [Google Scholar] [CrossRef] [PubMed]
- Rickert, P.G.; Antonio, M.R.; Firestone, M.A.; Kubatko, K.A.; Szreder, T.; Wishart, J.F.; Dietz, M.L. Tetraalkylphosphonium polyoxometalate ionic liquids: Novel, organic-inorganic hybrid materials. J. Phys. Chem. B 2007, 111, 4685–4692. [Google Scholar] [CrossRef] [PubMed]
- Rickert, P.G.; Antonio, M.R.; Firestone, M.; Kubatko, A.K.A.; Szreder, T.; Wishart, J.F.; Dietz, M.L. Tetraalkylphosphonium polyoxometalates: Electroactive, “task-specific” ionic liquids. Dalton Trans. 2007, 529–531. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Kai, D.; Ke, K.L.; Lin, M.; Jiang, L.; Jiang, Y.; Young, D.J.; Loh, X.J.; Li, X.; Hor, T.S.A. A triazolyl-pyridine-supported CuI dimer: Tunable luminescence and fabrication of composite fibers. ChemPlusChem 2015, 80, 1235–1240. [Google Scholar] [CrossRef]
- Teeuwen, R.L.M.; van Berkel, S.S.; van Dulmen, T.H.H.; Schoffelen, S.; Meeuwissen, S.A.; Zuilhof, H.; de Wolf, F.A.; van Hest, J.C.M. “Clickable” elastins: Elastin-like polypeptides functionalized with azide or alkyne groups. Chem. Commun. 2009, 0, 4022–4024. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; Wang, J.; Zhu, D.; Ren, X.; Ge, H.; Shen, L. Heteropolyanion-based ionic liquids: Reaction-induced self-separation catalysts for esterification. Angew. Chem. Int. Ed. 2009, 48, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Leng, Y.; Zhu, D.; Wu, Y.; Wang, J. Phosphotungstic acid salt of triphenyl(3-sulfopropyl)phosphonium: An efficient and reusable solid catalyst for esterification. Catal. Commun. 2009, 11, 151–154. [Google Scholar] [CrossRef]
- Li, H.; Qiao, Y.; Hua, L.; Hou, Z.; Feng, B.; Pan, Z.; Hu, Y.; Wang, X.; Zhao, X.; Yu, Y. Imidazolium polyoxometalate: An ionic liquid catalyst for esterification and oxidative esterification. ChemCatChem 2010, 2, 1165–1170. [Google Scholar] [CrossRef]
- Zhang, W.; Leng, Y.; Zhao, P.; Wang, J.; Zhu, D.; Huang, J. Heteropolyacid salts of N-methyl-2-pyrrolidonium as highly efficient and reusable catalysts for Prins reactions of styrenes with formalin. Green Chem. 2011, 13, 832–834. [Google Scholar] [CrossRef]
- Fang, D.; Wang, F.; Wang, L.; Wu, Y.; Yang, J.; Qian, C. Regioselective mononitration of aromatic compounds catalyzed by heteropolyanions-based acidic ionic liquids. Curr. Catal. 2012, 1, 197–201. [Google Scholar] [CrossRef]
- Zhang, X.; Mao, D.; Leng, Y.; Zhou, Y.; Wang, J. Heterogeneous beckmann rearrangements catalyzed by a sulfonated imidazolium salt of phosphotungstate. Catal. Lett. 2013, 143, 193–199. [Google Scholar] [CrossRef]
- Qiao, Y.; Hou, Z.; Li, H.; Hu, Y.; Feng, B.; Wang, X.; Hua, L.; Huang, Q. Polyoxometalate-based protic alkylimidazolium salts as reaction-induced phase-separation catalysts for olefin epoxidation. Green Chem. 2009, 11, 1955–1960. [Google Scholar] [CrossRef]
- Leng, Y.; Wang, J.; Zhu, D.; Zhang, M.; Zhao, P.; Long, Z.; Huang, J. Polyoxometalate-based amino-functionalized ionic solid catalysts lead to highly efficient heterogeneous epoxidation of alkenes with H2O2. Green Chem. 2011, 13, 1636–1639. [Google Scholar] [CrossRef]
- Leng, Y.; Wang, J.; Zhu, D.; Shen, L.; Zhao, P.; Zhang, M. Heteropolyanion-based ionic hybrid solid: A green bulk-type catalyst for hydroxylation of benzene with hydrogen peroxide. Chem. Eng. J. 2011, 173, 620–626. [Google Scholar] [CrossRef]
- Leng, Y.; Zhang, W.; Wang, J.; Jiang, P. A novel heteropolyanion-based amino-containing cross-linked ionic copolymer catalyst for epoxidation of alkenes with H2O2. Appl. Catal. A-Gen. 2012, 445–446, 306–311. [Google Scholar] [CrossRef]
- Leng, Y.; Liu, J.; Zhang, C.; Jiang, P. A polyhedral oligomeric silsesquioxane (POSS)-bridged oxo-molybdenum Schiff base complex with enhanced heterogeneous catalytic activity in epoxidation. Catal. Sci. Technol. 2014, 4, 997–1004. [Google Scholar] [CrossRef]
- Long, Z. Y.; Zhou, Y.; Chen, G. J.; Ge, W.L.; Wang, J. C3N4-H5PMo10V2O40: A dual-catalysis system for reductant-free aerobic oxidation of benzene to phenol. Sci. Rep. 2014, 4, 3651. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; Liu, J.; Jiang, P.; Wang, J. POSS-derived mesostructured amphiphilic polyoxometalate-based ionic hybrids as highly efficient epoxidation catalysts. ACS Sustain. Chem. Eng. 2015, 3, 170–176. [Google Scholar] [CrossRef]
