Bioprocessing of Functional Ingredients from Flaxseed
Abstract
:1. Introduction
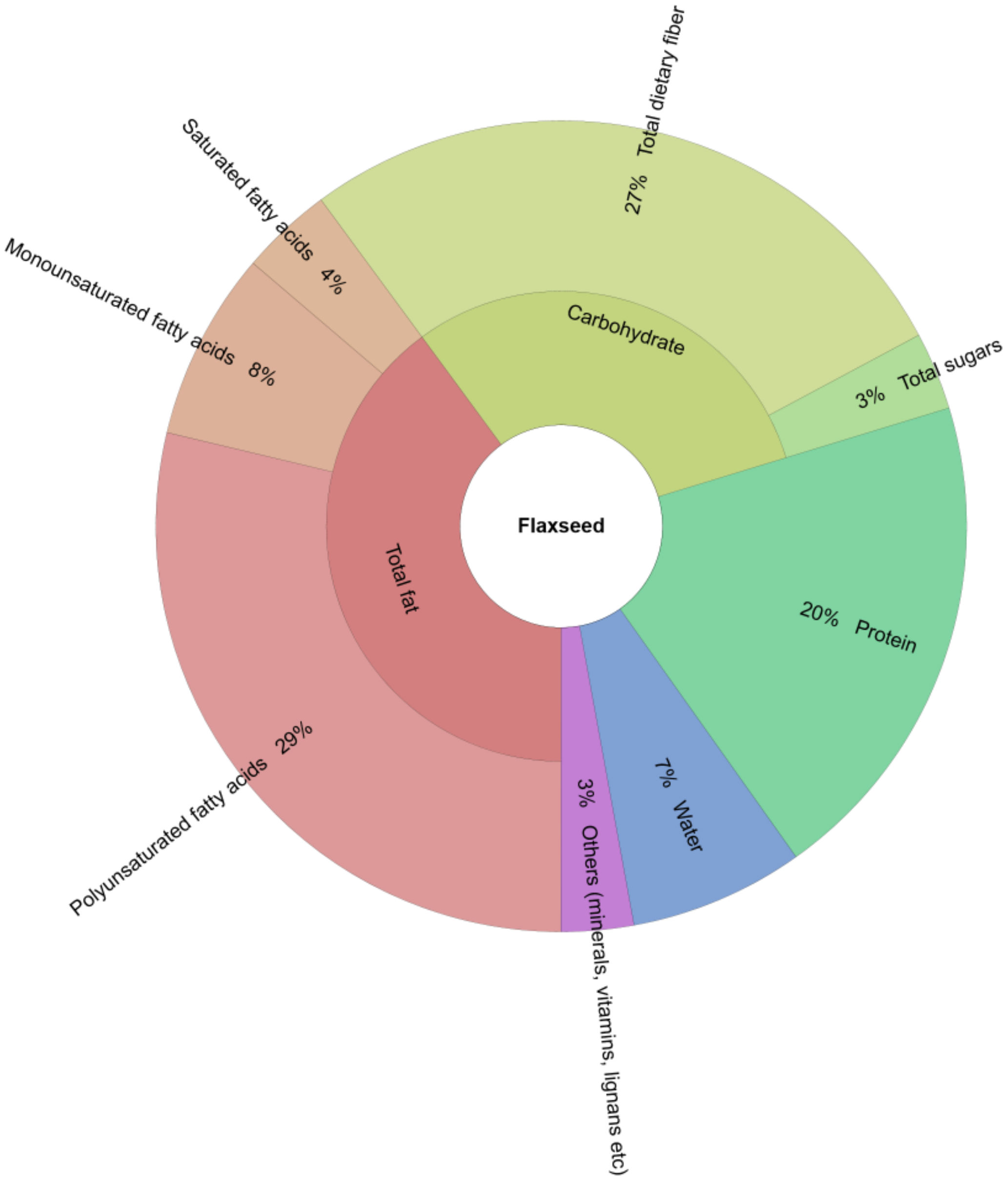
2. Functional Ingredients in Flaxseed and Their Use in Food
2.1. Lipids
2.2. Proteins, Peptides and Amino Acids
2.3. Carbohydrates
2.4. Dietary Fibers and Lignans and Other Components
2.5. Other Components (Vitamins and Minerals)
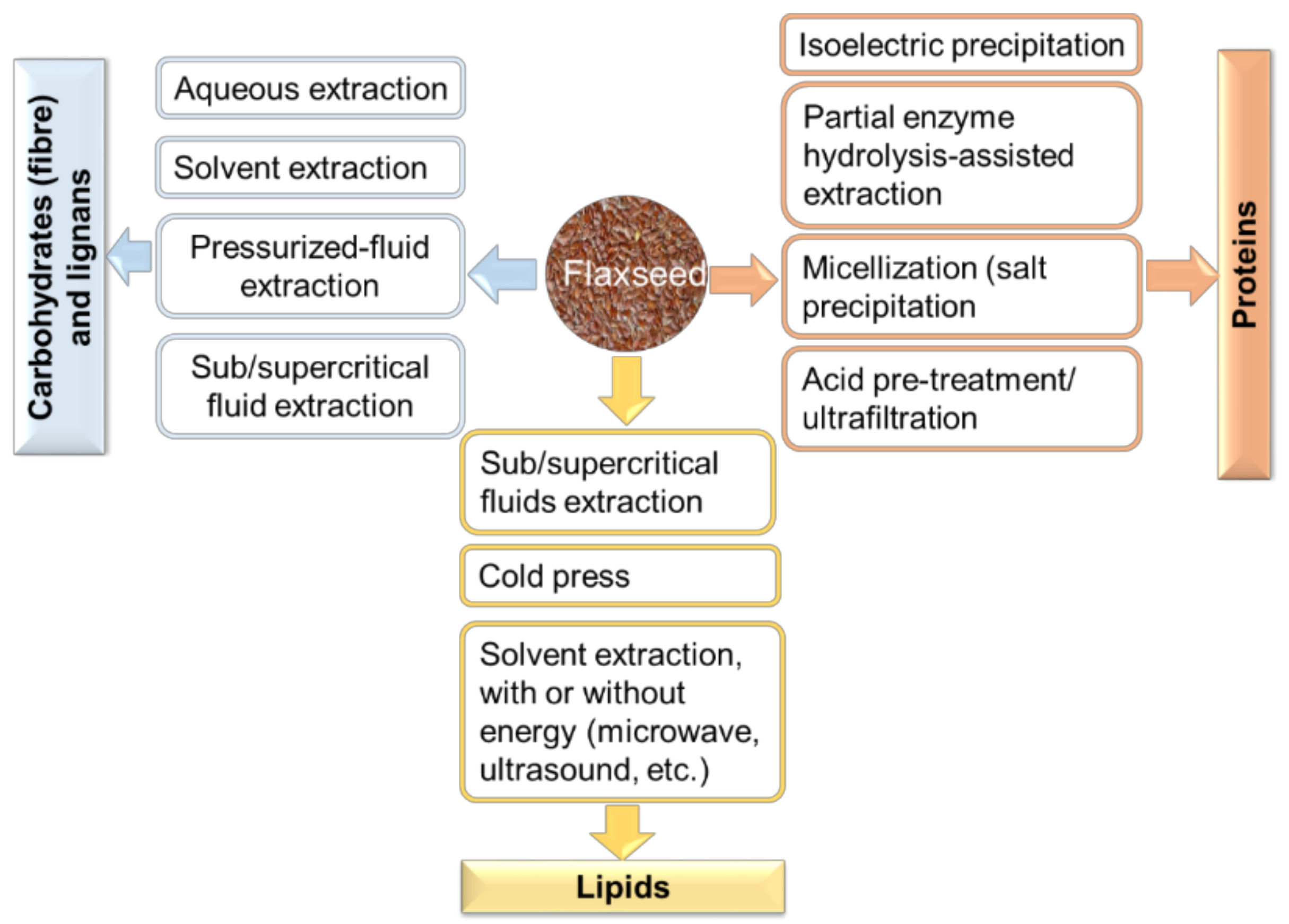
3. Bioprocess Techniques to Obtain Functional Flaxseed Ingredients
3.1. Processes for Extraction of Carbohydrates
3.1.1. Solid-Liquid Extraction
3.1.2. Pressurised Fluids
3.1.3. Sub/Supercritical Fluids
3.1.4. Ionic Liquids and Natural Deep Eutectic Solvents
3.2. Processes for Extraction of Proteins
3.2.1. Isoelectric Precipitation
3.2.2. Partial Enzyme Hydrolysis-Assisted Extraction
3.2.3. Micellization (Precipitation with Salts Such as Ammonium Sulfate)
3.2.4. Acid Pre-Treatment with Ultrafiltration
3.3. Processes for Extraction of Lipids
3.3.1. Cold Pressing
3.3.2. Solvent Extraction
3.3.3. Microwave-and Ultrasound-Assisted Extraction
3.3.4. Sub/Supercritical Fluids Extraction
4. Techniques for the Detoxification of Cyanogenic Glycosides
4.1. Solvent Extraction
4.2. Heat Treatment
4.3. Biological Treatment
5. Conclusion and Future Outlook
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goyal, A.; Sharma, V.; Upadhyay, N.; Gill, S.; Sihag, M. Flax and flaxseed oil: An ancient medicine & modern functional food. J. Food Sci. Technol. 2014, 51, 1633–1653. [Google Scholar] [PubMed]
- US-FDA. High linolenic acid flaxseed oil-grn no. 256. Available online: https://www.accessdata.fda.gov/scripts/fdcc/?set=GRASNotices&id=256 (accessed on 27 August 2018).
