Antibiofilm Activity and Mechanism of Action of the Disinfectant Chloramine T on Candida spp., and Its Toxicity against Human Cells
Abstract
:1. Introduction
2. Results
2.1. Determination of the MIC and MFC Values
2.2. Effects on C. Albicans Growth Kinetics
2.3. Mechanism of Action of CAT
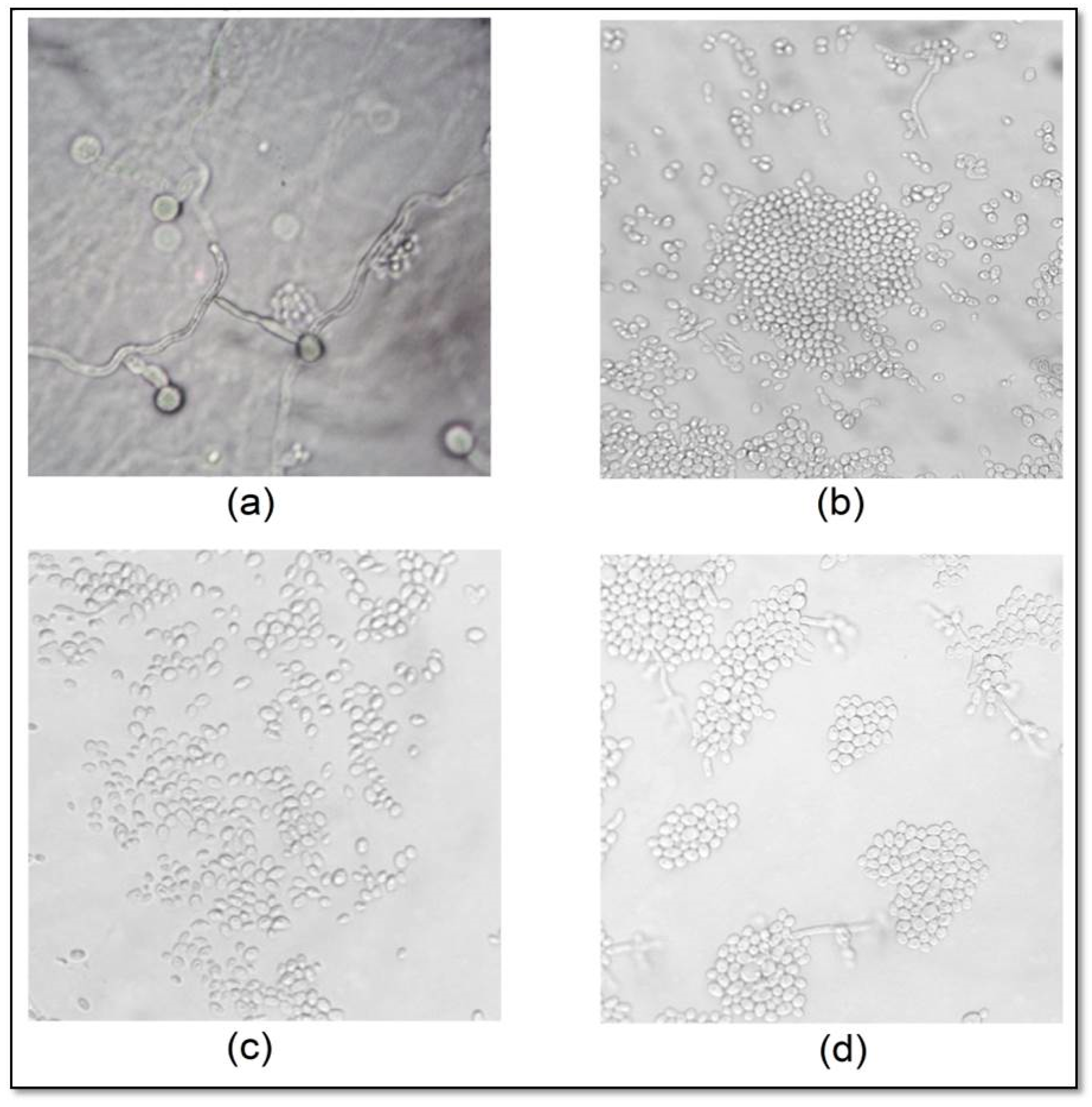
2.4. Effect of CAT on C. albicans Micromorphology
2.5. Effects of CAT on the Adherence, Formation and Reduction of C. Albicans Mature Biofilms
2.6. Cytotoxicity of CAT on Human Erythrocytes
3. Discussion
4. Materials and Methods
4.1. Microorganisms
4.2. Determination of the Minimum Inhibitory and Fungicidal Concentration (MIC)
4.3. Effects of CAT on Candida spp. Growth Kinetics
4.4. Mechanism(s) of Action of CAT
4.4.1. Action on Cell Wall Biosynthesis
4.4.2. Action on Cell Membrane Permeability
4.5. Effects of CAT on Fungal Micromorphology
4.6. Effects of CAT on C. Albicans Biofilms
4.6.1. Effects on Early Biofilm Adherence
4.6.2. Effects on Mature Biofilm Formation
4.6.3. Reduction of Preformed Mature Biofilm
4.7. Cytotoxic Effects of CAT on Human Erythrocytes
4.8. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gendreau, L.; Loewy, Z.G. Epidemiology and etiology of denture stomatitis. J. Prosthodont. 2011, 20, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Marinoski, J.; Bokor-Bratić, M.; Čanković, M. Is denture stomatitis always related with candida infection? A case control study. Med. Glas. (Zenica) 2014, 11, 379–384. [Google Scholar] [PubMed]
- Martori, E.; Ayuso-Montero, R.; Martinez-Gomis, J.; Viñas, M.; Peraire, M. Risk factors for denture-related oral mucosal lesions in a geriatric population. J. Prosthet. Dent. 2014, 111, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Navabi, N.; Gholamhoseinian, A.; Baghaei, B.; Hashemipour, M.A. Risk factors associated with denture stomatitis in healthy subjects attending a dental school in southeast Iran. Sultan Qaboos Univ. Med. J. 2013, 13, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Kabawat, M.; de Souza, R.F.; Badaró, M.M.; de Koninck, L.; Barbeau, J.; Rompré, P.; Emami, E. Phase 1 clinical trial on the effect of palatal brushing on denture stomatitis. Int. J. Prosthodont. 2014, 27, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Kilic, K.; Koc, A.N.; Tekinsen, F.F.; Yildiz, P.; Kilic, D.; Zararsiz, G.; Kilic, E. Assessment of Candida species colonization and denture-related stomatitis in bar- and locator- retained overdentures. J. Oral. Implantol. 2014, 40, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Felton, D.; Cooper, L.; Duqum, I.; Minsley, G.; Guckes, A.; Haug, S.; Meredith, P.; Solie, C.; Avery, D.; Chandler, N.D. Evidence-based guidelines for the care and maintenance of complete dentures: A publication of the American College of Prosthodontists. J. Am. Dent. Assoc. 2011, 20, S1–S20. [Google Scholar] [CrossRef]
