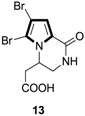
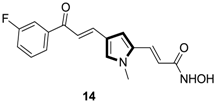
Synthesis of Multi-Substituted Pyrrole Derivatives Through [3+2] Cycloaddition with Tosylmethyl Isocyanides (TosMICs) and Electron-Deficient Compounds
Abstract
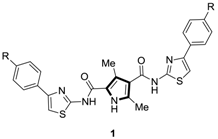
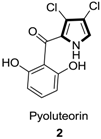
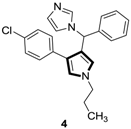
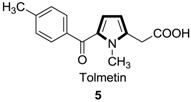
1. Introduction
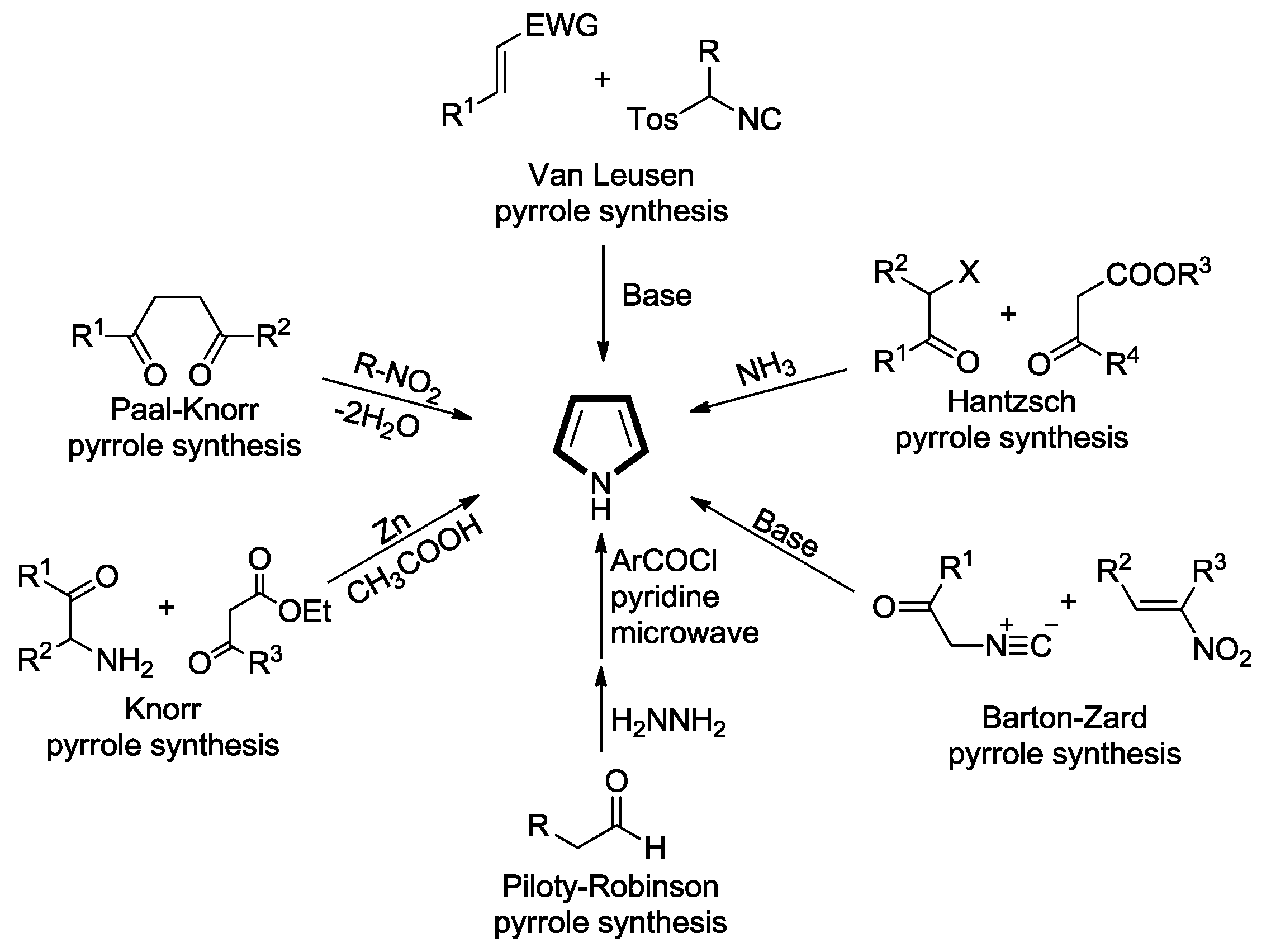
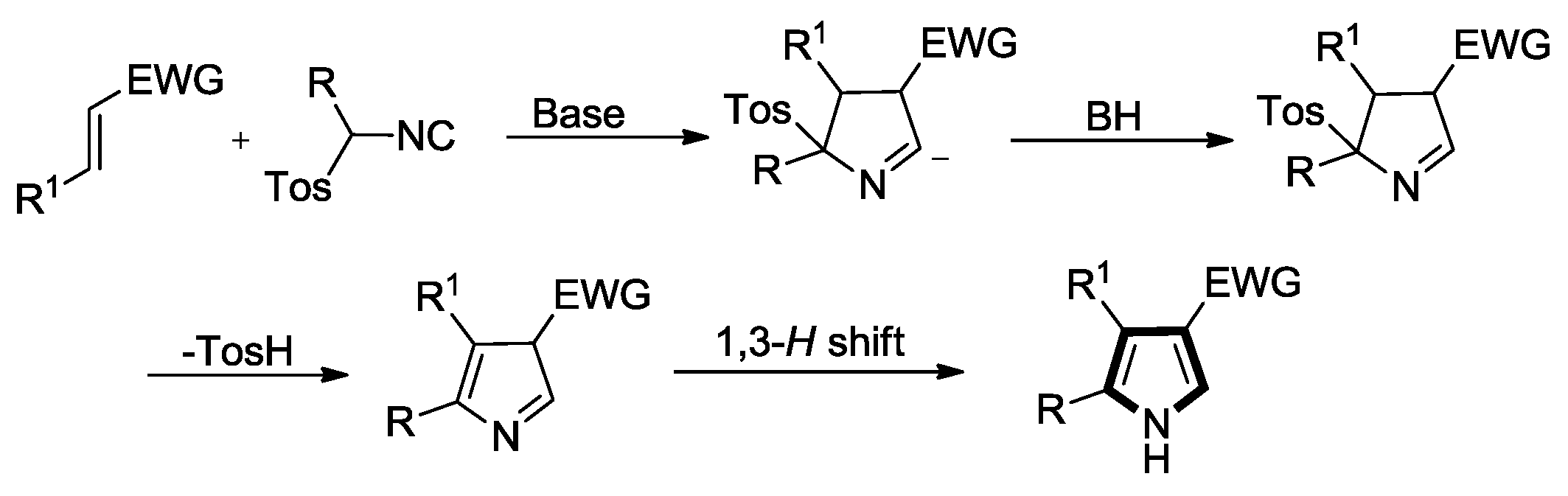
