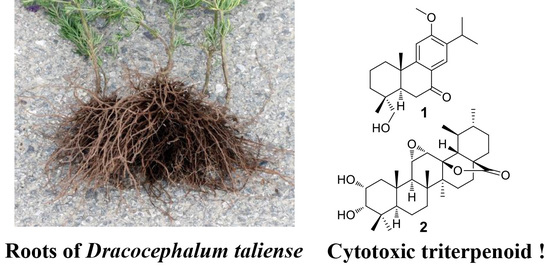
Cytotoxic Terpenoids from the Roots of Dracocephalum taliense
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Elucidation of Compounds
2.2. Bioactivities
3. Experimental
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Spectroscopic Data
3.5. Cytotoxic Assay
3.6. Anti-Inflammatory Assay
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Christianson, D.W. Structural and chemical biology of terpenoid cyclases. Chem. Rev. 2017, 117, 11570–11648. [Google Scholar] [CrossRef] [PubMed]
- Gershenzon, J.; Dudareva, N. The function of terpene natural products in the natural world. Nat. Chem. Biol. 2007, 3, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, W.G.; Sun, H.D.; Pu, J.X. Diterpenoids from Isodon species: An update. Nat. Prod. Rep. 2017, 34, 1090–1140. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.P.; Li, C.; Yang, H.Z.; Lu, Y.Q.; Yu, H.Y.; Gao, H.M.; Wang, Z.M. Three new cytotoxic ent-kaurane diterpenes from Isodon excisoides. Molecules 2015, 20, 17544–17556. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Jiang, H.Y.; Tang, J.W.; Li, X.R.; Du, X.; Li, Y.; Sun, H.D.; Pu, J.X. ent-Abietanoids isolated from Isodon serra. Molecules 2017, 22, 309. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.B.; Ni, Z.Y.; Shi, Q.W.; Dong, M.; Kiyota, H.; Gu, Y.C.; Cong, B. Constituents from Salvia species and their biological activities. Chem. Rev. 2012, 112, 5967–6026. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.H.; Qian, L.; Niu, X.M.; Xie, M.J.; Xu, Z.; Schneider, B.; Gershenzon, J.; Li, S.H. Glandular trichomes of Leucosceptrum canum harbor defensive sesterterpenoids. Angew. Chem. Int. Ed. 2010, 122, 4471–4475. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.H.; Hua, J.; Li, C.H.; Jing, S.X.; Liu, Y.; Li, X.N.; Zhao, X.; Li, S.H. New antifeedant C20 terpenoids from Leucosceptrum canum. Org. Lett. 2012, 14, 5768–5771. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.H.; Hugelshofer, C.L.; Hua, J.; Jing, S.X.; Li, C.H.; Liu, Y.; Li, X.N.; Zhao, X.; Magauer, T.; Li, S.H. Unraveling the metabolic pathway in Leucosceptrum canum by isolation of new defensive leucosceptroid degradation products and biomimetic model synthesis. Org. Lett. 2014, 16, 6416–6419. [Google Scholar] [CrossRef] [PubMed]
- Li, C.H.; Jing, S.X.; Luo, S.H.; Shi, W.; Hua, J.; Liu, Y.; Li, X.N.; Schneider, B.; Gershenzon, J.; Li, S.H. Peltate glandular trichomes of Colquhounia coccinea var. mollis harbor a new class of defensive sesterterpenoids. Org. Lett. 2013, 15, 1694–1697. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Jin, H.Z.; Qin, J.J.; Fu, J.J.; Hu, X.J.; Liu, J.H.; Yan, L.; Chen, M.; Zhang, W.D. Chemical constituents of plants from the genus Dracocephalum. Chem. Biodivers. 2010, 7, 1911–1929. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.M.; Guo, H.F.; Yu, W.T.; Wang, S.Q.; Ji, M.; Lou, H.X. Stereochemistry of flavonoidal alkaloids from Dracocephalum rupestre. Phytochemistry 2008, 69, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Selenge, E.; Murata, T.; Kobayashi, K.; Batkhuu, J.; Yoshizaki, F. Flavone tetraglycosides and benzyl alcohol glycosides from the Mongolian medicinal plant Dracocephalum ruyschiana. J. Nat. Prod. 2013, 76, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Saeidnia, S.; Gohari, A.R.; Ito, M.; Kiuchi, F.; Honda, G. Bioactive constituents from Dracocephalum subcapitatum (O. Kuntze) Lipsky. Z. Naturforsch. C 2005, 60, 22–24. [Google Scholar] [CrossRef]