- Gornitsky, M.; Paradisl, I.; Landaverde, G.; Malo, A.M.; Velly, A.M. A clinical and microbiological evaluation of denture cleansers for geriatric patients in long-term care institutions. J. Can. Dent. Assoc. 2002, 68, 39–45. [Google Scholar] [PubMed]
- Yildirim-Bicer, A.Z.; Peker, I.; Akca, G.; Celik, I. In vitro antifungal evaluation of seven different disinfectants on acrylic resins. Biomed. Res. Int. 2014, 2014, e519098. [Google Scholar] [CrossRef] [PubMed]
- Lima, E.M.; Moura, J.S.; Del Bel Cury, A.A.; Garcia, R.C.; Cury, J.A. Effect of enzymatic and NaOCl treatments on acrylic roughness and on biofilm accumulation. J. Oral. Rehabil. 2006, 33, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Amin, F.; Iqbal, S.; Azizuddin, S.; Afrid, F.I. Effect of denture cleansers on the color stability of heat cure acrylic resin. J. Coll. Phys. Surg. Pak. 2014, 24, 787–790. [Google Scholar]
- Neppelenbroek, K.H.; Kurokawa, L.A.; Procópio, A.L.F.; Pegoraro, T.A.; Hotta, J.; Mello Lima, J.F.; Urban, V.M. Hardness and surface roughness of enamel and base layers of resin denture teeth after long-term repeated chemical disinfection. J. Contemp. Dent. Pract. 2015, 16, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Arnitz, R.; Nagl, M.; Gottardi, W. Microbicidal activity of monochloramine and chloramine T compared. J. Hosp. Infect. 2009, 73, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Gottardi, W.; Debabov, D.; Nagl, M. N-chloramines, a promising class of well-tolerated topical anti-infectives. Antimicrob. Agents Chemother. 2013, 57, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Austin, J.H.; Taylor, H.D. Behavior of hypochlorite and of chloramine-T solutions in contact with necrotic and normal tissues in vivo. J. Exp. Med. 1918, 27, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Dakin, H.D.; Cohen, J.B.; Kenyon, J. Studies in antiseptics (II): On chloramine: Its preparation, properties and use. Br. Med. J. 1916, 1, 160–162. [Google Scholar] [CrossRef] [PubMed]
- Sweet, J.B.; Macynski, A.A. Effect of antimicrobial mouth rinses on the incidence of localized alveolitis and infection following mandibular third molar oral surgery. Oral Surg. Oral Med. Oral Pathol. 1985, 59, 24–26. [Google Scholar] [CrossRef]
- Miyagi, S.P.H.; Mello, I.; Bussadori, S.K.; Márcia, M.M. Response of cultured pulpal fibroblasts to Papacárie® gel. Rev. Odontol. Univ. Cid. São Paulo 2006, 18, 245–249. [Google Scholar]