2. Synthesis of Pyrrole Derivatives by [3+2] Cycloaddition of TosMICs with Alkenes
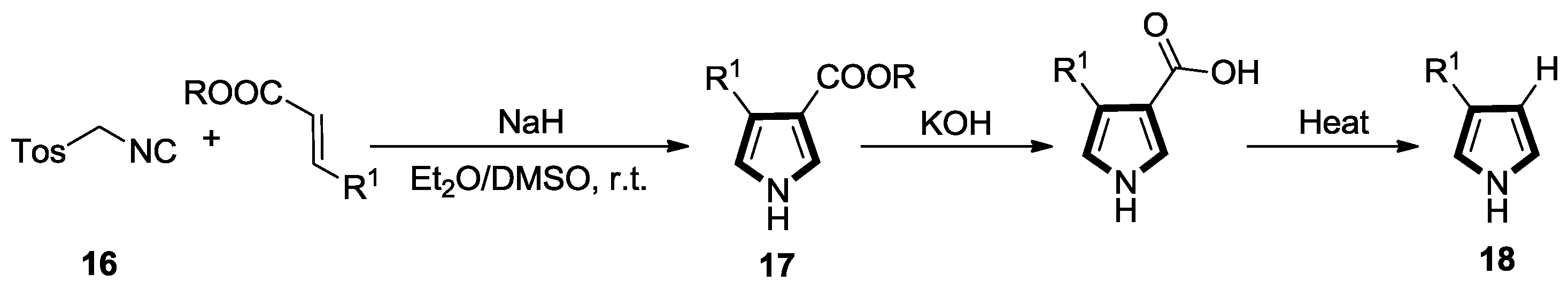
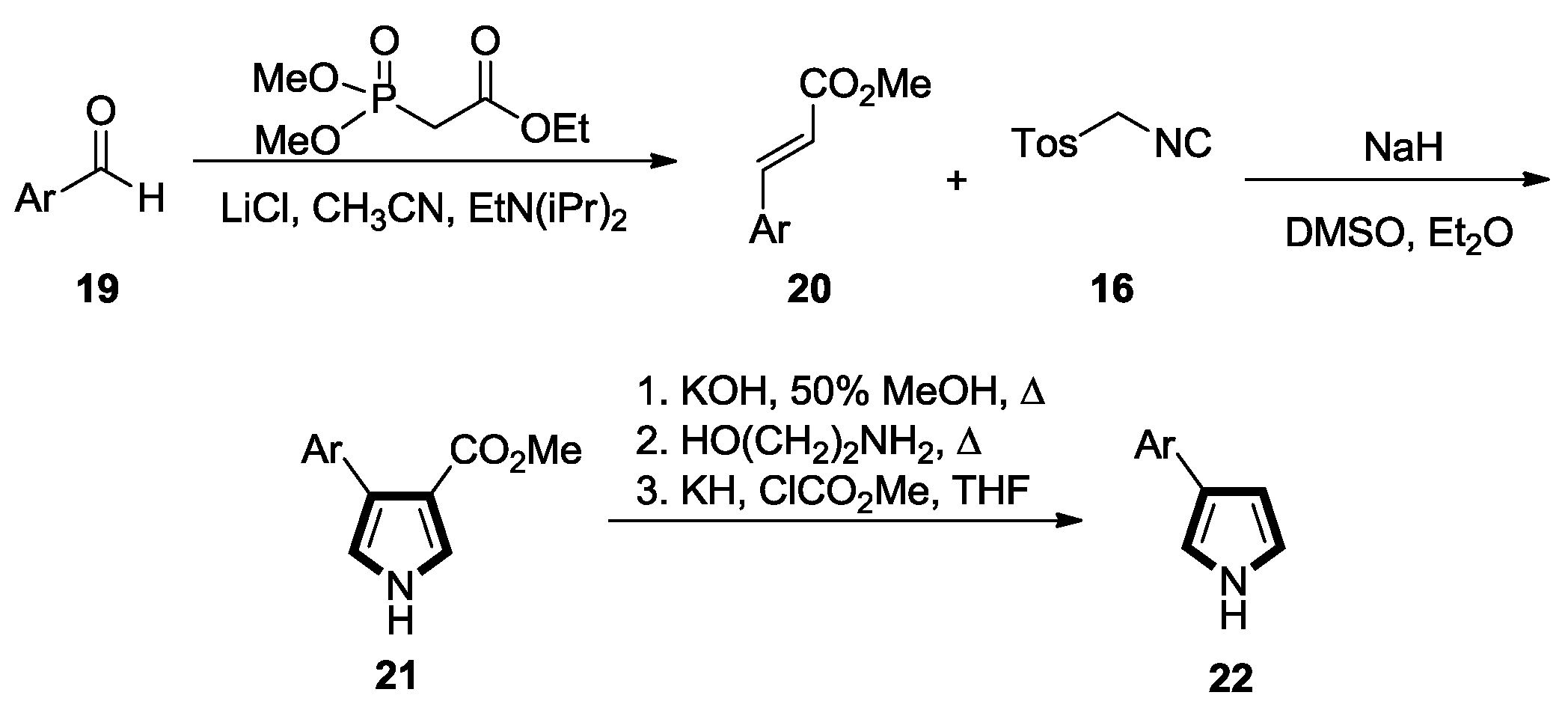
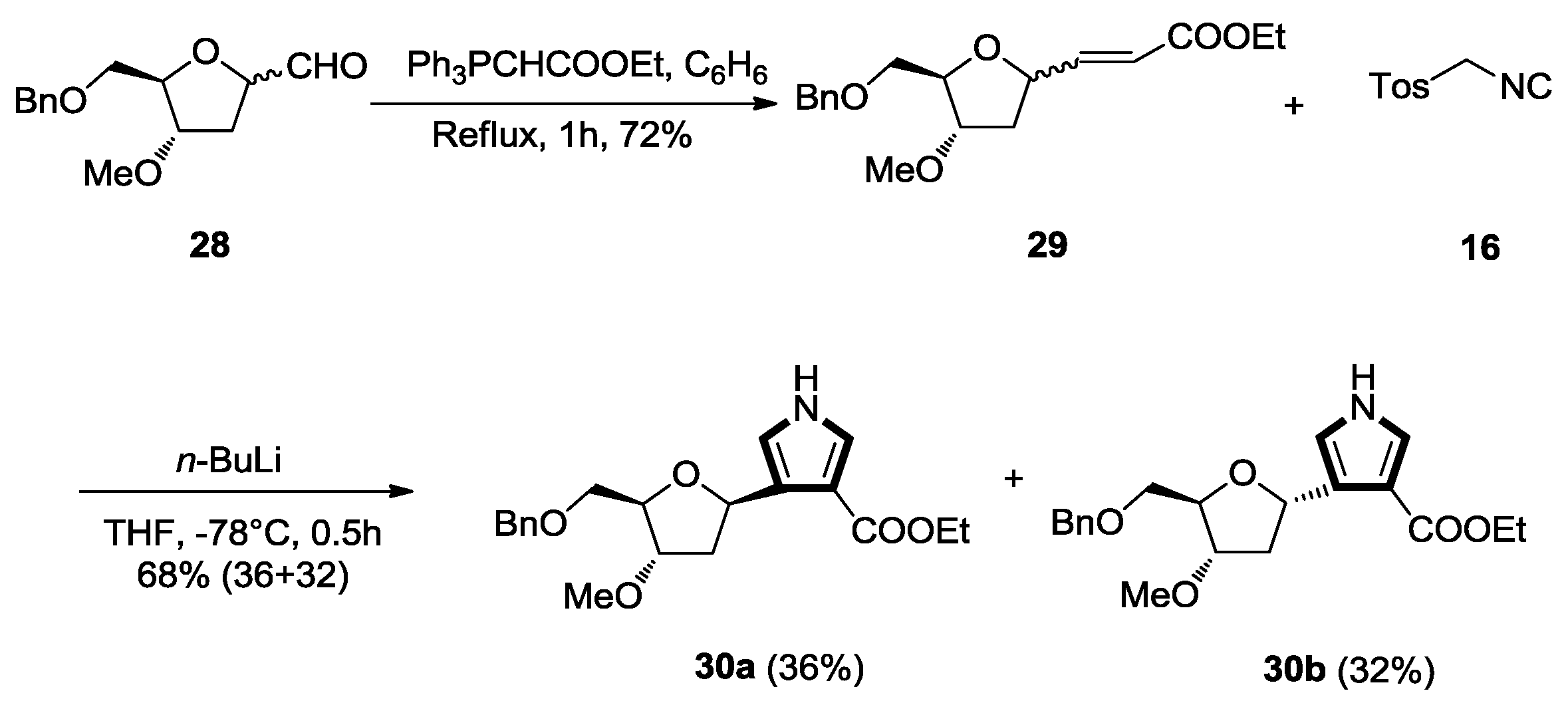