- Jahaniani, F.; Ebrahimi, S.A.; Rahbar-Roshandel, N.; Mahmoudian, M. Xanthomicrol is the main cytotoxic component of Dracocephalum kotschyii and a potential anti-cancer agent. Phytochemistry 2005, 66, 1581–1592. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, N.; Kiuchi, F.; Ito, M.; Honda, G.; Takeda, Y.; Khodzhimatov, O.K.; Ashurmetov, O.A. New icetexane and 20-norabietane diterpenes with trypanocidal activity from Dracocephalum komarovi. J. Nat. Prod. 2003, 66, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Golshani, S.; Karamkhani, F.; Monsefesfehani, H.R.; Abdollahi, M. Antinociceptive effects of the essential oil of Dracocephalum kotschyi in the mouse writhing test. J. Pham. Sci. 2004, 7, 76–79. [Google Scholar]
- Saeidnia, S.; Gohari, A.R.; Uchiyama, N.; Ito, M.; Honda, G.; Kiuchi, F. Two new monoterpene glycosides and trypanocidal terpenoids from Dracocephalum kotschyi. Chem. Pharm. Bull. 2004, 52, 1249–1250. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, N.; Ito, M.; Kiuchi, F.; Honda, G.; Takeda, Y.; Khodzhimatov, O.K.; Ashurmetov, O.A. A trypanocidal diterpene with novel skeleton from Dracocephalum komarovi. Tetrahedron Lett. 2004, 45, 531–533. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Li, X.W. Dracocephalum Linnaeus; Science Press: Beijing, China, 1994; Volume 17, pp. 127–133. [Google Scholar]
- Yang, Z.Y. Illustrated Handbook for Medicinal Materials from Nature in Yunnan; Yunnan Science and Technology Press: Yunnan, China, 2007; Volume 4, p. 35. [Google Scholar]
- Frontana, B.; Cárdenas, J.; Rodríguez-Hahn, L. Diterpenoids from Salvia coulteri. Phytochemistry 1994, 36, 739–741. [Google Scholar] [CrossRef]
- Gao, J.J.; Han, G.Q. Cytotoxic abietane diterpenoids from Caryopteris incana. Phytochemistry 1997, 44, 759–761. [Google Scholar]
- Jiao, K.; Li, H.Y.; Zhang, P.; Pi, H.F.; Ruan, H.L.; Wu, J.Z. Three new ursane-type triterpenoids from the aerial parts of Isodon excisoides. J. Asian Nat. Prod. Res. 2013, 15, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Corral, J.M.M. D.; Gordaliza, M.; Salinero, M.A.; Feliciano, A.S. 13C NMR data for abieta-8,11,13-triene diterpenoids. Magn. Reson. Chem. 1994, 32, 774–781. [Google Scholar] [CrossRef]
- Fraga, B.M.; Hernández, M.G.; Artega, J.M.; Suárez, S. The microbiological transformation of the diterpenes dehydroabietanol and teideadiol by Mucor plumbeus. Phytochemistry 2003, 63, 663–668. [Google Scholar] [CrossRef]
- Kelecom, A. An abietane diterpene from the labiate Coleus barbatus. Phytochemistry 1984, 23, 1677–1679. [Google Scholar] [CrossRef]
- Rodríguez, B. 1H and 13C NMR spectral assignments of some natural abietane diterpenoids. Magn. Reson. Chem. 2003, 41, 741–746. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, H. Diterpenoids from Rabdosia kunmingensis. Phytochemistry 1989, 28, 3405–3409. [Google Scholar]
- Furukawa, M.; Makino, M.; Ohkoshi, E.; Uchiyama, T.; Fujimoto, Y. Terpenoids and phenethyl glucosides from Hyssopus cuspidatus (Labiatae). Phytochemistry 2011, 72, 2244–2252. [Google Scholar] [CrossRef] [PubMed]
- Ying, B.P.; Kubo, I. Complete 1H and 13C NMR assignments of totarol and its derivatives. Phytochemistry 1991, 30, 1951–1955. [Google Scholar] [CrossRef]
- Pettit, G.R.; Tan, R.; Northen, J.S.; Herald, D.L.; Chapuis, J.C.; Pettit, R.K. Antineoplastic agents. 529. Isolation and structure of nootkastatins 1 and 2 from the Alaskan yellow cedar Chamaecyparis nootkatensis. J. Nat. Prod. 2004, 67, 1476–1482. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, L.; Caputo, R. Sempervirol, a novel type of diterpene phenol. Tetrahedron Lett. 1967, 8, 673–675. [Google Scholar] [CrossRef]
- Song, L.; Shi, J.G.; Lin, S.; Yang, Y.C.; Yao, C.S. Chemical constituents from the linseed meal. Fitoterapia 2014, 97, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Li, S.; Peng, L.; Lin, Z.; Rao, G.; Sun, H. Constituents from Limonia Crenulata. J. Asian Nat. Prod. Res. 2001, 3, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Niu, S.; Ding, Z.; Wang, R.; Dai, Q.; Wei, W.; Luo, R.; Liu, L. Isolation and characterization of aphidicolin derivatives from Tolypocladium inflatum. Molecules 2017, 22, 1168. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Liu, Y.C.; Jing, S.X.; Luo, S.H.; Li, S.H. Macrocyclic diterpenoids from the latex of Euphorbia helioscopia. Nat. Prod. Commun. 2015, 10, 2037–2039. [Google Scholar] [PubMed]