- Andrade, I.M.; Silva-Lovato, C.H.; Souza, R.F.; Pisani, M.X.; Andrade, K.M.; Paranhos, H.F. Trial of experimental toothpastes regarding quality for cleaning dentures. Int. J. Prosthodont. 2012, 25, 157–159. [Google Scholar] [PubMed]
- Panzeri, H.; Lara, E.H.; Paranhos, H.F.; Lovato da Silva, C.H.; Souza, R.F.; Souza Gugelmin, M.C.; Tirapelli, C.; Cruz, P.C.; Andrade, I.M. In vitro and clinical evaluation of specific dentifrices for complete denture hygiene. Gerodontology 2009, 26, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Tortorano, A.M.; Viviani, M.A.; Biraghi, E.; Rigoni, A.L.; Prigitano, A.; Grillot, R. In vitro testing of fungicidal activity of biocides against Aspergillus fumigatus. J. Med. Microbiol. 2005, 54, 955–957. [Google Scholar] [CrossRef] [PubMed]
- Fuursted, K.; Hjort, A.; Knudsen, L. Evaluation of bactericidal activity and lag of regrowth (postantibiotic effect) of five antiseptics on nine bacterial pathogens. J. Antimicrob. Chemother. 1997, 40, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Gottardi, W.; Nagl, M. Chlorine covers on living bacteria: The initial step in antimicrobial action of active chlorine compounds. J. Antimicrob. Chemother. 2005, 55, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Ernst, J.F. Transcription factors in Candida albicans—environmental control of morphogenesis. Microbiology 2000, 146, 1763–1774. [Google Scholar] [CrossRef] [PubMed]
- Gow, N.A.; Brown, A.J.; Odds, F.C. Fungal morphogenesis and host invasion. Curr. Opin. Microbiol. 2002, 5, 366–371. [Google Scholar] [CrossRef]
- Jackson, S.; Coulthwaite, L.; Loewy, Z.; Scallan, A.; Verran, J. Biofilm development by blastospores and hyphae of Candida albicans on abraded denture acrylic resin surfaces. J. Prosthet. Dent. 2014, 112, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Chandra, J.; Kuhn, D.M.; Mukherjee, P.K.; Hoyer, L.L.; McCormick, T.; Ghannoum, M.A. Biofilm formation by the fungal pathogen Candida albicans: Development, architecture, and drug resistance. J. Bacteriol. 2001, 183, 5385–5394. [Google Scholar] [CrossRef] [PubMed]
- Nagl, M.; Nguyen, V.A.; Gottardi, W.; Ulmer, H.; Höpfl, R. Tolerability and efficacy of N-chlorotaurine in comparison with chloramine T for the treatment of chronic leg ulcers with a purulent coating: A randomized phase II study. Br. J. Dermatol. 2003, 149, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Protocol M27-A2. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 2nd ed.; Pennsylvania NCCLS: Wayne, PA, USA, 2002; p. 51. [Google Scholar]