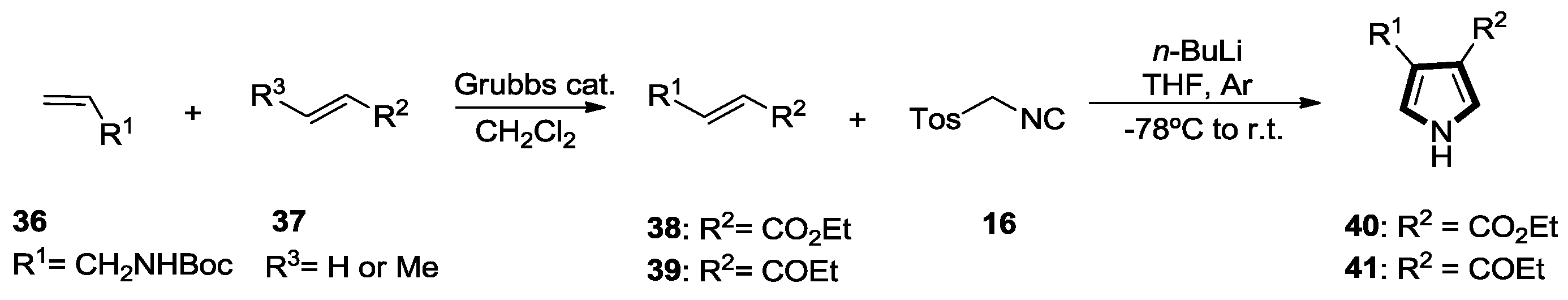
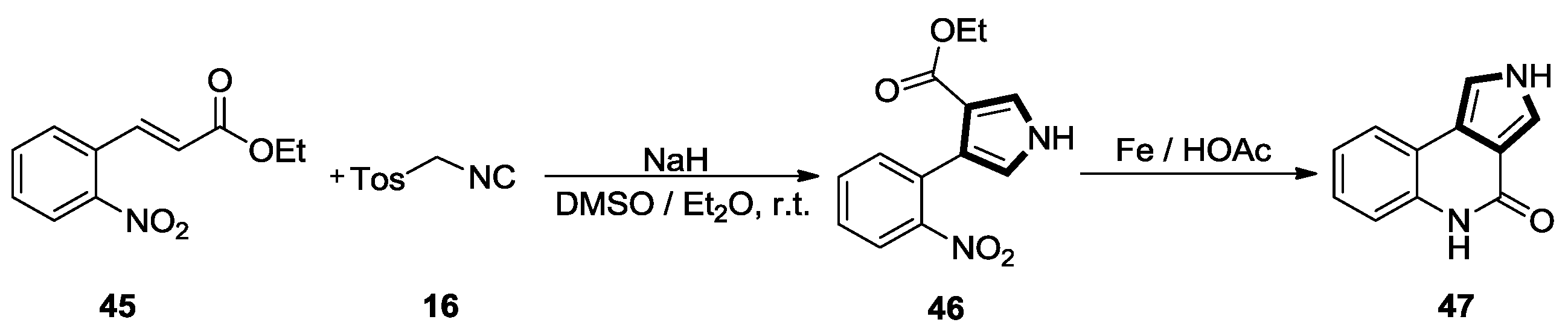
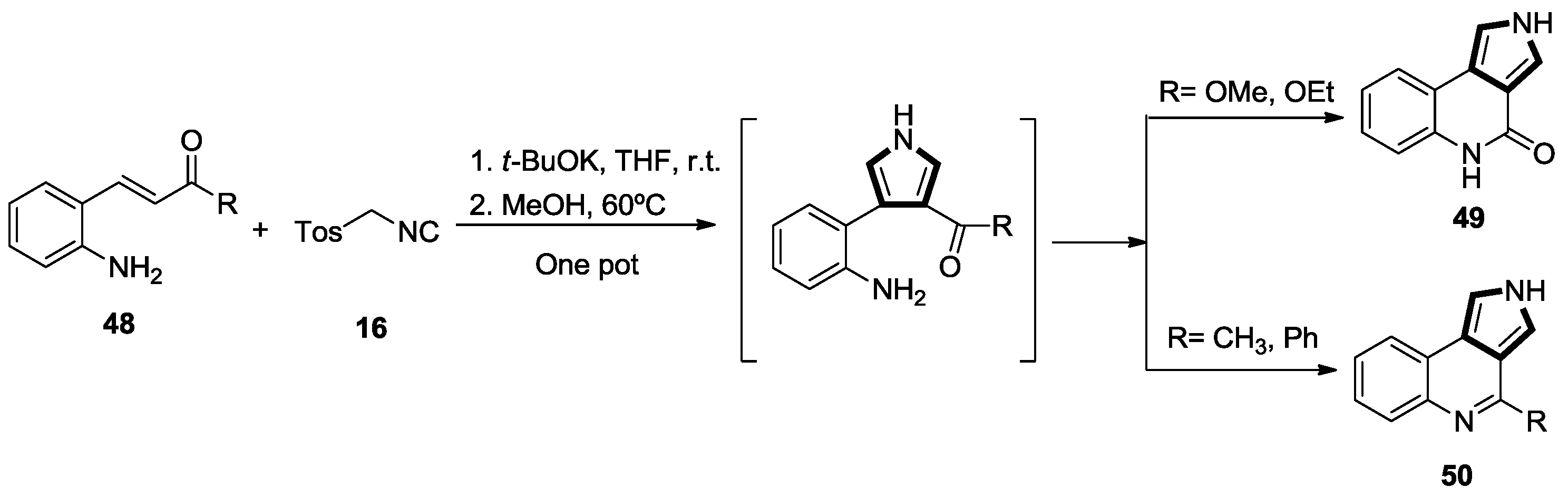
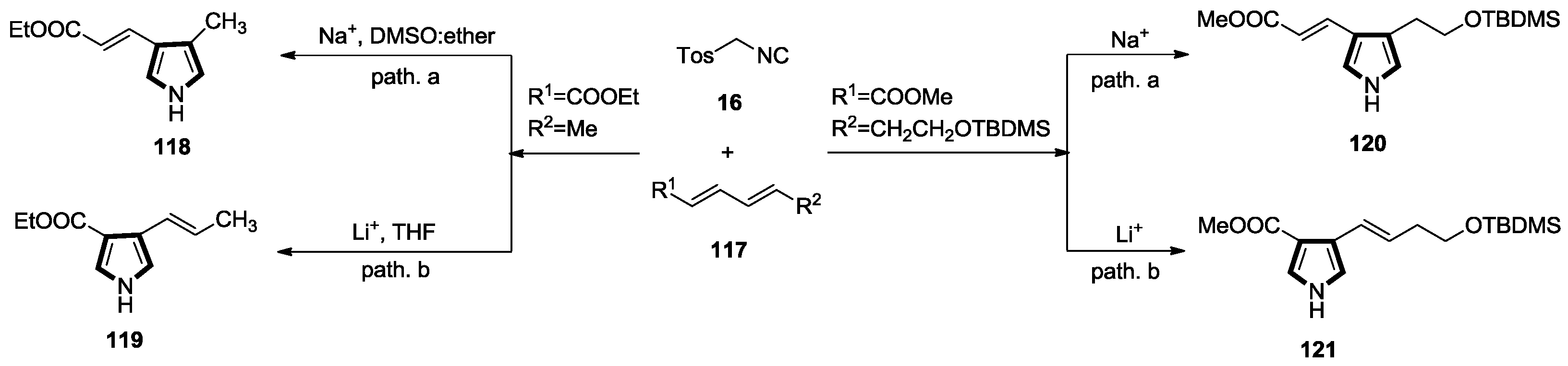