- Morita, H.; Shishido, A.; Matsumoto, T.; Itokawa, H.; Takeya, K. Cyclolinopeptides b-e, new cyclic peptides from linum usitatissimum. Tetrahedron 1999, 55, 967–976. [Google Scholar] [CrossRef]
- Rabetafika, H.N.; Van Remoortel, V.; Danthine, S.; Paquot, M.; Blecker, C. Flaxseed proteins: Food uses and health benefits. Int. J. Food Sci. Technol. 2011, 46, 221–228. [Google Scholar] [CrossRef]
- Bekhit, A.E.-D.A.; Shavandi, A.; Jodjaja, T.; Birch, J.; Teh, S.; Mohamed, A.I.A.; Al-Juhaimi, F.Y.; Saeedi, P.; Bekhit, A.A. Flaxseed: Composition, detoxification, utilization, and opportunities. Biocatal. Agric. Biotechnol. 2018, 13, 129–152. [Google Scholar] [CrossRef]
- Shim, Y.Y.; Gui, B.; Arnison, P.G.; Wang, Y.; Reaney, M.J.T. Flaxseed (Linum usitatissimum L.) bioactive compounds and peptide nomenclature: A review. Trends Food Sci. Technol. 2014, 38, 5–20. [Google Scholar] [CrossRef]
- Martinchik, A.N.; Baturin, A.K.; Zubtsov, V.V.; Molofeev, V. [Nutritional value and functional properties of flaxseed]. Vopr. Pitan. 2012, 81, 4–10. [Google Scholar] [PubMed]
- Repin, N.; Kay, B.A.; Cui, S.W.; Wright, A.J.; Duncan, A.M.; Douglas Goff, H. Investigation of mechanisms involved in postprandial glycemia and insulinemia attenuation with dietary fibre consumption. Food Funct. 2017, 8, 2142–2154. [Google Scholar] [CrossRef] [PubMed]
- Fodje, A.M.L.; Chang, P.R.; Leterme, P. In vitro bile acid binding and short-chain fatty acid profile of flax fiber and ethanol co-products. J. Med. Food 2009, 12, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Kremer, J.M. N− 3 fatty acid supplements in rheumatoid arthritis. Am. J. Clin. Nutr. 2000, 71, 349–351. [Google Scholar] [CrossRef] [PubMed]
- Shakir, K.F.; Madhusudhan, B. Hypocholesterolemic and hepatoprotective effects of flaxseed chutney: Evidence from animal studies. Indian J. Clin. Biochem. 2007, 22, 117. [Google Scholar] [CrossRef] [PubMed]
- Marambe, P.; Shand, P.; Wanasundara, J. An in-vitro investigation of selected biological activities of hydrolysed flaxseed (Linum usitatissimum L.) proteins. J. Am. Oil Chem. Soc. 2008, 85, 1155–1164. [Google Scholar] [CrossRef]
- Kaneda, T.; Yoshida, H.; Nakajima, Y.; Toishi, M.; Nugroho, A.E.; Morita, H. Cyclolinopeptides, cyclic peptides from flaxseed with osteoclast differentiation inhibitory activity. Bioorg. Med. Chem. Lett. 2016, 26, 1760–1761. [Google Scholar] [CrossRef] [PubMed]
- Sharav, O.; Shim, Y.Y.; Okinyo-Owiti, D.P.; Sammynaiken, R.; Reaney, M.J.T. Effect of cyclolinopeptides on the oxidative stability of flaxseed oil. J. Agric. Food Chem. 2014, 62, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Leyva, D.R.; Zahradka, P.; Ramjiawan, B.; Guzman, R.; Aliani, M.; Pierce, G.N. The effect of dietary flaxseed on improving symptoms of cardiovascular disease in patients with peripheral artery disease: Rationale and design of the flax-pad randomized controlled trial. Contemporary Clin. trials 2011, 32, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Austria, J.A.; Aliani, M.; Malcolmson, L.J.; Dibrov, E.; Blackwood, D.P.; Maddaford, T.G.; Guzman, R.; Pierce, G.N. Daily choices of functional foods supplemented with milled flaxseed by a patient population over one year. J. Funct. Foods 2016, 26, 772–780. [Google Scholar] [CrossRef]
- Technavio. Global flaxseeds market-key drivers and forecast from technavio. Available online: https://www.businesswire.com/news/home/20170530006108/en/Global-Flaxseeds-Market---Key-Drivers-Forecast (accessed on 27 August 2018).
- Tolkachev, O.N.; Zhuchenko, A.A. Biologically active substances of flax: Medicinal and nutritional properties (a review). Pharm. Chem. J. 2004, 34, 360–367. [Google Scholar] [CrossRef]
- Basiri, S.; Haidary, N.; Shekarforoush, S.S.; Niakousari, M. Flaxseed mucilage: A natural stabilizer in stirred yogurt. Carbohydr. Polym. 2018, 187, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Li, Y.; Mai, Y.; Gao, L.; Ou, S.; Wang, Y.; Liu, L.; Peng, X. Flaxseed gum reduces body weight by regulating gut microbiota. J. Funct. Foods 2018, 47, 136–142. [Google Scholar] [CrossRef]
- Kajla, P.; Sharma, A.; Sood, D.R. Flaxseed—A potential functional food source. J. Food Sci. Technol. 2015, 52, 1857–1871. [Google Scholar] [CrossRef] [PubMed]
- USDA. Report 12220, Seeds, Flaxseed. Available online: https://ndb.nal.usda.gov/ndb/foods/show/12220 (accessed on 27 August 2018).