- Siddiqui, Z.N.; Farooq, F.; Musthafa, T.N.M.; Ahmad, A.; Khan, A.U. Synthesis, characterization and antimicrobial evaluation of novel halopyrazole derivatives. J. Saudi Chem. Soc. 2013, 17, 237–243. [Google Scholar] [CrossRef]
- Castro, R.D.; Lima, E.O.; Freires, I.A.; Alves, L.A. Combined effect of Cinnamomum zeylanicum blume essential oil and nystatin on Candida albicans growth and micromorphology. Rev. Ciênc. Méd. Biol. 2013, 12, 149–156. [Google Scholar]
- Leite, M.C.A.; Bezerra, A.P.B.; Sousa, J.P.; Guerra, F.Q.S.; Lima, E.O. Evaluation of antifungal activity and action mechanism of citral against Candida albicans. Evid. Based Complement. Alternat. Med. 2004, 2004, e378280. [Google Scholar] [CrossRef]
- Hao, B.; Cheng, S.; Clancy, C.J.; Nguyen, M.H. Caspofungin kills Candida albicans by causing both cellular apoptosis and necrosis. Antimicrob. Agents Chemother. 2013, 57, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Letscher-Bru, V.; Herbrecht, R. Caspofungin: The first representative of a new antifungal class. J. Antimicrob. Chemother. 2003, 51, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Freires, I.A.; Murata, R.M.; Furletti, V.F.; Sartoratto, A.; Alencar, S.M.; Figueira, G.M.; Rodrigues, J.A.O.; Duarte, M.C.T.; Rosalen, P.L. Coriandrum sativum L. (coriander) Essential oil: Antifungal activity and mode of action on Candida spp., and molecular targets affected in human whole-genome expression. PLoS ONE 2014, 9, e99086. [Google Scholar] [CrossRef] [PubMed]
- Frost, D.J.; Brandt, K.D.; Cugier, D.; Goldman, R. A whole-cell Candida albicans assay for the detection of inhibitors towards fungal cell wall synthesis and assembly. J. Antibiot. 1995, 48, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Ellepola, A.N.; Samaranayake, L.P. Impact of brief and sequential exposure to nystatin on the germ tube formation and cell surface hydrophobicity of oral Candida albicans isolates from human immunodeficiency virus-infected patients. Med. Princ. Pract. 2014, 23, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Kinsky, S.C. The effect of polyene antibiotics on permeability in Neurospora chassa. Biochem. Biophys. Res. Commun. 1961, 4, 353–357. [Google Scholar] [CrossRef]
- Dalmau, L.M. Remarques sur la technique mycologique. Ann. Parasitol. Hum. Comp. 1929, 7, 536–545. [Google Scholar]
- Furletti, V.F.; Teixeira, I.P.; Obando-Pereda, G.; Mardegan, R.C.; Sartoratto, A.; Figueira, G.M.; Duarte, R.M.; Rehder, V.L.; Duarte, M.C.; Höfling, J.F. Action of Coriandrum sativum L. essential oil upon oral Candida albicans biofilm formation. Evid. Based Complement. Alternat. Med. 2011, 2011, e985832. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.B.; Pharma, S.P. Use of chitosan as a biomaterial: Studies on its safety and hemostatic potential. J. Biomed. Mater. Res. 1997, 34, 21–28. [Google Scholar] [CrossRef]