- Selenge, E.; Murata, T.; Tanaka, S.; Sasaki, K.; Batkhuu, J.; Yoshizaki, F. Monoterpene glycosides, phenylpropanoids, and acacetin glycosides from Dracocephalum foetidum. Phytochemistry 2014, 101, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Chirikova, N.K.; Okhlopkova, Z.M.; Zulfugarov, I.S. Chemical composition and antioxidant activity of Tanara Oto (Dracocephalum palmatum Stephan), a medicinal plant used by the North-Yakutian nomads. Molecules 2013, 18, 14105–14121. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–17 are available from the authors. |



| Position | 1 a | 2 b | ||
|---|---|---|---|---|
| δH (ppm), (Hz) | δC (ppm) | δH (ppm), (Hz) | δC (ppm) | |
| 1a | 2.44, m | 38.8, t | 1.91, m | 41.5, t |
| 1b | 1.55, m | 1.48, m | ||
| 2a | 1.79, m | 19.4, t | 4.07, m | 66.0, d |
| 2b | 1.61, m | |||
| 3a | 1.96, m | 36.2, t | 3.46, d (1.9) | 78.7, d |
| 3b | 1.04, m | |||
| 4 | - | 39.2, s | - | 38.2, s |
| 5 | 1.94, m | 51.1, d | 1.20, m | 47.4, d |
| 6a | 2.70, m | 36.5, t | 1.47, m (2H) | 17.3, t |
| 6b | 2.64, m | |||
| 7a | - | 197.2, s | 1.29, m | 31.2, t |
| 7b | 1.14, m | |||
| 8 | - | 124.7, s | - | 41.6, s |
| 9 | - | 157.3, s | 1.70, d (1.5) | 51.0, d |
| 10 | - | 39.0, s | - | 37.7, s |
| 11 | 6.95, s | 106.0, d | 3.14, dd (1.5, 3.7) | 54.4, d |
| 12 | - | 162.3, s | 2.94, d (3.7) | 56.2, d |
| 13 | - | 135.4, s | - | 89.2, s |
| 14 | 7.78, s | 125.5, d | - | 41.2, s |
| 15a | 3.24, m | 27.1, d | 1.71, m | 26.7, t |
| 15b | 1.54, m | |||
| 16a | 1.17, d (3H, 7.0) | 22.7, q | 2.22, m | 22.4, t |
| 16b | 1.31, m | |||
| 17 | 1.19, d (3H, 7.0) | 22.8, q | - | 45.0, s |
| 18a | 3.81, d (10.7) | 64.7, t | 1.92, m | 53.9, d |
| 18b | 3.61, d (10.7) | |||
| 19 | 1.02, s (3H) | 27.2, q | 2.30, m | 32.9, d |
| 20 | 1.27, s (3H) | 23.9, q | 1.87, m | 34.6, d |
| 21a | 1.51, m | 28.2, t | ||
| 21b | 1.05, m | |||
| 22a | 1.68, m | 25.5, t | ||
| 22b | 1.58, m | |||
| 23 | 1.01, s (3H) | 28.2, q | ||
| 24 | 0.87, s (3H) | 21.3, q | ||
| 25 | 1.07, s (3H) | 18.5, q | ||
| 26 | 1.05, s (3H) | 20.3, q | ||
| 27 | 1.15, s (3H) | 16.0, q | ||
| 28 | - | 179.5, s | ||
| 29 | 1.14, d (3H, 6.6) | 18.2, q | ||
| 30 | 0.84, d (3H, 7.2) | 11.1, q | ||
| 12-OMe | 3.94, s (3H) | 56.0, q | ||
| No | Cytotoxicity to Different Cell Lines/IC50 (μM) | Inhibitory Activity on Inflammatory Cytokine (μM) | ||
|---|---|---|---|---|
| NCI-H1975 | HepG2 | MCF-7 | IL-2 | |
| 1 | >80 | >80 | >80 | >40 |
| 2 | 7.17 ± 0.26 | 6.58 ± 0.14 | >80 | >5 |
| PC a | (6.82 ± 0.24) × 10−3 | (34.72 ± 2.31) × 10−3 | (54.35 ± 7.72) × 10−3 | (2.38 ± 0.28) × 10−2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Y.; Hua, J.; Wang, W.; Zhan, Z.; Wang, A.; Luo, S. Cytotoxic Terpenoids from the Roots of Dracocephalum taliense. Molecules 2018, 23, 57. https://doi.org/10.3390/molecules23010057
Deng Y, Hua J, Wang W, Zhan Z, Wang A, Luo S. Cytotoxic Terpenoids from the Roots of Dracocephalum taliense. Molecules. 2018; 23(1):57. https://doi.org/10.3390/molecules23010057
Chicago/Turabian StyleDeng, Yanyan, Juan Hua, Wenjia Wang, Zhonglang Zhan, Anqi Wang, and Shihong Luo. 2018. "Cytotoxic Terpenoids from the Roots of Dracocephalum taliense" Molecules 23, no. 1: 57. https://doi.org/10.3390/molecules23010057
APA StyleDeng, Y., Hua, J., Wang, W., Zhan, Z., Wang, A., & Luo, S. (2018). Cytotoxic Terpenoids from the Roots of Dracocephalum taliense. Molecules, 23(1), 57. https://doi.org/10.3390/molecules23010057