2.1. Alkenes with an Ester Group
2.2. Alkenes with an Amide Group
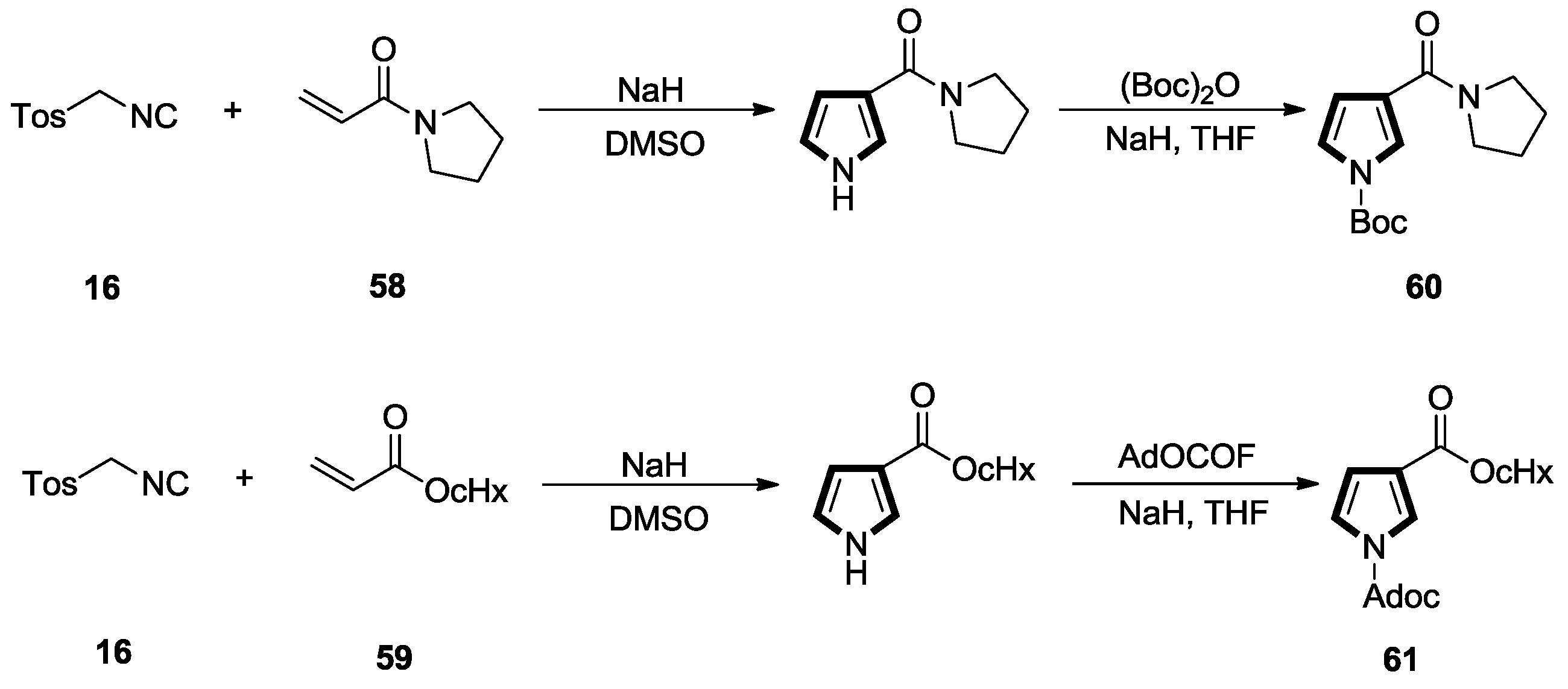
2.3. Alkenes with a Keto Group
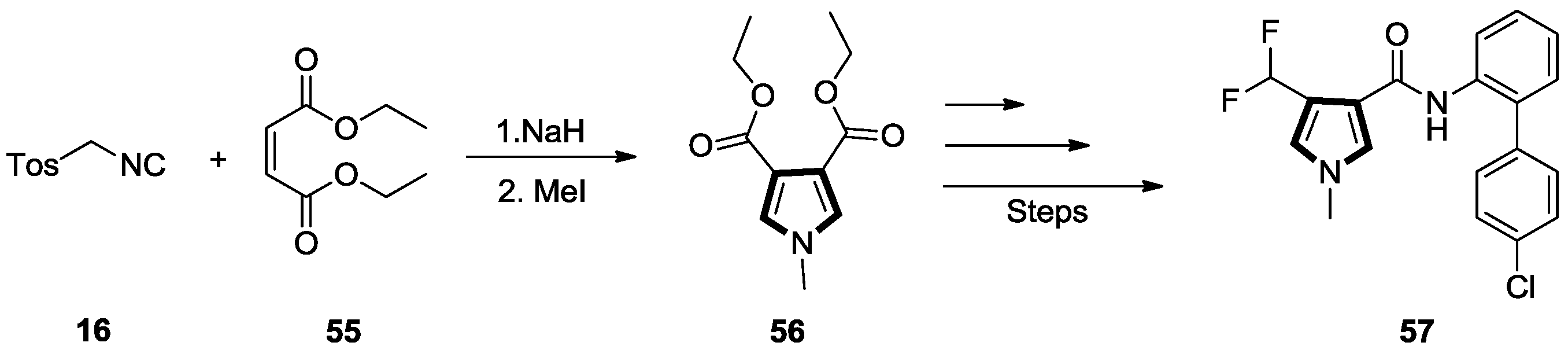