Sample Availability: Samples of the of chloramine T trihydrate (INLAB-CAS 7080-50-4, São Paulo, Brazil) are available from the authors. |




| Strain | CAT | NaOCl | Nystatin | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MIC | MFC | MFC/MIC Ratio | MIC | MFC | MFC/MIC Ratio | MIC | MFC | MFC/MIC Ratio | |
| Candida albicans ATCC 60193 | 2.77 | 5.54 | 2 (Fungicidal) | 8.39 | 8.39 | 1 (Fungicidal) | 0.0004 | 0.0008 | 2 (Fungicidal) |
| Candida albicans CBS 562 | 0.69 | 2.77 | 4 (Fungistatic) | 4.19 | 8.39 | 2 (Fungicidal) | 0.0004 | 0.0008 | 2 (Fungicidal) |
| Candida tropicalis ATCC 750 | 2.77 | 2.77 | 1 (Fungicidal) | 8.39 | 8.39 | 1 (Fungicidal) | 0.0004 | 0.0004 | 1 (Fungicidal) |
| Candida tropicalis CBS 94 | 5.54 | 5.54 | 1 (Fungicidal) | 16.79 | 16.79 | 1 (Fungicidal) | 0.0004 | 0.0004 | 1 (Fungicidal) |
| Candida krusei ATCC 3413 | 1.38 | 1.38 | 1 (Fungicidal) | 4.19 | 4.19 | 1 (Fungicidal) | 0.001 | 0.001 | 1 (Fungicidal) |
| Candida krusei CBS 73 | 1.38 | 1.38 | 1 (Fungicidal) | 4.19 | 4.19 | 1 (Fungicidal) | 0.0008 | 0.0008 | 1 (Fungicidal) |
| Candida glabrata IZ 07 | 5.54 | 11.09 | 2 (Fungicidal) | 16.79 | 16.79 | 1 (Fungicidal) | 0.0004 | 0.0008 | 2 (Fungicidal) |
| Strain | MIC (mmol/L) | |||||
|---|---|---|---|---|---|---|
| CAT | NaOCl | Caspofungin | ||||
| Without Sorbitol | With Sorbitol | Without Sorbitol | With Sorbitol | Without Sorbitol | With Sorbitol | |
| Candida albicans ATCC 60193 | 2.77 | 22.18 | 8.39 | 33.58 | <0.0003 | >0.0045 |
| Candida albicans CBS 562 | 0.69 | 11.09 | 4.19 | 33.58 | <0.0003 | 0.0045 |
| CAT | NaOCl | Nystatin | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Absence of Ergosterol | Presence of Ergosterol | Absence of Ergosterol | Presence of Ergosterol | Absence of Ergosterol | Presence of Ergosterol | |||||||
| 100 µg/mL | 200 µg/mL | 400 µg/mL | 100 µg/mL | 200 µg/mL | 400 µg/mL | 100 µg/mL | 200 µg/mL | 400 µg/mL | ||||
| Candida albicans ATCC 60193 | 2.77 | 5.54 | 5.54 | 5.54 | 8.39 | 16.79 | 16.79 | 16.79 | 0.0004 | 0.001 | 0.003 | 0.003 |
| Candida albicans CBS 562 | 0.69 | 5.54 | 5.54 | 5.54 | 4.19 | 16.79 | 33.58 | 33.58 | 0.0004 | 0.001 | 0.001 | 0.001 |
| Concentration | Inhibition of Initial Adherence | Inhibition Of Mature Biofilm Formation | ||||
|---|---|---|---|---|---|---|
| Group 1 (Exposure time: 2 h) | Group 2 (Exposure time: 1 min) | Group 3 (Exposure time: 8 h) | ||||
| CAT | NaOCl | CAT | NaOCl | CAT | NaOCl | |
| MIC | 25% < %I ≤ 50% Aa | 25% < %I ≤ 50% Aa | 25% < %I ≤ 50% Aa | 25% < %I ≤ 50% Aa | %I ≤ 25% Aa | 25% < %I ≤ 50% Aa |
