Dibasic Derivatives of Phenylcarbamic Acid against Mycobacterial Strains: Old Drugs and New Tricks?
Abstract
:1. Introduction
2. Results and Discussion
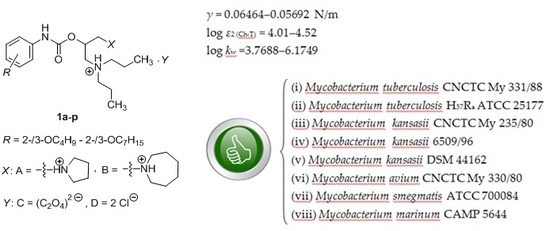
2.1. Synthesis of the Compounds 1a–p
2.2. Determination and Prediction of Some Physicochemical Properties of the Compounds 1a–p
2.2.1. Surface Tension
2.2.2. Electronic Properties
2.2.3. Lipohydrophilic Properties
RMSE = 0.2169, NR = 0.8115, F = 131.75, Prob > F = 0.0001 ***
RMSE = 8.022 × 10−4, NR = 0.0030, F = 103.02, Prob > F = 0.0001 ***
2.3. In Vitro Antimycobacterial Assays
2.4. Structure–Activity Relationships
2.4.1. Models Related to Mycobacterium tuberculosis CNCTC My 331/88
RMSE = 0.0761, NR = 0.1863, F = 86.06, Prob > F = 0.0001 ***
RMSE = 0.1153, NR = 0.2825, F = 34.03, Prob > F = 0.0011 **
RMSE = 0.1103, NR = 0.2702, F = 30.30, Prob > F = 0.0015 **
RMSE = 0.0989, NR = 0.2424, F = 39.13, Prob > F = 0.0008 ***
2.4.2. Models Related to the Mycobacterium kansasii Species
RMSE = 0.1016, NR = 0.2489, F = 45.76, Prob > F = 0.0001 ***
RMSE = 0.1185, NR = 0.2901, F = 44.86, Prob > F = 0.0001 ***
RMSE = 0.1153, NR = 0.2825, F = 47.12, Prob > F = 0.0005 ***
RMSE = 0.1971, NR = 0.4827, F = 32.71, Prob > F = 0.0012 **
RMSE = 0.1277, NR = 0.3129, F = 86.15, Prob > F = 0.0001 ***
2.4.3. Models Related to Mycobacterium avium CNCTC My 330/80
RMSE = 0.1745, NR = 0.4275, F = 25.61, Prob > F = 0.0032 **
RMSE = 0.1061, NR = 0.2599, F = 79.57, Prob > F = 0.0001 ***
RMSE = 0.1596, NR = 0.3909, F = 28.62, Prob > F = 0.0081 **
2.4.4. Models Related to Mycobacterium smegmatis ATCC 700084
3. Materials and Methods
3.1. General Information
3.2. Synthesis of Compounds
3.3. Determination and Prediction of Physicochemical Properties
3.3.1. Estimation of Surface Tension
3.3.2. Estimation of Electronic Properties
3.3.3. Estimation and In Silico Investigation of Lipohydrophilic Properties
3.4. In Vitro Antimycobacterial Assays
3.5. Calculations and Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-PrOH | Propan-2-ol |
| 3-d | 3-Day cultivation |
| 7-d | 7-Day cultivation |
| 14-d | 14-Day cultivation |
| 21-d | 21-Day cultivation |
| λe | Eigenvalues (principal component analysis) |
| χ,2red | Reduced chi-square (statistical analysis) |
| ϕM | Volume fraction of a mobile phase modifier (RP-HPLC) |
| CPX | Ciprofloxacin |
| Adj. R2 | Adjusted coefficient of determination (statistical analysis) |
| DEE | Diethyl ether |
| DF | Degrees of freedom (statistical analysis) |
| DMSO | Dimethyl sulfoxide |
| EtOH | Ethanol |
| EMB | Ethambutol |
| F | Fisher’s F-test (Fisher’s significance ratio; statistical analysis) |
| INH | Isoniazid |
| IR | Infrared |
| k | Retention (capacity) factor (RP-HPLC) |
| LA/LAs | Local anesthetic/local anesthetics |
| log kw | Lipophilicity index; values extrapolated from intercepts of a linear relationship between the logarithm of retention factor k (log k) and volume fraction of a mobile phase modifier (ϕM; RP-HPLC) |
| MeOH | Methanol |
| MA | Mycobacterium avium |
| MIC | Minimum inhibitory concentration (in μΜ units) |
| MK | Mycobacterium kansasii |
| MM | Mycobacterium marinum |
| MS | Mycobacterium smegmatis |
| MT | Mycobacterium tuberculosis |
| nc | Number of carbon atoms forming an 2-/3-alkoxy side chain (R) |
| NR | Norm of residuals (statistical analysis) |
| OFLX | Ofloxacin |
| PC | Principal Component (principal component analysis) |
| PCA/PCAs | Principal component analysis/principal component analyses |
| Prob > F | Probability of obtaining the F Ratio (statistical analysis) |
| RMSE | Root mean squared error (standard deviation; statistical analysis) |
| RSS | Residual sum of squares (statistical analysis) |
| S | Slope (RP-HPLC) |
| SAR/SARs | Structure–activity relationship/Structure–activity relationships |
| tr | Retention time of a compound (RP-HPLC) |
References
- Squeglia, F.; Romano, M.; Ruggiero, A.; Berisio, R. Molecular players in tuberculosis drug development: Another break in the cell wall. Curr. Med. Chem. 2017, 24, 3954–3969. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, P.B.; Campos, D.L.; Ribeiro, C.M.; da Silva, I.C.; Pavan, F.R. New antimycobacterial agents in the pre-clinical phase or beyond: Recent advances in patent literature (2001–2016). Expert Opin. Ther. Pat. 2017, 27, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.L.; Aziz, D.B.; Dartois, V.; Dick, T. NTM Drug discovery: Status, gaps and the way forward. Drug Discov. Today 2018, 23, 1502–1519. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, D.; Srivastava, S.; Chapagain, M.L.; Lee, P.S.; Cirrincione, K.N.; Pasipanodya, J.G.; Gumbo, T. The discovery of ceftazidime/avibactam as an anti-Mycobacterium avium agent. J. Antimicrob. Chemother. 2017, 72 (Suppl. 2), i36–i42. [Google Scholar] [CrossRef] [PubMed]