- Bernacchia, R.; Preti, R.; Vinci, G. Chemical composition and health benefits of flaxseed. Austin J. Nutr. Food Sci. 2014, 2, 1045. [Google Scholar]
- Riediger, N.D.; Othman, R.; Fitz, E.; Pierce, G.N.; Suh, M.; Moghadasian, M.H. Low n-6:n-3 fatty acid ratio, with fish-or flaxseed oil, in a high fat diet improves plasma lipids and beneficially alters tissue fatty acid composition in mice. Eur. J. Nutr. 2008, 47, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Dubois, V.; Breton, S.; Linder, M.; Fanni, J.; Parmentier, M. Fatty acid profiles of 80 vegetable oils with regard to their nutritional potential. Eur. J. Lipid Sci. Technol. 2007, 109, 710–732. [Google Scholar] [CrossRef]
- Singh, K.; Mridula, D.; Rehal, J.; Barnwal, P. Flaxseed: A potential source of food, feed and fiber. Crit. Rev. Food Sci. Nutr. 2011, 51, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.U.; Rickard, S.E.; Orcheson, L.J.; Seidl, M.M. Flaxseed and its lignan and oil components reduce mammary tumor growth at a late stage of carcinogenesis. Carcinogenesis 1996, 17, 1373–1376. [Google Scholar] [CrossRef] [PubMed]
- Oomah, B.D. Flaxseed as a functional food source. J. Sci. Food Agric. 2001, 81, 889–894. [Google Scholar] [CrossRef]
- Marambe, H.K.; Shand, P.J.; Wanasundara, J.P.D. In vitro digestibility of flaxseed (Linum usitatissimum L.) protein: Effect of seed mucilage, oil and thermal processing. Int. J. Food Sci. Tech. 2012, 48, 628–635. [Google Scholar] [CrossRef]
- Xu, Y.; Hall, C.; Wolf-Hall, C. Antifungal activity stability of flaxseed protein extract using response surface methodology. J. Food Sci. 2008, 73, M9–M14. [Google Scholar] [CrossRef] [PubMed]
- Omoni, A.O.; Aluko, R.E. Mechanism of the inhibition of calmodulin-dependent neuronal nitric oxide synthase by flaxseed protein hydrolysates. J. Am. Oil Chem. Soc. 2006, 83, 335–340. [Google Scholar] [CrossRef]
- Bhathena, S.J.; Ali, A.A.; Haudenschild, C.; Latham, P.; Ranich, T.; Mohamed, A.I.; Hansen, C.T.; Velasquez, M.T. Dietary flaxseed meal is more protective than soy protein concentrate against hypertriglyceridemia and steatosis of the liver in an animal model of obesity. J. Am. Coll. Nutr. 2003, 22, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.; McSteen, P.M.; Cebrat, M.; Picur, B.; Siemion, I.Z. Antimalarial activity of cyclolinopeptide a and its analogues. Acta Poloniae Pharm.-Drug Res. 2000, 57, 134–136. [Google Scholar]
- Silva, F.G.D.e.; Hernández-Ledesma, B.; Amigo, L.; Netto, F.M.; Miralles, B. Identification of peptides released from flaxseed (Linum usitatissimum) protein by alcalase® hydrolysis: Antioxidant activity. LWT Food Sci. Technol. 2017, 76, 140–146. [Google Scholar] [CrossRef]
- Hwang, C.-F.; Chen, Y.-A.; Luo, C.; Chiang, W.-D. Antioxidant and antibacterial activities of peptide fractions from flaxseed protein hydrolysed by protease from bacillus altitudinis HK02. Int. J. Food Sci. Technol. 2016, 51, 681–689. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Aluko, R.E. Antioxidant and angiotensin converting enzyme-inhibitory properties of a flaxseed protein-derived high fischer ratio peptide mixture. J. Agric. Food Chem. 2010, 58, 4762–4768. [Google Scholar] [CrossRef] [PubMed]
- Nwachukwu, I.D.; Girgih, A.T.; Malomo, S.A.; Onuh, J.O.; Aluko, R.E. Thermoase-derived flaxseed protein hydrolysates and membrane ultrafiltration peptide fractions have systolic blood pressure-lowering effects in spontaneously hypertensive rats. Int. J. Mol. Sci. 2014, 15, 18131–18147. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.H.; Cacace, J.E.; Mazza, G. Extraction of lignans, proteins and carbohydrates from flaxseed meal with pressurized low polarity water. LWT Food Sci. Technol. 2007, 40, 1637–1647. [Google Scholar] [CrossRef]
- Johnsson, P.; Kamal-Eldin, A.; Lundgren, L.N.; Åman, P. Hplc method for analysis of secoisolariciresinol diglucoside in flaxseeds. J. Agric. Food Chem. 2000, 48, 5216–5219. [Google Scholar] [CrossRef] [PubMed]