| 2 × MIC | %I ≤ 25% Aa | 50% < %I ≤ 75% Ba | 25% < %I ≤ 50% Aa | 25% < %I ≤ 50% Aa | 25% < %I ≤ 50% Aa | 25% < %I ≤ 50% Aa |
| 4 × MIC | 25% < %I ≤ 50% Aa | 25% < %I ≤ 50% Aa | 25% < %I ≤ 50% Aa | 25% < %I ≤ 50% Aa | 25% < %I ≤ 50% Aa | 25% < %I ≤ 50% Aa |
| Concentration | Group 4 (Exposure time: 3 × 1 min) | Group 5 (Exposure Time: 6 × 1 min) | Group 6 (Exposure time: 8 h) | Group 7 (Exposure time: 2 × 8 h) | ||||
|---|---|---|---|---|---|---|---|---|
| CAT | NaOCl | CAT | NaOCl | CAT | NaOCl | CAT | NaOCl | |
| MIC | 50% < %I ≤ 75% Aa | %I ≤ 25% Aa | %I ≤ 25% Aa | %I ≤ 25% Aa | 50% < %I ≤ 75% Aa | 50% < %I ≤ 75% Aa | 25% < %I ≤ 50% Aa | 25% < %I ≤ 50% Aa |
| 2 × MIC | 50% < %I ≤ 75% Aa | %I ≤ 25% Ba | %I ≤ 25% Aa | %I ≤ 25% Aa | 50% < %I ≤ 75% Aa | 25% < %I ≤ 50% Aa | 25% < %I ≤ 50% Aa | 25% < %I ≤ 50% Aa |
| 4 × MIC | 50%< %I ≤ 75% Aa | %I ≤ 50% Aa | 25% <%I ≤ 50% Aa | %I ≤ 25% Ba | 50% < %I ≤ 75% Aa | 25% < %I ≤ 50% Aa | 25% < %I ≤ 50% Aa | 25% < %I ≤ 50% Aa |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, G.L.S.; Rosalen, P.L.; Peixoto, L.R.; Pérez, A.L.A.d.L.; Carlo, F.G.d.C.; Castellano, L.R.C.; Lima, J.M.d.; Freires, I.A.; Lima, E.D.O.; Castro, R.D.d. Antibiofilm Activity and Mechanism of Action of the Disinfectant Chloramine T on Candida spp., and Its Toxicity against Human Cells. Molecules 2017, 22, 1527. https://doi.org/10.3390/molecules22091527
Ferreira GLS, Rosalen PL, Peixoto LR, Pérez ALAdL, Carlo FGdC, Castellano LRC, Lima JMd, Freires IA, Lima EDO, Castro RDd. Antibiofilm Activity and Mechanism of Action of the Disinfectant Chloramine T on Candida spp., and Its Toxicity against Human Cells. Molecules. 2017; 22(9):1527. https://doi.org/10.3390/molecules22091527
Chicago/Turabian StyleFerreira, Gabriela Lacet Silva, Pedro Luiz Rosalen, Larissa Rangel Peixoto, Ana Luiza Alves de Lima Pérez, Fabíola Galbiatti de Carvalho Carlo, Lúcio Roberto Cançado Castellano, Jefferson Muniz de Lima, Irlan Almeida Freires, Edeltrudes De Oliveira Lima, and Ricardo Dias de Castro. 2017. "Antibiofilm Activity and Mechanism of Action of the Disinfectant Chloramine T on Candida spp., and Its Toxicity against Human Cells" Molecules 22, no. 9: 1527. https://doi.org/10.3390/molecules22091527
APA StyleFerreira, G. L. S., Rosalen, P. L., Peixoto, L. R., Pérez, A. L. A. d. L., Carlo, F. G. d. C., Castellano, L. R. C., Lima, J. M. d., Freires, I. A., Lima, E. D. O., & Castro, R. D. d. (2017). Antibiofilm Activity and Mechanism of Action of the Disinfectant Chloramine T on Candida spp., and Its Toxicity against Human Cells. Molecules, 22(9), 1527. https://doi.org/10.3390/molecules22091527