- Amaral, L.; Viveiros, M.; Kristiansen, J.E. ‘Non-Antibiotics’: Alternative therapy for the management of MDRTB and MRSA in economically disadvantaged countries. Curr. Drug Targets 2006, 7, 887–891. [Google Scholar] [CrossRef] [PubMed]
- Amaral, L.; Viveiros, M. Thioridazine: A non-antibiotic drug highly effective, in combination with first line anti-tuberculosis drugs, against any form of antibiotic resistance of Mycobacterium tuberculosis due to its multi-mechanisms of action. Antibiotics 2017, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.M.; Rosenkranz, H.S. Antimicrobial activity of local anesthetics: Lidocaine and procaine. J. Infect. Dis. 1970, 121, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Nakajima, H.; Hirose, T.; Mochizuki, T.; Fukaura, A.; Ota, S.; Sugihara, S.; Tazawa, H.; Takahashi, T. Growth inhibition of mycobacteria by lidocaine in fiberoptic bronchoscopic aspirates. JJSRE 1993, 15, 108–115. [Google Scholar] [CrossRef]
- Goldberg, L. Pharmacological properties of xylocaine. Sven. Tandl. Tidskr. 1947, 40, 819–830. [Google Scholar]
- Gordh, T. Xylocain—A new local analgesic. Anesthesia 1949, 4, 4–9. [Google Scholar] [CrossRef]
- Miescher, K. Studies of local anesthetics. Helv. Chim. Acta 1932, 15, 163–190. [Google Scholar] [CrossRef]
- Agarwal, N.; Kalra, V.K. Studies on the mechanism of action of local anesthetics on proton translocating ATPase from Mycobacterium phlei. Biochim. Biophys. Acta 1984, 764, 316–323. [Google Scholar] [CrossRef]
- Nakayama, Y. The electrophoretical analysis of esterase and catalase and its use in taxonomical studies of mycobacteria. Jpn. J. Microbiol. 1967, 11, 95–101. [Google Scholar] [CrossRef]
- Prigozhin, D.M.; Mavrici, D.; Huizar, J.P.; Vansell, H.J.; Alber, T. Structural and biochemical analyses of Mycobacterium tuberculosis N-acetylmuramyl-l-alanine amidase Rv3717 point to a role in peptidoglycan fragment recycling. J. Biol. Chem. 2013, 288, 31549–31555. [Google Scholar] [CrossRef] [PubMed]
- Sultana, R.; Vemula, M.H.; Banerjee, S.; Guruprasad, L. The PE16 (Rv1430) of Mycobacterium tuberculosis is an esterase belonging to serine hydrolase superfamily of proteins. PLoS ONE 2013, 8, e55320. [Google Scholar] [CrossRef] [PubMed]
- Vacondio, F.; Silva, C.; Mor, M.; Testa, B. Qualitative structure–metabolism relationships in the hydrolysis of carbamates. Drug Metab. Rev. 2010, 42, 551–589. [Google Scholar] [CrossRef] [PubMed]
- Moraczewski, A.L.; Banaszynski, L.A.; From, A.M.; White, C.E.; Smith, B.D. Using hydrogen bonding to control carbamate C−N rotamer equilibria. J. Org. Chem. 1998, 63, 7258–7262. [Google Scholar] [CrossRef] [PubMed]
- Combrink, K.D.; Denton, D.A.; Harran, S.; Ma, Z.; Chapo, K.; Yan, D.; Bonventre, E.; Roche, E.D.; Doyle, T.B.; Robertson, G.T.; et al. New C25 carbamate rifamycin derivatives are resistant to inactivation by ADP-ribosyl transferases. Bioorg. Med. Chem. Lett. 2007, 17, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Velikorodov, A.V.; Urlyapova, N.G.; Daudova, A.D. Synthesis and antimycobacterial activity of carbamate derivatives of 1,2-oxazine. Pharm. Chem. J. 2006, 40, 380–382. [Google Scholar] [CrossRef]
- Férriz, J.M.; Vávrová, K.; Kunc, F.; Imramovský, A.; Stolaříková, J.; Vavříková, E.; Vinšová, J. Salicylanilide carbamates: Antitubercular agents active against multidrug-resistant Mycobacterium tuberculosis strains. Bioorg. Med. Chem. 2010, 18, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Csöllei, J.; Búčiová, Ľ.; Čižmárik, J.; Kopáčová, L. Studies of local anaesthetics CXII. Preparation and activity of dibasic alkylesters of 2-, and 3-alkoxy-substituted phenylcarbamic acids. Českoslov. Farm. 1993, 42, 127–129. (In Slovak) [Google Scholar]
- Pavelčík, F.; Remko, M.; Čižmárik, J.; Majer, J. Crystal and molecular structure of heptacain hydrochloride. Collect. Czechoslov. Chem. Commun. 1986, 51, 264–270. [Google Scholar] [CrossRef]
- Waisser, K.; Doležal, R.; Palát, K., Jr.; Čizmárik, J.; Kaustová, J. QSAR Study of antimycobacterial activity of quaternary ammonium salts of piperidinylethyl esters of alkoxysubstituted phenylcarbamic acids. Folia Microbiol. 2006, 51, 21–24. [Google Scholar] [CrossRef]
- Blaser, A.; Palmer, B.D.; Sutherland, H.S.; Kmentova, I.; Franzblau, S.G.; Wan, B.; Wang, Y.; Ma, Z.; Thompson, A.M.; Denny, W.A. Structure−activity relationships for amide-, carbamate-, and urea-linked analogues of the tuberculosis drug (6S)-2-nitro-6-{[4-(trifluoromethoxy)benzyl]oxy}-6,7-dihydro-5H-imidazo[2,1-b][1,3]oxazine (PA-824). J. Med. Chem. 2012, 55, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Gonec, T.; Pospisilova, S.; Holanova, L.; Stranik, J.; Cernikova, A.; Pudelkova, V.; Kos, J.; Oravec, M.; Kollar, P.; Cizek, A.; et al. Synthesis and antimicrobial evaluation of 1-[(2-substituted phenyl)carbamoyl]naphthalen-2-yl carbamates. Molecules 2016, 21, 1189. [Google Scholar] [CrossRef] [PubMed]