- Sok, D.-E.; Cui, H.S.; Kim, M.R. Isolation and boactivities of furfuran type lignan compounds from edible plants. Recent Patents Food Nutr. Agric. 2009, 1, 87–95. [Google Scholar] [CrossRef]
- Korkina, L.; Kostyuk, V.; Luca, C.D.; Pastore, S. Plant phenylpropanoids as emerging anti-inflammatory agents. Mini-Rev. Med. Chem. 2011, 11, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Smeds, A.I.; Eklund, P.C.; Sjöholm, R.E.; Willför, S.M.; Nishibe, S.; Deyama, T.; Holmbom, B.R. Quantification of a broad spectrum of lignans in cereals, oilseeds, and nuts. J. Agric. Food Chem. 2007, 55, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Evans, D.A.; Tangney, C.C.; Bienias, J.L.; Wilson, R.S.; Aggarwal, N.T.; Scherr, P.A. Relation of the tocopherol forms to incident alzheimer disease and to cognitive change. Am. J. Clin. Nutr. 2005, 81, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.H. Flax: A Health and Nutrition Primer; Flax Council of Canada: Winnipeg, MB, Canada, 2007. [Google Scholar]
- Carter, J. Potential of flaxseed and flaxseed oil in baked goods and other products in human nutrition. Cereal Foods World 1993, 38, 753–759. [Google Scholar]
- Elboutachfaiti, R.; Delattre, C.; Quéro, A.; Roulard, R.; Duchêne, J.; Mesnard, F.; Petit, E. Fractionation and structural characterization of six purified rhamnogalacturonans type i from flaxseed mucilage. Food Hydrocoll. 2017, 62, 273–279. [Google Scholar] [CrossRef]
- Farrán, A.; Cai, C.; Sandoval, M.; Xu, Y.; Liu, J.; Hernáiz, M.J.; Linhardt, R.J. Green solvents in carbohydrate chemistry: From raw materials to fine chemicals. Chem. Rev. 2015, 115, 6811–6853. [Google Scholar] [CrossRef] [PubMed]
- Barbary, O.; Al-Sohaimy, S.; El-Saadani, M.; Zeitoun, A. Extraction, composition and physicochemical properties of flaxseed mucilage. J. Adv. Agric. Res. 2009, 14, 605–620. [Google Scholar]
- Udenigwe, C.C.; Lin, Y.-S.; Hou, W.-C.; Aluko, R.E. Kinetics of the inhibition of renin and angiotensin i-converting enzyme by flaxseed protein hydrolysate fractions. J. Funct. Foods 2009, 1, 199–207. [Google Scholar] [CrossRef]
- Tripodo, G.; Ibáñez, E.; Cifuentes, A.; Gilbert-López, B.; Fanali, C. Optimization of pressurized liquid extraction by response surface methodology of goji berry (Lycium barbarum L.) phenolic bioactive compounds. Electrophoresis 2018, 39, 1673–1682. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Todd, R.; Baroutian, S. A techno-economic comparison of subcritical water, supercritical CO2 and organic solvent extraction of bioactives from grape marc. J. Clean. Prod. 2017, 158, 349–358. [Google Scholar] [CrossRef]
- Radošević, K.; Ćurko, N.; Gaurina Srček, V.; Cvjetko Bubalo, M.; Tomašević, M.; Kovačević Ganić, K.; Radojčić Redovniković, I. Natural deep eutectic solvents as beneficial extractants for enhancement of plant extracts bioactivity. LWT Food Sci. Technol. 2016, 73, 45–51. [Google Scholar] [CrossRef]
- Plaza, M.; Turner, C. Pressurized hot water extraction of bioactives. TrAC Trends Anal. Chem. 2015, 71, 39–54. [Google Scholar] [CrossRef]
- Hawthorne, S.B.; Grabanski, C.B.; Martin, E.; Miller, D.J. Comparisons of soxhlet extraction, pressurized liquid extraction, supercritical fluid extraction and subcritical water extraction for environmental solids: Recovery, selectivity and effects on sample matrix. J. Chromatogr. A 2000, 892, 421–433. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef] [PubMed]
- González, C.G.; Mustafa, N.R.; Wilson, E.G.; Verpoorte, R.; Choi, Y.H. Application of natural deep eutectic solvents for the “green” extraction of vanillin from vanilla pods. Flavour Fragr. J. 2018, 33, 91–96. [Google Scholar] [CrossRef]
- Malomo, S.A.; Aluko, R.E. A comparative study of the structural and functional properties of isolated hemp seed (cannabis sativa l.) albumin and globulin fractions. Food Hydrocolloids 2015, 43, 743–752. [Google Scholar] [CrossRef]