- Leng, Y.; Zhao, J.; Jiang, P.; Wang, J. Amphiphilic porous polyhedral oligomeric silsesquioxanes (POSS) incorporated polyoxometalate-paired polymeric hybrids: Interfacial catalysts for epoxidation reactions. RSC Adv. 2015, 5, 17709–17715. [Google Scholar] [CrossRef]
- Dallinger, D.; Kappe, C.O. Microwave-assisted synthesis in water as solvent. Chem. Rev. 2007, 107, 2563–2591. [Google Scholar] [CrossRef] [PubMed]
- Kappe, C.O. Microwave dielectric heating in synthetic organic chemistry. Chem. Soc. Rev. 2008, 37, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- Polshettiwar, V.; Varma, R.S. Microwave-Assisted Organic Synthesis and Transformations using Benign Reaction Media. Acc. Chem. Res. 2008, 41, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Fu, R.; Chai, W.; Zheng, H.; Sun, L.; Lu, Q.; Yuan, R. An eco-benign and highly efficient procedure for N-acylation catalyzed by heteropolyanion-based ionic liquids using carboxylic acid under solvent-free conditions. Tetrahedron 2014, 70, 2237–2245. [Google Scholar] [CrossRef]
- Fu, R.; Yang, Y.; Chen, Z.; Lai, W.; Ma, Y.; Wang, Q.; Yuan, R. Microwave-assisted heteropolyanion-based ionic liquids catalyzed transamidation of non-activated carboxamides with amines under solvent-free conditions. Tetrahedron 2014, 70, 9492–9499. [Google Scholar] [CrossRef]
- Wang, Q.; Feng, J.; Chai, W.; Geng, H.; Xu, M.; Wang, K.; Xu, C.; Fu, R.; Yuan, R. Bu4NI-catalyzed construction of C–O bonds by oxidative coupling of alcohols with ethers. Tetrahedron Lett. 2014, 55, 4785–4789. [Google Scholar] [CrossRef]
- Wang, Q.; Zheng, H.; Chai, W.; Chen, D.; Zeng, X.; Fu, R.; Yuan, R. Copper catalyzed C–O bond formation via oxidative cross-coupling reaction of aldehydes and ethers. Org. Biomol. Chem. 2014, 12, 6549–6553. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Geng, H.; Chai, W.; Zeng, X.; Xu, M.; Zhu, C.; Fu, R.; Yuan, R. Copper-catalyzed formation of C–O bonds by oxidative coupling of benzylic alcohols with ethers. Eur. J. Org. Chem. 2014, 6850–6853. [Google Scholar] [CrossRef]
- Fu, R.; Yang, Y.; Ma, Y.; Yang, F.; Li, J.; Chai, W.; Wang, Q.; Yuan, R. Microwave-promoted direct amidation of unactivated esters catalyzed by heteropolyanion-based ionic liquids under solvent-free conditions. Tetrahedron Lett. 2015, 56, 4527–4531. [Google Scholar] [CrossRef]
- Fu, R.; Yang, Y.; Lai, W.; Ma, Y.; Chen, Z.; Zhou, J.; Chai, W.; Wang, Q.; Yuan, R. Efficient and green microwave-assisted multicomponent Biginelli reaction for the synthesis of dihydropyrimidinones catalyzed by heteropolyanion-based ionic liquids under solvent-free conditions. Synth. Commun. 2015, 45, 477–487. [Google Scholar] [CrossRef]
- Fu, R.; Yang, Y.; Zhang, J.; Shao, J.; Xia, X.; Ma, Y.; Yuan, R. Direct oxidative amidation of aldehydes with amines catalyzed by heteropolyanion-based ionic liquids under solvent-free conditions via a dual-catalysis process. Org. Biomol. Chem. 2016, 14, 1784–1793. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Yang, Y.; Jin, W.; Gu, H.; Zeng, X.; Chai, W.; Ma, Y.; Wang, Q.; Yi, J.; Yuan, R. Microwave-assisted heteropolyanion-based ionic liquid promoted sustainable protocol to N-heteroaryl amides via N-directing dual catalyzed oxidative amidation of aldehydes. RSC Adv. 2016, 6, 107699–107707. [Google Scholar] [CrossRef]
- Fu, R.; Yang, Y.; Feng, W.; Ge, Q.; Feng, Y.; Zeng, X.; Chai, W.; Yi, J.; Yuan, R. An efficient, eco-friendly and sustainable tandem oxidative amidation of alcohols with amines catalyzed by heteropolyanion-based ionic liquids via a bifunctional catalysis process. Tetrahedron 2016, 72, 8319–8326. [Google Scholar] [CrossRef]
- Folkers, K.; Harwood, H.J.; Johnson, T.B. Researches on pyrimidines. Cxxx. Synthesis of 2-keto-1,2,3,4-tetrahydropyrimidines. J. Am. Chem. Soc. 1932, 54, 3751–3758. [Google Scholar] [CrossRef]
- Ling, R.; Yoshida, M.; Mariano, P.S. Exploratory investigations probing a preparatively versatile, pyridinium salt photoelectrocyclization-solvolytic aziridine ring opening sequence. J. Org. Chem. 1996, 61, 4439–4449. [Google Scholar] [CrossRef] [PubMed]
- Kappe, C.O. A reexamination of the mechanism of the Biginelli dihydropyrimidine synthesis. Support for an N-acyliminium ion intermediate. J. Org. Chem. 1997, 62, 7201–7204. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: All samples are available from the authors. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).