- Malík, I.; Sedlárová, E.; Csöllei, J.; Andriamainty, F.; Čižmárik, J.; Kečkéšová, S. The physicochemical properties of dibasic alkyl esters of 2- and 3-alkyloxy substituted phenylcarbamic acid. Acta Fac. Pharm. Univ. Comen. 2007, 54, 136–145. [Google Scholar]
- Čižmárik, J.; Borovanský, A.; Švec, P. Study of local anesthetics LII. Piperidinoethyl esters of alkoxyphenylcarbamic acids. Acta Fac. Pharm. 1976, 29, 53–79. [Google Scholar]
- Heywood, D.L.; Phillips, B. The reaction of epichlorohydrin with secondary amines. J. Am. Chem. Soc. 1958, 80, 1257–1259. [Google Scholar] [CrossRef]
- Schleppnik, A.A.; Gutsche, C.D. Synthesis of polysubstituted triptych-boroxazolidines. J. Org. Chem. 1962, 27, 3684–3686. [Google Scholar] [CrossRef]
- Dilmohamud, B.A.; Seeneevassen, J.; Rughooputh, S.D.D.V.; Ramasami, P. Surface tension and related thermodynamic parameters of alcohols using the Traube stalagmometer. Eur. J. Phys. 2005, 26, 1079–1084. [Google Scholar] [CrossRef]
- Vazquez, G.; Alvarez, E.; Navaza, J.M. Surface tension of alcohol water + water from 20 to 50 °C. J. Chem. Eng. Data 1995, 40, 611–614. [Google Scholar] [CrossRef]
- Stopková, L.; Gališinová, J.; Šuchtová, Z.; Čižmárik, J.; Andriamainty, F. Determination of critical micellar concentration of homologous 2-alkoxyphenylcarbamoyloxyethylmorpholinium chlorides. Molecules 2018, 23, 1064. [Google Scholar] [CrossRef] [PubMed]
- Čižmárik, J.; Borovanský, A.; Švec, P. Study of local anesthetics. LVII. Perhydroazepinylethyl esters of alkoxyphenylcarbamic acids. Česk. Farm. 1976, 25, 118–121. (In Slovak) [Google Scholar] [PubMed]
- Blešová, M.; Čižmárik, J.; Bachratá, M.; Bezáková, Ž.; Borovanský, A. Chromatographic parameters, pKa, and surface activity of a series of hydrochlorides of perhydroazepinylethyl esters of alkoxyphenylcarbamic acids and their relation to anaesthetic activity. Collect. Czechoslov. Chem. Commun. 1985, 50, 1133–1140. [Google Scholar] [CrossRef]
- Yadav, L.D.S. Ultraviolet and Visible Spectroscopy. In Organic Spectroscopy; Yadav, L.D.S., Ed.; Springer: Amsterdam, The Netherlands, 2005; pp. 7–51. [Google Scholar] [CrossRef]
- Čižmárik, J.; Borovanský, A. Synthesis, u.v. and i.r. spectra of 2-piperidnoethyl esters of alkyloxyphenylcarbamic acids. Chem. Zvesti 1975, 29, 119–123. [Google Scholar]
- Rutkowska, E.; Pajak, K.; Jóźwiak, K. Lipophilicity–methods of determination and its role in medicinal chemistry. Acta Pol. Pharm. 2013, 70, 3–18. [Google Scholar] [PubMed]
- Waisser, K.; Doležal, R.; Čižmárik, J. Graphic demonstration of the structure–antimycobacterial activity relationships in the series of ester phenylcarbamic acid with piperidine or pyrrolidine moiety. Folia Pharm. Univ. Carol. 2008, 37, 65–76. [Google Scholar]
- Van de Waterbeemd, H.; Kansy, M.; Wagner, B.; Fischer, H. Lipophilicity Measurement by Reversed-Phase High Performance Liquid Chromatography (RP-HPLC). In Lipophilicity in Drug Action and Toxicology. Methods and Principles in Medicinal Chemistry; Pliška, V., Testa, B., van de Waterbeemd, H., Eds.; VCh Verlag: Weinheim, Germany, 1996; pp. 73–88. ISBN 3-527-29383-3. [Google Scholar]
- Valkó, K.; Slégel, P. New chromatographic hydrophobicity index (ϕ0) based on the slope and the intercept of the log k′ versus organic phase concentration plot. J. Chromatogr. A 1993, 631, 49–61. [Google Scholar] [CrossRef]
- Du, Ch.M.; Valko, K.; Bevan, Ch.; Reynolds, D.; Abraham, M.H. Rapid method for estimating octanol–water partition coefficient (log Poct) from isocratic RP-HPLC and a hydrogen bond acidity term (A). J. Liq. Chrom. Rel. Technol. 2001, 24, 635–649. [Google Scholar] [CrossRef]
- El Tayar, N.; van de Waterbeemd, H.; Testa, B. Lipophilicity measurements of protonated basic compounds by reversed-phase high-performance liquid chromatography: I. Relationship between capacity factors and the methanol concentration in methanol–water eluents. J. Chromatogr. A 1985, 320, 293–304. [Google Scholar] [CrossRef]
- El Tayar, N.; van de Waterbeemd, H.; Testa, B. Lipophilicity measurements of protonated basic compounds by reversed-phase high-performance liquid chromatography: II. Procedure for the determination of a lipophilic index measured by reversed-phase high-performance liquid chromatography. J. Chromatogr. A 1985, 320, 305–312. [Google Scholar] [CrossRef]
- Paschke, A.; Manz, M.; Schüürmann, G. Application of different RP-HPLC methods for the determination of the octanol/water partition coefficient of selected tetrachlorobenzyltoluenes. Chemosphere 2001, 45, 721–728. [Google Scholar] [CrossRef]
- Montanari, M.L.C.; Montanari, C.A.; Piló-Veloso, D.; Cass, Q.B. Estimation of the RP-HPLC lipophilicity parameters log k′, and log kw, a comparison with the hydrophobicity index ϕ0. J. Liq. Chromatogr. Relat. Technol. 1997, 20, 1703–1715. [Google Scholar] [CrossRef]