- Rommi, K.; Hakala, T.K.; Holopainen, U.; Nordlund, E.; Poutanen, K.; Lantto, R. Effect of enzyme-aided cell wall disintegration on protein extractability from intact and dehulled rapeseed (Brassica rapa L. And Brassica napus L.) press cakes. J. Agric. Food Chem. 2014, 62, 7989–7997. [Google Scholar] [CrossRef] [PubMed]
- Hadnadjev, M.; Dapcevic-Hadnadjev, T.; Pojic, M.; Saric, B.; Misan, A.; Jovanov, P.; Sakac, M. Progress in vegetable proteins isolation techniques: A review. Food Feed Res. 2017, 44, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, C.; Rubilar, M.; Jara, C.; Verdugo, M.; Sineiro, J.; Shene, C. Flaxseed and flaxseed cake as a source of compounds for food industry. J. Soil Sci. Plant Nutr. 2010, 10, 454–463. [Google Scholar] [CrossRef]
- Castel, V.; Andrich, O.; Netto, F.M.; Santiago, L.G.; Carrara, C.R. Comparison between isoelectric precipitation and ultrafiltration processes to obtain amaranth mantegazzianus protein concentrates at pilot plant scale. J. Food Eng. 2012, 112, 288–295. [Google Scholar] [CrossRef]
- Avramenko, N.A.; Chang, C.; Low, N.H.; Nickerson, M.T. Encapsulation of flaxseed oil within native and modified lentil protein-based microcapsules. Food Res. Int. 2016, 81, 17–24. [Google Scholar] [CrossRef]
- Karamać, M.; Kosińska-Cagnazzo, A.; Kulczyk, A. Use of different proteases to obtain flaxseed protein hydrolysates with antioxidant activity. Int. J. Mol. Sci. 2016, 17, 1027. [Google Scholar]
- Bajaj, P.R.; Bhunia, K.; Kleiner, L.; Joyner, H.S.; Smith, D.; Ganjyal, G.; Sablani, S.S. Improving functional properties of pea protein isolate for microencapsulation of flaxseed oil. J. Microencaps. 2017, 34, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Burgess, R.R. Protein Precipitation Techniques. Methods Enzymol. 2009, 463, 331–342. [Google Scholar] [PubMed]
- Krause, J.-P.; Schultz, M.; Dudek, S. Effect of extraction conditions on composition, surface activity and rheological properties of protein isolates from flaxseed (Linum usitativissimum L). J. Sci. Food Agric. 2002, 82, 970–976. [Google Scholar] [CrossRef]
- Marambe, H.K.; Wanasundara, J.P.D. Chapter 8—protein from flaxseed (Linum usitatissimum L.). In Sustainable Protein Sources; Nadathur, S.R., Wanasundara, J.P.D., Scanlin, L., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 133–144. [Google Scholar]
- Gutte, K.B.; Sahoo, A.K.; Ranveer, R.C. Effect of ultrasonic treatment on extraction and fatty acid profile of flaxseed oil. OCL 2015, 22, D606. [Google Scholar] [CrossRef] [Green Version]
- Shim, Y.Y.; Gui, B.; Wang, Y.; Reaney, M.J.T. Flaxseed (Linum usitatissimum L.) oil processing and selected products. Trends Food Sci. Technol. 2015, 43, 162–177. [Google Scholar] [CrossRef]
- Bhargavi, G.; Nageswara Rao, P.; Renganathan, S. Review on the extraction methods of crude oil from all generation biofuels in last few decades. IOP Conference Ser. Mater. Sci. Eng. 2018, 330, 1–20. [Google Scholar] [CrossRef]
- Rommi, K. Enzyme-aided recovery of protein and protein hydrolyzates from rapeseed cold-press cake. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 2016. [Google Scholar]
- Rubilar, M.; Gutiérrez, C.; Verdugo, M.; Shene, C.; Sineiro, J. Flaxseed as a source of functional ingredients. J. Soil Sci. Plant Nutr. 2010, 10, 373–377. [Google Scholar] [CrossRef]
- Campbell, K.A. Protein and Oil Recoveries from Enzyme-Assisted Aqueous Extraction of Soybeans and Sunflower Seed. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 2010. [Google Scholar]
- Galvão, E.L.; Martínez, J.; de Oliveira, H.N.M.; de Sousa, E.M.B.D. Supercritical extraction of linseed oil: Economical viability and modeling extraction curves. Chem. Eng. Commun. 2013, 200, 205–221. [Google Scholar] [CrossRef]
- Akanda, M.J.H.; Sarker, M.Z.I.; Ferdosh, S.; Manap, M.Y.A.; Ab Rahman, N.N.N.; Ab Kadir, M.O. Applications of supercritical fluid extraction (sfe) of palm oil and oil from natural sources. Molecules 2012, 17, 1764–1794. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, N.; Xu, L.; Yu, X. A novel process for the aqueous extraction of linseed oil based on nitrogen protection. Adv. J. Food Sci. Technol. 2015, 9, 606–613. [Google Scholar] [CrossRef]