2.4. Alkenes with a Nitro Group
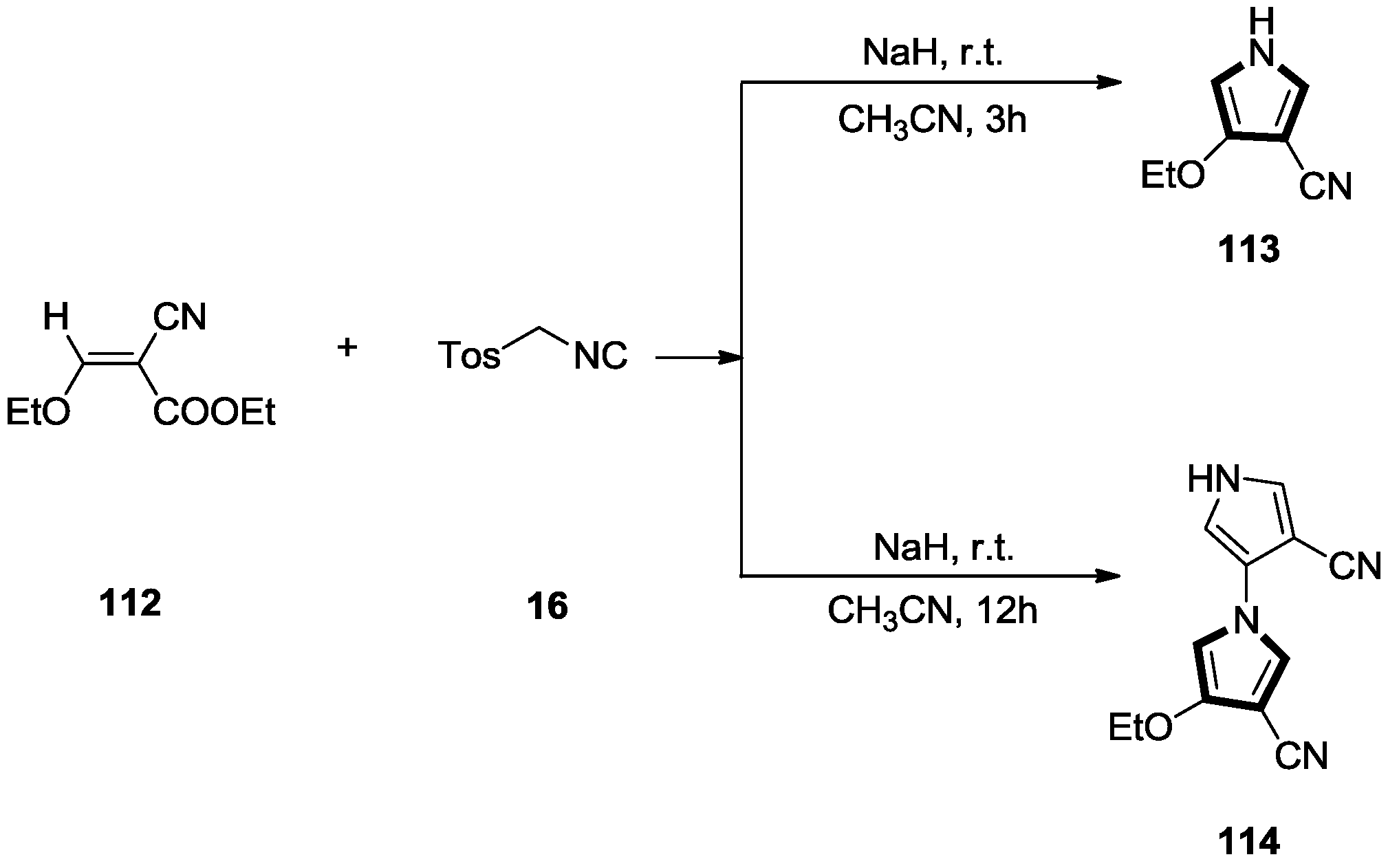
2.5. Alkenes with a Cyano Group
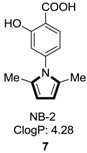
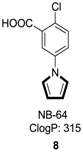