- Flieger, J.; Tatarczak-Michalewska, M.; Wujec, M.; Pitucha, M.; Świeboda, R. RP-HPLC Analysis and in vitro identification of antimycobacterial activity of novel thiosemicarbazides and 1,2,4-triazole derivatives. J. Pharm. Biomed. Anal. 2015, 107, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Goněc, T.; Malík, I.; Csöllei, J.; Jampílek, J.; Stolaříková, J.; Solovič, I.; Mikuš, P.; Keltošová, S.; Kollár, P.; O’Mahony, J.; et al. Synthesis and in vitro antimycobacterial activity of novel N-arylpiperazines containing an ethane-1,2-diyl connecting chain. Molecules 2017, 22, 2100. [Google Scholar] [CrossRef] [PubMed]
- Carta, A.; Paglietti, G.; Rahbar Nikookar, M.E.; Sanna, P.; Sechi, L.; Zanetti, S. Novel substituted quinoxaline 1,4-dioxides with in vitro antimycobacterial and anticandida activity. Eur. J. Med. Chem. 2002, 37, 355–366. [Google Scholar] [CrossRef]
- Moreno, E.; Gabano, E.; Torres, E.; Platts, J.A.; Ravera, M.; Aldana, I.; Monge, A.; Pérez-Silanes, S. Studies on log Po/w of quinoxaline di-N-oxides: A comparison of RP-HPLC experimental and predictive approaches. Molecules 2011, 16, 7893–7908. [Google Scholar] [CrossRef] [PubMed]
- Snyder, L.R.; Poppe, H. Mechanism of solute retention in liquid–solid chromatography and the role of the mobile phase in affecting separation: Competition versus ‘sorption’. J. Chromatogr. A 1980, 184, 363–413. [Google Scholar] [CrossRef]
- Valkó, K.; Snyder, L.R.; Glajch, J.L. Retention in reversed-phase liquid chromatography as a function of mobile-phase composition. J. Chromatogr. A 1993, 656, 501–520. [Google Scholar] [CrossRef]
- Soczewiński, E. Mechanistic molecular model of liquid–solid chromatography: Retention–eluent composition relationships. J. Chromatogr. A 2002, 965, 109–116. [Google Scholar] [CrossRef]
- Chen, N.; Yhang, Y.; Lu, P. Effects of molecular structure on the S index in the retention equation in reversed-phase high-performance liquid chromatography. J. Chromatogr. A 1992, 606, 35–42. [Google Scholar] [CrossRef]
- Ghose, A.K.; Crippen, G.M. Atomic physicochemical parameters for three-dimensional structure-directed quantitative structure–activity relationships. I. Partition coefficients as a measure of hydrophobicity. J. Comput. Chem. 1986, 7, 565–577. [Google Scholar] [CrossRef]
- Ghose, A.K.; Crippen, G.M. Atomic physicochemical parameters for three-dimensional structure-directed quantitative structure–activity relationships. II. Modeling dispersive and hydrophobic interactions. J. Chem. Inform. Comput. Sci. 1987, 27, 21–35. [Google Scholar] [CrossRef]
- Viswanadhan, N.V.; Ghose, K.A.; Reyankar, R.G.; Robins, K.R. Atomic physicochemical parameters for three-dimensional structure-directed quantitative structure–activity relationships 4. Additional parameters for hydrophobic and dispersive interactions and their application for an automated superposition of certain naturally occurring nucleoside antibiotics. J. Chem. Inform. Comput. Sci. 1989, 29, 163–172. [Google Scholar] [CrossRef]
- Broto, P.; Moreau, G.; Vandycke, C. Molecular structures: Perception, autocorrelation descriptor and SAR studies. System of atomic contributions for the calculation of the n-octanol/water partition coefficients. Eur. J. Med. Chem. 1984, 19, 71–78. [Google Scholar]
- Leo, A. The octanol–water partition coefficient of aromatic solutes: The effect of electronic interactions, alkyl chains, hydrogen bonds, and ortho-substitution. J. Chem. Soc. Perkin Trans. II 1983, 20, 825–838. [Google Scholar] [CrossRef]
- Tetko, I.V.; Gasteiger, J.; Todeschini, R.; Mauri, A.; Livingstone, D.; Ertl, P.; Palyulin, V.A.; Radchenko, E.V.; Zefirov, N.S.; Makarenko, A.S.; et al. Virtual computational chemistry laboratory–design and description. J. Comput. Aided Mol. Des. 2005, 19, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Gao, Y.; Lai, L. Calculating partition coefficient by atom-additive method. Perspect. Drug Discov. Des. 2000, 19, 47–66. [Google Scholar] [CrossRef]
- Cheng, T.; Zhao, Y.; Li, X.; Lin, F.; Xu, Y.; Zhang, X.; Li, Y.; Wang, R.; Lai, L. Computation of octanol–water partition coefficients by guiding an additive model with knowledge. J. Chem. Inf. Model. 2007, 47, 2140–2148. [Google Scholar] [CrossRef] [PubMed]
- Moriguchi, I.; Hirono, S.; Liu, Q.; Nakagome, I.; Matsushita, Y. Simple method of calculating octanol/water partition coefficient. Chem. Pharm. Bull. 1992, 40, 127–130. [Google Scholar] [CrossRef]
- Sander, T.; Freyss, J.; von Korff, M.; Reich, J.R.; Rufener, C. OSIRIS, an entirely in-house developed drug discovery informatics system. J. Chem. Inf. Model. 2009, 49, 232–246. [Google Scholar] [CrossRef] [PubMed]
- Molinspiration Cheminformatics, Slovenský Grob, Slovak Republic. Available online: http://www.molinspiration.com/cgi-bin/properties (accessed on 7 June 2018).