- Nikiforidis, C.V.; Kiosseoglou, V. Aqueous extraction of oil bodies from maize germ (Zea mays) and characterization of the resulting natural oil-in-water emulsion. J. Agric. Food Chem. 2009, 57, 5591–5596. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yu, X.; Zhao, Z.; Xu, L.; Zhang, R. Efficient salt-aided aqueous extraction of bitter almond oil. J. Sci. Food Agric. 2017, 97, 3814–3821. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Pordesimo, L.; Weiss, J. High intensity ultrasound-assisted extraction of oil from soybeans. Food Res. Int. 2004, 37, 731–738. [Google Scholar] [CrossRef]
- Zhang, Z.-S.; Wang, L.-J.; Li, D.; Jiao, S.-S.; Chen, X.D.; Mao, Z.-H. Ultrasound-assisted extraction of oil from flaxseed. Sep. Purif. Technol. 2008, 62, 192–198. [Google Scholar] [CrossRef]
- Hernández-Santos, B.; Rodríguez-Miranda, J.; Herman-Lara, E.; Torruco-Uco, J.G.; Carmona-García, R.; Juárez-Barrientos, J.M.; Chávez-Zamudio, R.; Martínez-Sánchez, C.E. Effect of oil extraction assisted by ultrasound on the physicochemical properties and fatty acid profile of pumpkin seed oil (Cucurbita pepo). Ultrason. Sonochem. 2016, 31, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Sicaire, A.-G.; Vian, M.A.; Fine, F.; Carré, P.; Tostain, S.; Chemat, F. Ultrasound induced green solvent extraction of oil from oleaginous seeds. Ultrason. Sonochem. 2016, 31, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Omar, K.A.; Liu, R.; Wang, X. Effects of ultrasound-assisted extraction on yield of flaxseed oil, β- and γ- tocopherols optimized by orthogonal array design. Eur. J. Lipid Sci. Technol. 2014, 116, 1412–1420. [Google Scholar] [CrossRef]
- Ali, M.; Watson, I.A. Comparison of oil extraction methods, energy analysis and biodiesel production from flax seeds. Int. J. Energy Res. 2013, 38, 614–625. [Google Scholar] [CrossRef]
- Long, J.-j.; Fu, Y.-j.; Zu, Y.-g.; Li, J.; Wang, W.; Gu, C.-b.; Luo, M. Ultrasound-assisted extraction of flaxseed oil using immobilized enzymes. Bioresour. Technol. 2011, 102, 9991–9996. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Zhang, W.; Sun, S.; Duan, X.; Zhang, Z. Enhanced extraction of oil from flaxseed (Linum usitatissimum L.) using microwave pre-treatment. J. Oleo Sci. 2015, 64, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- Rombaut, N.; Savoire, R.; Van Hecke, E.; Thomasset, B. Supercritical CO2 extraction of linseed: Optimization by experimental design with regards to oil yield and composition. Eur. J. Lipid Sci. Technol. 2017, 119, 1600078. [Google Scholar] [CrossRef]
- Özkal, S.G.; Yener, M.E. Supercritical carbon dioxide extraction of flaxseed oil: Effect of extraction parameters and mass transfer modeling. J. Supercrit. Fluids 2016, 112, 76–80. [Google Scholar] [CrossRef]
- Pradhan, R.C.; Meda, V.; Rout, P.K.; Naik, S.; Dalai, A.K. Supercritical CO2 extraction of fatty oil from flaxseed and comparison with screw press expression and solvent extraction processes. J. Food Eng. 2010, 98, 393–397. [Google Scholar] [CrossRef]
- Zanqui, A.B.; de Morais, D.R.; da Silva, C.M.; Santos, J.M.; Gomes, S.T.M.; Visentainer, J.V.; Eberlin, M.N.; Cardozo-Filho, L.; Matsushita, M. Subcritical extraction of flaxseed oil with n-propane: Composition and purity. Food Chem. 2015, 188, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Kratzer, F.H. The treatment of linseed meal to improve its feeding value for chicks. Poult. Sci. 1946, 25, 541–542. [Google Scholar] [CrossRef]
- Bolarinwa, I.F.; Oke, M.O.; Olaniyan, S.A.; Ajala, A.S. A review of cyanogenic glycosides in edible plants. In Toxicology-New Aspects to This Scientific Conundrum; InTech: London, UK, 2016. [Google Scholar]
- Dhas, P.; Jayakumar, S.; Chitra, P.; Mary, A. Study of the effects of hydrogen cyanide exposure in cassava workers. In. J. Occup. Environ. Med. 2011, 15, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Oomah, B.D.; Mazza, G.; Kenaschuk, E.O. Cyanogenic compounds in flaxseed. J. Agric. Food Chem. 1992, 40, 1346–1348. [Google Scholar] [CrossRef]