- Viswanadhan, V.N.; Rami Reddy, M.; Bacquet, R.J.; Erion, M.D. Assessment of methods used for predicting lipophilicity: Application to nucleosides and nucleoside bases. J. Comput. Chem. 1993, 14, 1019–1026. [Google Scholar] [CrossRef]
- Tetko, I.V.; Tanchuk, V.Y. Application of associative neural networks for prediction of lipophilicity in ALOGPS 2.1 program. J. Chem. Inf. Comput. Sci. 2002, 42, 1136–1145. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, E42717–E42729. [Google Scholar] [CrossRef] [PubMed]
- Abdi, H.; Williams, L.J. Principal component analysis. WIREs Comp. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef] [Green Version]
- Carpenter, T.S.; Kirshner, D.A.; Lau, E.Y.; Wong, S.E.; Nilmeier, J.P.; Lightstone, F.C. A method to predict blood–brain barrier permeability of drug-like compounds using molecular dynamics simulations. Biophys. J. 2014, 107, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Norinder, U.; Haeberlein, M. Computational approaches to the prediction of blood–brain distribution. Adv. Drug Deliv. Rev. 2002, 54, 291–313. [Google Scholar] [CrossRef]
- Van de Waterbeemd, H.; Camenisch, G.; Folkers, G.; Chretien, J.R.; Raevsky, O.A. Estimation of blood–brain barrier crossing of drugs using molecular size and shape, and H-bonding descriptors. J. Drug Targets 1998, 6, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Imramovsky, A.; Pesko, M.; Kralova, K.; Vejsova, M.; Stolarikova, J.; Vinsova, J.; Jampilek, J. Investigating spectrum of biological activity of 4- and 5-chloro-2-hydroxy-N-[2-(arylamino)-1-alkyl-2-oxoethyl]benz-amides. Molecules 2011, 16, 2414–2430. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria; Approved Standard, 8th ed.; CLSI Document M11-A8; CLSI: Wayne, NJ, USA, 2012; pp. 10–56. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 24th ed.; Informational Supplement M100-S24; CLSI: Wayne, NJ, USA, 2014; pp. 106–211. [Google Scholar]
- Brown-Elliott, B.A.; Cohen, S.; Wallace, R.J., Jr. Susceptibility Testing of Mycobacteria. In Antimicrobial Susceptibility Testing Protocols; Schwalbe, R., Steele-Moore, L., Goodwin, A.C., Eds.; CRC Press (Taylor and Francis Group): Boca Raton, FL, USA, 2007; pp. 243–274. [Google Scholar]
- Waisser, K.; Dražková, K.; Čižmárik, J.; Kaustová, J. Antimycobacterial activity of basic ethylesters of alkoxy-substituted phenylcarbamic acids. Folia Microbiol. 2003, 48, 45–50. [Google Scholar] [CrossRef]
- Jackson, D.A. Stopping rules in principal components analysis: A comparison of heuristical and statistical approaches. Ecology 1993, 74, 2204–2214. [Google Scholar] [CrossRef]
- Čižmárik, J.; Waisser, K.; Doležal, R. QSAR Study of antimycobacterial activity of esters of phenylcarbamic acids. Acta Fac. Pharm. Univ. Comen. 2008, 55, 90–95. [Google Scholar]
- Čižmárik, J.; Waisser, K. Review of potential antituberculotics: Esters of substituted phenylcarbamic acids. Folia Pharm. Univ. Carol. 2008, 37, 91–94. [Google Scholar]
- Grzegorzewicz, A.E.; de Sousa-d’Auria, C.; McNeil, M.R.; Huc-Claustre, E.; Jones, V.; Petit, C.; Angala, S.K.; Zemanová, J.; Wang, Q.; Belardinelli, J.M.; et al. Assembling of the Mycobacterium tuberculosis cell wall core. J. Biol. Chem. 2016, 291, 18867–18879. [Google Scholar] [CrossRef] [PubMed]
- Kubinyi, H. Quantitative structure–activity relationships. 7. The bilinear model, a new model for nonlinear dependence of biological activity on hydrophobic character. J. Med. Chem. 1977, 20, 625–629. [Google Scholar] [CrossRef] [PubMed]
- David, H.L.; Rastogi, N.; Clavel-Sérès, S.; Clément, F.; Thorel, M.-F. Structure of the cell envelope of Mycobacterium avium. Zent. Bakteriol. Mikrobiol. Hyg. A 1987, 264, 49–66. [Google Scholar] [CrossRef]
- Hansch, C.; Clayton, J.M. Lipophilic character and biological activity of drugs II. The parabolic case. J. Pharm. Sci. 1973, 62, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Šula, L. WHO Co-operative studies on a simple culture technique for the isolation of mycobacteria. 1. Preparation, lyophilization and reconstitution of a simple semi-synthetic concentrated liquid medium; culture technique; growth pattern of different mycobacteria. Bull. World Health Organ. 1963, 29, 589–606. [Google Scholar] [PubMed]
- De Voss, J.J.; Rutter, K.; Schroeder, B.G.; Su, H.; Zhu, Y.; Barry, C.E., III. The salicylate-derived mycobactin siderophores of Mycobacterium tuberculosis are essential for growth in macrophages. Proc. Natl. Acad. Sci. USA 2000, 97, 1252–1257. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, B.D.; De Voss, J.J. Structure and absolute configuration of mycobactin J. Tetrahedron Lett. 2001, 42, 3653–3655. [Google Scholar] [CrossRef]
- Atlas, R.; Snyder, J. Reagents, Stains, and Media: Bacteriology. In Manual of Clinical Microbiology (2-Volume Set), 11th ed.; Jorgensen, J.H., Pfaller, M.A., Carroll, K.C., Funke, G., Landry, M.L., Richter, S.S., Warnock, D.W., Eds.; ASM Press: Washington, DC, USA, 2015; Volume 1, pp. 316–349. [Google Scholar] [CrossRef]
- Franzblau, S.G.; Witzig, R.S.; McLaughlin, J.C.; Torres, P.; Madico, G.; Hernandez, A.; Degnan, M.T.; Cook, M.B.; Quenzer, V.K.; Ferguson, R.M.; et al. Rapid, low-technology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate Alamar Blue assay. J. Clin. Microbiol. 1998, 36, 362–366. [Google Scholar] [PubMed]
- Long, J.E.; DeJesus, M.; Ward, D.; Baker, R.E.; Ioerger, T.; Sassetti, C.M. Identifying Essential Genes in Mycobacterium Tuberculosis by Global Phenotypic Profiling. In Gene Essentiality. Methods and Protocols; Lu, L.J., Ed.; Humana Press (Springer): New York, NY, USA, 2015; pp. 79–98. [Google Scholar] [CrossRef]
- Ringnér, M. What is principal component analysis? Nat. Biotechnol. 2008, 26, 303–304. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1a–p are available from the authors Ivan Malík and Jozef Csöllei. |









 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Comp. | R | X | Y | γ (N/m) | λ1 | log ε1 | λ2 (Ch-T) | 1 log ε2 (Ch-T) | λ3 | log ε3 |
| 1a | 2-OC4H9 | A | C | 0.06464 | 208 | 4.51 | 236 | 4.19 | 280 | 3.63 |
| 1b | 2-OC5H11 | A | C | 0.06366 | 208 | 4.39 | 236 | 4.05 | 280 | 3.51 |
| 1c | 2-OC6H13 | A | C | 0.06222 | 208 | 4.58 | 238 | 4.52 | 278 | 3.49 |
| 1d | 2-OC7H15 | A | C | 0.05985 | 208 | 4.43 | 236 | 4.08 | 280 | 3.55 |
| 1e | 3-OC4H9 | A | D | 0.06316 | 210 | 4.65 | 238 | 4.24 | 279 | 3.59 |
| 1f | 3-OC5H11 | A | D | 0.06285 | 210 | 4.55 | 237 | 4.27 | 279 | 3.62 |
| 1g | 3-OC6H13 | A | D | 0.06105 | 210 | 4.54 | 237 | 4.13 | 279 | 3.48 |
| 1h | 3-OC7H15 | A | D | 0.05786 | 210 | 4.66 | 237 | 4.27 | 279 | 3.63 |
| 1i | 2-OC4H9 | B | C | 0.06302 | 208 | 4.44 | 236 | 4.08 | 280 | 3.57 |
| 1j | 2-OC5H11 | B | C | 0.06206 | 208 | 4.54 | 236 | 4.22 | 280 | 3.71 |
| 1k | 2-OC6H13 | B | C | 0.06065 | 208 | 4.42 | 236 | 4.10 | 280 | 3.57 |
| 1l | 2-OC7H15 | B | C | 0.05853 | 208 | 4.44 | 236 | 4.14 | 280 | 3.59 |
| 1m | 3-OC4H9 | B | D | 0.06298 | 210 | 4.52 | 238 | 4.09 | 279 | 3.44 |
| 1n | 3-OC5H11 | B | D | 0.06154 | 210 | 4.56 | 238 | 4.18 | 279 | 3.52 |
| 1o | 3-OC6H13 | B | D | 0.05925 | 210 | 4.52 | 238 | 4.01 | 279 | 3.37 |
| 1p | 3-OC7H15 | B | D | 0.05692 | 210 | 4.65 | 238 | 4.20 | 279 | 3.56 |
| Comp. | Mobile Phase MeOH/Water (v/v) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 80:20 | 85:15 | 90:10 | 95:5 | Pure MeOH | ||||||
| tr (min) | log k | tr (min) | log k | tr (min) | log k | tr (min) | log k | tr (min) | log k | |
| 1a | 13.883 | 0.7165 | 10.694 | 0.5756 | 7.830 | 0.3958 | 5.766 | 0.2008 | 4.224 | −0.0403 |
| 1b | 17.200 | 0.8254 | 12.647 | 0.6656 | 9.449 | 0.5064 | 6.514 | 0.2841 | 4.526 | 0.0203 |
| 1c | 25.694 | 1.0206 | 16.950 | 0.8162 | 11.292 | 0.6053 | 7.431 | 0.3683 | 5.246 | 0.1287 |
| 1d | 31.000 | 1.1092 | 19.600 | 0.8882 | 13.553 | 0.7022 | 7.892 | 0.4052 | 5.385 | 0.1573 |
| 1e | 10.330 | 0.5584 | 6.917 | 0.3183 | 5.400 | 0.1478 | 3.822 | −0.1454 | 3.329 | −0.2956 |
| 1f | 12.400 | 0.6574 | 9.200 | 0.4911 | 6.280 | 0.2546 | 4.022 | −0.0941 | 3.340 | −0.2913 |
| 1g | 17.200 | 0.8254 | 11.402 | 0.6104 | 7.483 | 0.3679 | 5.153 | 0.1182 | 3.583 | −0.2067 |