- Wanasundara, P.K.J.P.D.; Amarowicz, R.; Kara, M.T.; Shahidi, F. Removal of cyanogenic glycosides of flaxseed meal. Food Chem. 1993, 48, 263–266. [Google Scholar] [CrossRef]
- Wanasundara, J.P.D.; Shahidi, F. Alkanol-ammonia-water/hexane extraction of flaxseed. Food Chem. 1994, 49, 39–44. [Google Scholar] [CrossRef]
- Varga, T.K.; Diosady, L.L. Simultaneous extraction of oil and antinutritional compounds from flaxseed. J. Am. Oil Chem. Soc. 1994, 71, 603–607. [Google Scholar] [CrossRef]
- Yamashita, T.; Sano, T.; Hashimoto, T.; Kanazawa, K. Development of a method to remove cyanogen glycosides from flaxseed meal. Int. J. Food Sci. Technol. 2007, 42, 70–75. [Google Scholar] [CrossRef]
- Bradbury, J.H.; Denton, I.C. Simple method to reduce the cyanogen content of gari made from cassava. Food Chem. 2010, 123, 840–845. [Google Scholar] [CrossRef]
- Bradbury, J.H.; Denton, I.C. Rapid wetting method to reduce cyanogen content of cassava flour. Food Chem. 2010, 121, 591–594. [Google Scholar] [CrossRef]
- Wu, C.F.; Xu, X.M.; Huang, S.H.; Deng, M.C.; Feng, A.J.; Peng, J.; Yuan, J.P.; Wang, J.H. An efficient fermentation method for the degradation of cyanogenic glycosides in flaxseed. Food Addit. Contam Part A 2012, 29, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Sornyotha, S.; Kyu, K.L.; Ratanakhanokchai, K. An efficient treatment for detoxification process of cassava starch by plant cell wall-degrading enzymes. J. Biosci. Bioeng. 2010, 109, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Tąta, A.; Sokołowska, K.; Świder, J.; Konieczna-Molenda, A.; Proniewicz, E.; Witek, E. Study of cellulolytic enzyme immobilization on copolymers of N-vinylformamide. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 149, 494–504. [Google Scholar] [CrossRef] [PubMed]



| Type | Structure or Primary Sequence | Production Method | Biological Property | Reference(s) |
|---|---|---|---|---|
| Peptide | Gly-Phe-Pro-Gly-Arg-Leu-Asp-His-Trp-Cys-Ala-Ser-Glu | Hydrolysis by Alcalase | Antioxidant | [34] |
| Hydrolysate | <1 kDa peptides fractions | Hydrolysis by protease from Bacillus altitudinis HK02 | Antimicrobial activity | [35] |
| Hydrolysate | 1–3 kDa peptides fractions | Hydrolysis by protease from Bacillus altitudinis HK02 | Antioxidant activity | [35] |
| Hydrolysate | Less than 4 kDa peptide fractions | Hydrolysis by thermolysin and pronase | Antioxidant; Antihypertensive (angiotensin I-converting enzyme, ACE-inhibitory) activity | [36] |
| Hydrolysate | Less than 1, and 1–3 kDa peptide fractions | Hydrolysis by thermoase and membrane ultrafiltration | Antihypertensive (ACE-inhibitory); Renin-inhibitory activity | [37] |
| Cyclolinopeptide -A | cyclo-(Pro-Pro-Phe-Phe-Leu-Ile-Ile-Leu-Val) | Extraction | Immunosuppressive activity; Antioxidant; Antimalarial activity | [13,14] |
| Cyclolinopeptide -B | cyclo-(Pro-Pro-Phe-Phe-Val-Ile-Met-Leu-Ile) | Extraction | Immunosuppressive activity | [13] |
| Cyclolinopeptide -E | cyclo-(Pro-Leu-Phe-Ile-MetO-Leu-Val-Phe) | Extraction | Immunosuppressive activity | [14] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzuvor, C.K.O.; Taylor, J.T.; Acquah, C.; Pan, S.; Agyei, D. Bioprocessing of Functional Ingredients from Flaxseed. Molecules 2018, 23, 2444. https://doi.org/10.3390/molecules23102444
Dzuvor CKO, Taylor JT, Acquah C, Pan S, Agyei D. Bioprocessing of Functional Ingredients from Flaxseed. Molecules. 2018; 23(10):2444. https://doi.org/10.3390/molecules23102444
Chicago/Turabian StyleDzuvor, Christian Kwesi Ofotsu, Jordan Tauai Taylor, Caleb Acquah, Sharadwata Pan, and Dominic Agyei. 2018. "Bioprocessing of Functional Ingredients from Flaxseed" Molecules 23, no. 10: 2444. https://doi.org/10.3390/molecules23102444
APA StyleDzuvor, C. K. O., Taylor, J. T., Acquah, C., Pan, S., & Agyei, D. (2018). Bioprocessing of Functional Ingredients from Flaxseed. Molecules, 23(10), 2444. https://doi.org/10.3390/molecules23102444