| 1h | 22.003 | 0.9521 | 13.701 | 0.7159 | 8.532 | 0.4565 | 5.788 | 0.2092 | 3.556 | −0.2153 |
| 1i | 24.020 | 0.9881 | 16.917 | 0.8153 | 11.691 | 0.6240 | 7.765 | 0.3954 | 5.127 | 0.1205 |
| 1j | 29.525 | 1.0863 | 20.021 | 0.8981 | 13.060 | 0.6828 | 8.520 | 0.4509 | 5.295 | 0.1449 |
| 1k | 47.108 | 1.3023 | 27.001 | 1.0424 | 16.533 | 0.8038 | 9.641 | 0.5221 | 6.938 | 0.3303 |
| 1l | 70.012 | 1.4831 | 38.800 | 1.2152 | 22.636 | 0.9619 | 11.670 | 0.6271 | 7.756 | 0.3996 |
| 1m | 15.731 | 0.7805 | 9.218 | 0.4922 | 6.420 | 0.2695 | 4.685 | 0.0424 | 3.519 | −0.2275 |
| 1n | 22.183 | 0.9502 | 12.370 | 0.6542 | 8.156 | 0.4205 | 4.995 | 0.0941 | 3.947 | −0.1047 |
| 1o | 29.174 | 1.0807 | 13.651 | 0.7059 | 9.634 | 0.5174 | 5.095 | 0.1095 | 4.127 | −0.0618 |
| 1p | 33.719 | 1.1484 | 17.129 | 0.8215 | 11.332 | 0.6072 | 5.801 | 0.2051 | 3.925 | −0.1102 |
| Comp. | log kw | 1S | 2χ2red | 3RSS | 4R | 5Adj. R2 | 6RMSE | 7NR | 8F | 9Prob > F |
|---|---|---|---|---|---|---|---|---|---|---|
| 1a | 3.7688 | 3.7768 | 0.0011 | 0.0034 | 0.9953 | 0.9875 | 0.0335 | 0.0580 | 317.64 | 0.0001 *** |
| 1b | 4.0454 | 3.9834 | 0.0018 | 0.0055 | 0.9931 | 0.9817 | 0.0429 | 0.0744 | 215.17 | 0.0007 *** |
| 1c | 4.6049 | 4.4634 | 0.0002 | 0.0007 | 0.9993 | 0.9982 | 0.0152 | 0.0263 | 2155.37 | 0.0001 *** |
| 1d | 4.9487 | 4.7736 | 0.0011 | 0.0033 | 0.9971 | 0.9923 | 0.0332 | 0.0574 | 518.15 | 0.0002 *** |
| 1e | 4.0258 | 4.3434 | 0.0013 | 0.0038 | 0.9960 | 0.9894 | 0.0354 | 0.0614 | 375.47 | 0.0003 *** |
| 1f | 4.6722 | 4.9652 | 0.0028 | 0.0084 | 0.9932 | 0.9821 | 0.0530 | 0.0917 | 219.79 | 0.0007 *** |
| 1g | 4.9446 | 5.1128 | 0.0013 | 0.0040 | 0.9969 | 0.9919 | 0.0366 | 0.0634 | 487.75 | 0.0002 *** |
| 1h | 5.5384 | 5.6830 | 0.0042 | 0.0126 | 0.9923 | 0.9795 | 0.0648 | 0.1122 | 192.51 | 0.0008 *** |
| 1i | 4.4679 | 4.3102 | 0.0014 | 0.0042 | 0.9955 | 0.9879 | 0.0376 | 0.0652 | 328.27 | 0.0004 *** |
| 1j | 4.8466 | 4.6600 | 0.0016 | 0.0048 | 0.9956 | 0.9882 | 0.0401 | 0.0695 | 337.19 | 0.0004 *** |
| 1k | 5.2359 | 4.9286 | 0.0005 | 0.0017 | 0.9987 | 0.9964 | 0.0234 | 0.0406 | 1106.57 | 0.0001 *** |
| 1l | 5.8966 | 5.5102 | 0.0007 | 0.0020 | 0.9988 | 0.9965 | 0.0259 | 0.0449 | 1127.92 | 0.0001 *** |
| 1m | 4.7099 | 4.9316 | 0.0004 | 0.0013 | 0.9990 | 0.9972 | 0.0205 | 0.0356 | 1442.73 | 0.0001 *** |
| 1n | 5.2087 | 5.3398 | 0.0011 | 0.0032 | 0.9978 | 0.9941 | 0.0325 | 0.0564 | 673.05 | 0.0001 *** |
| 1o | 5.6569 | 5.7628 | 0.0045 | 0.0134 | 0.9920 | 0.9789 | 0.0668 | 0.1157 | 186.23 | 0.0009 *** |
| 1p | 6.1749 | 6.2672 | 0.0022 | 0.0067 | 0.9966 | 0.9909 | 0.0474 | 0.0820 | 438.042 | 0.0001 *** |
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Comp. | R | X | log PCr | log PV | log PB | CLOGP 4.0 | XLOGP 2.0 | XLOGP 3.0 |
| 9′a | 2-OC4H9 | A | 4.59 | 4.45 | 4.51 | 6.23 | 4.52 | 5.14 |
| 9′b | 2-OC5H11 | A | 5.00 | 4.85 | 4.97 | 6.76 | 5.09 | 5.68 |
| 9′c | 2-OC6H13 | A | 5.42 | 5.24 | 5.42 | 7.29 | 5.66 | 6.22 |
| 9′d | 2-OC7H15 | A | 5.84 | 5.64 | 5.88 | 7.82 | 6.23 | 6.77 |
| 9′e | 3-OC4H9 | A | 4.59 | 4.45 | 4.51 | 6.23 | 4.52 | 5.14 |
| 9′f | 3-OC5H11 | A | 5.00 | 4.85 | 4.97 | 6.76 | 5.09 | 5.68 |
| 9′g | 3-OC6H13 | A | 5.42 | 5.24 | 5.42 | 7.29 | 5.66 | 6.22 |
| 9′h | 3-OC7H15 | A | 5.84 | 5.64 | 5.88 | 7.82 | 6.23 | 6.77 |
| 9′i | 2-OC4H9 | B | 5.42 | 5.24 | 5.42 | 7.35 | 5.24 | 5.86 |
| 9′j | 2-OC5H11 | B | 5.84 | 5.64 | 5.88 | 7.88 | 5.81 | 6.40 |
| 9′k | 2-OC6H13 | B | 6.26 | 6.04 | 6.33 | 8.41 | 6.38 | 6.84 |
| 9′l | 2-OC7H15 | B | 6.67 | 6.43 | 6.79 | 8.94 | 6.95 | 7.48 |
| 9′m | 3-OC4H9 | B | 5.42 | 5.24 | 5.42 | 7.35 | 5.24 | 5.86 |
| 9′n | 3-OC5H11 | B | 5.84 | 5.64 | 5.88 | 7.88 | 5.81 | 6.40 |
| 9′o | 3-OC6H13 | B | 6.26 | 6.04 | 6.33 | 8.41 | 6.38 | 6.84 |
| 9′p | 3-OC7H15 | B | 6.67 | 6.43 | 6.79 | 8.94 | 6.95 | 7.48 |
| Comp. | MLOGP | ACLOGP | miLogP 2.2 | ALOGP | log PS-IT | ALOGPs 2.1 |
|---|---|---|---|---|---|---|
| 9′a | 2.75 | 5.05 | 5.30 | 5.42 | 4.27 | 5.18 |
| 9′b | 2.96 | 5.52 | 5.80 | 5.87 | 4.68 | 5.57 |
| 9′c | 3.16 | 5.98 | 6.32 | 6.33 | 5.10 | 5.93 |
| 9′d | 3.36 | 6.45 | 6.81 | 6.79 | 5.53 | 6.25 |
| 9′e | 2.75 | 5.05 | 5.32 | 5.42 | 4.27 | 5.21 |
| 9′f | 2.96 | 5.52 | 5.83 | 5.87 | 4.68 | 5.61 |
| 9′g | 3.16 | 5.98 | 6.33 | 6.33 | 5.10 | 5.95 |
| 9′h | 3.36 | 6.45 | 6.84 | 6.79 | 5.53 | 6.32 |
| 9′i | 3.16 | 5.69 | 6.31 | 6.33 | 4.78 | 5.93 |
| 9′j | 3.36 | 6.16 | 6.81 | 6.79 | 5.20 | 6.23 |
| 9′k | 3.56 | 6.62 | 7.32 | 7.24 | 5.62 | 6.54 |
| 9′l | 3.76 | 7.08 | 7.82 | 7.70 | 6.04 | 6.77 |
| 9′m | 3.16 | 5.69 | 6.33 | 6.33 | 4.78 | 5.97 |
| 9′n | 3.36 | 6.16 | 6.84 | 6.79 | 5.20 | 6.27 |
| 9′o | 3.56 | 6.62 | 7.34 | 7.24 | 5.62 | 6.59 |
| 9′p | 3.76 | 7.08 | 7.85 | 7.70 | 6.04 | 6.83 |
| Comp. | MIC [μM] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MTv H37Rv | MTa H37Ra | MK 235/80 | MK 6509/96 | MK DSM | ||||||
| 1 14-d | 2 21-d | 3 7-d | 7-d | 14-d | 21-d | 7-d | 14-d | 21-d | 7-d | |
| 1a | 32 | 32 | 213 | 62.5 | 62.5 | 62.5 | 32 | 62.5 | 62.5 | 53 |
| 1b | 16 | 32 | 52 | 32 | 32 | 62.5 | 32 | 32 | 62.5 | 26.1 |
| 1c | 16 | 16 | 25 | 32 | 32 | 32 | 16 | 16 | 16 | 12.7 |
| 1d | 8 | 8 | 12.5 | 16 | 16 | 32 | 8 | 16 | 16 | 6.2 |
| 1e | 16 | 32 | 8.1 | 32 | 32 | 32 | 16 | 16 | 32 | 16.2 |
| 1f | 16 | 16 | <3.9 | 16 | 16 | 32 | 8 | 16 | 16 | 7.9 |
| 1g | 8 | 16 | <3.8 | 16 | 16 | 16 | 8 | 8 | 16 | 3.8 |
| 1h | 4 | 8 | <3.7 | 8 | 8 | 8 | 4 | 4 | 8 | 1.9 |
| 1i | 16 | 32 | 203 | 32 | 62.5 | 62.5 | 16 | 32 | 32 | 51 |
| 1j | 16 | 32 | 199 | 16 | 32 | 62.5 | 16 | 32 | 32 | 50 |
| 1k | 8 | 16 | 195 | 16 | 32 | 62.5 | 16 | 32 | 32 | 98 |
| 1l | 4 | 8 | 96 | 16 | 32 | 32 | 16 | 16 | 32 | 382 |
| 1m | 8 | 16 | 15.4 | 16 | 16 | 32 | 8 | 16 | 16 | 7.7 |
| 1n | 4 | 8 | 7.5 | 8 | 8 | 8 | 4 | 8 | 8 | 7.5 |
| 1o | 8 | 16 | 7.3 | 8 | 16 | 16 | 4 | 8 | 8 | 3.6 |
| 1p | 4 | 8 | 7.1 | 8 | 8 | 8 | 4 | 8 | 8 | 3.6 |
| INH | 0.5 | 1 | 36.5 | >250 | >250 | >250 | 2 | 4 | 8 | 29.2 |
| EMB | 1 | 2 | – | – | – | – | 1 | 2 | 2 | – |
| OFLX | 1 | 2 | – | 0.5 | 1 | 1 | 0.5 | 0.5 | 1 | – |
| CPX | – | – | 48.3 | – | – | – | – | – | – | 3.0 |
| Comp. | MIC [μM] | |||
|---|---|---|---|---|
| MA 330/80 | MS | MM | ||
| 1 14-d | 2 21-d | 3 3-d | 21-d | |
| 1a | 125 | 125 | 213 | 106 |
| 1b | 62.5 | 125 | 104 | 52 |
| 1c | 32 | 62.5 | 51 | 25.5 |
| 1d | 32 | 32 | 24.9 | 12.5 |
| 1e | 62.5 | 62.5 | 16.2 | 32.5 |
| 1f | 32 | 32 | 15.8 | <3.9 |
| 1g | 16 | 16 | 15.4 | <3.8 |
| 1h | 8 | 16 | 15.0 | <3.7 |
| 1i | 62.5 | 125 | 203 | 51 |
| 1j | 62.5 | 125 | 6.2 | 50 |
| 1k | 32 | 62.5 | 390 | 98 |
| 1l | 32 | 62.5 | 382 | 191 |
| 1m | 16 | 32 | 30.7 | 15.4 |
| 1n | 16 | 16 | 15.0 | 15.0 |
| 1o | 16 | 16 | 7.3 | 7.3 |
| 1p | 16 | 16 | 7.1 | 7.1 |
| INH | > 250 | > 250 | 117 | 467 |
| EMB | 16 | 16 | – | – |
| OFLX | 32 | 62.5 | – | – |
| CPX | – | – | 0.4 | 0.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malík, I.; Csöllei, J.; Solovič, I.; Pospíšilová, Š.; Michnová, H.; Jampílek, J.; Čížek, A.; Kapustíková, I.; Čurillová, J.; Pecháčová, M.; et al. Dibasic Derivatives of Phenylcarbamic Acid against Mycobacterial Strains: Old Drugs and New Tricks? Molecules 2018, 23, 2493. https://doi.org/10.3390/molecules23102493
Malík I, Csöllei J, Solovič I, Pospíšilová Š, Michnová H, Jampílek J, Čížek A, Kapustíková I, Čurillová J, Pecháčová M, et al. Dibasic Derivatives of Phenylcarbamic Acid against Mycobacterial Strains: Old Drugs and New Tricks? Molecules. 2018; 23(10):2493. https://doi.org/10.3390/molecules23102493
Chicago/Turabian StyleMalík, Ivan, Jozef Csöllei, Ivan Solovič, Šárka Pospíšilová, Hana Michnová, Josef Jampílek, Alois Čížek, Iva Kapustíková, Jana Čurillová, Mária Pecháčová, and et al. 2018. "Dibasic Derivatives of Phenylcarbamic Acid against Mycobacterial Strains: Old Drugs and New Tricks?" Molecules 23, no. 10: 2493. https://doi.org/10.3390/molecules23102493
APA StyleMalík, I., Csöllei, J., Solovič, I., Pospíšilová, Š., Michnová, H., Jampílek, J., Čížek, A., Kapustíková, I., Čurillová, J., Pecháčová, M., Stolaříková, J., Pecher, D., & Oravec, M. (2018). Dibasic Derivatives of Phenylcarbamic Acid against Mycobacterial Strains: Old Drugs and New Tricks? Molecules, 23(10), 2493. https://doi.org/10.3390/molecules23102493