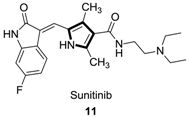
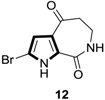
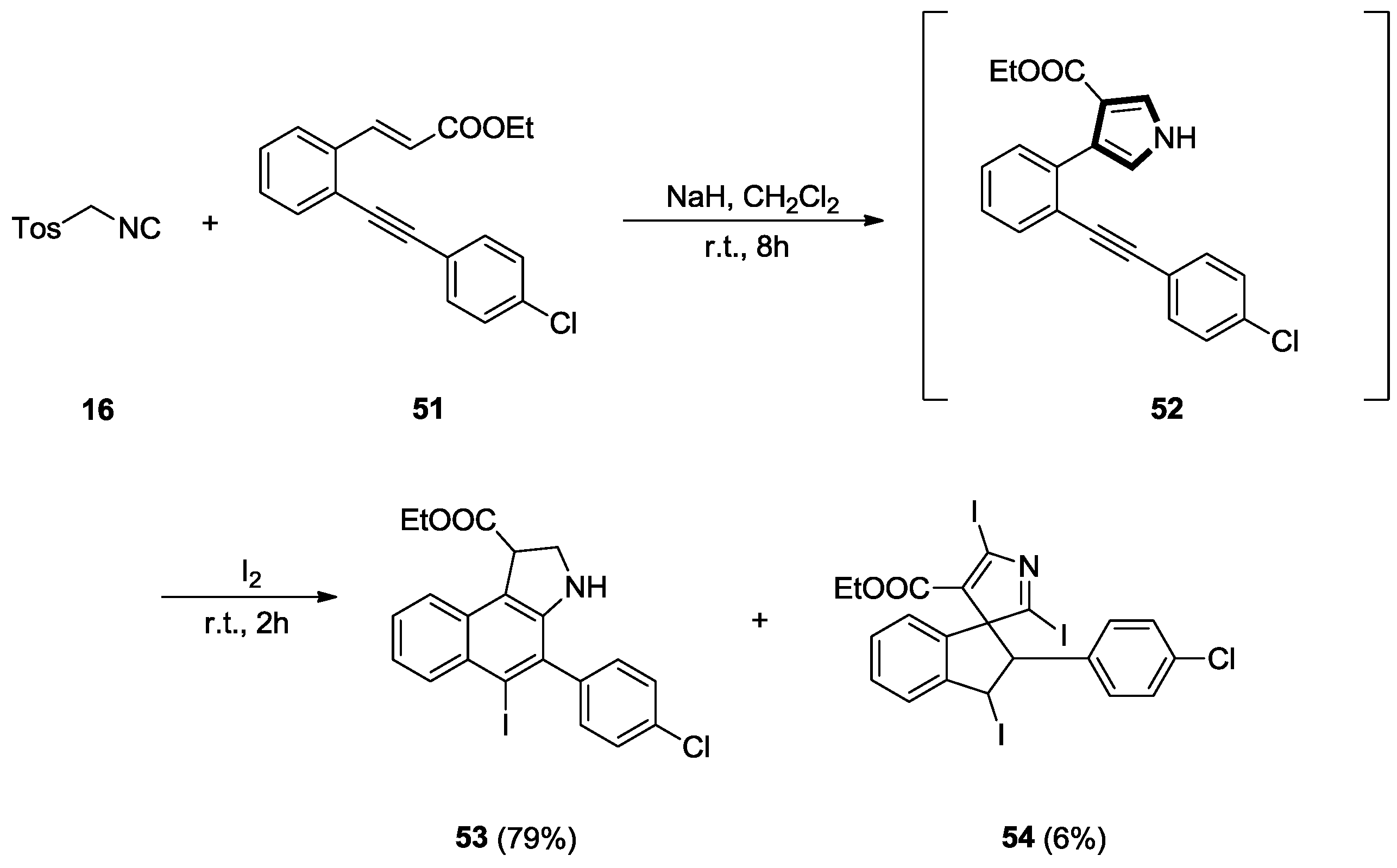
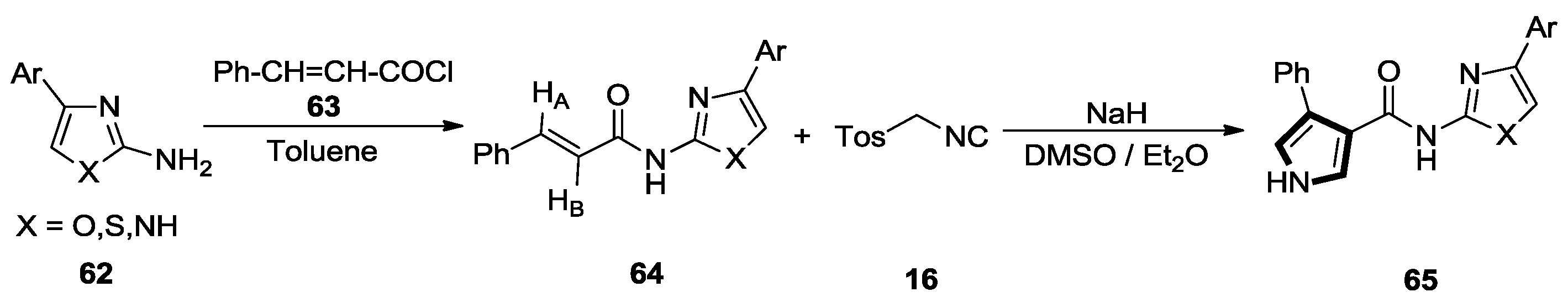
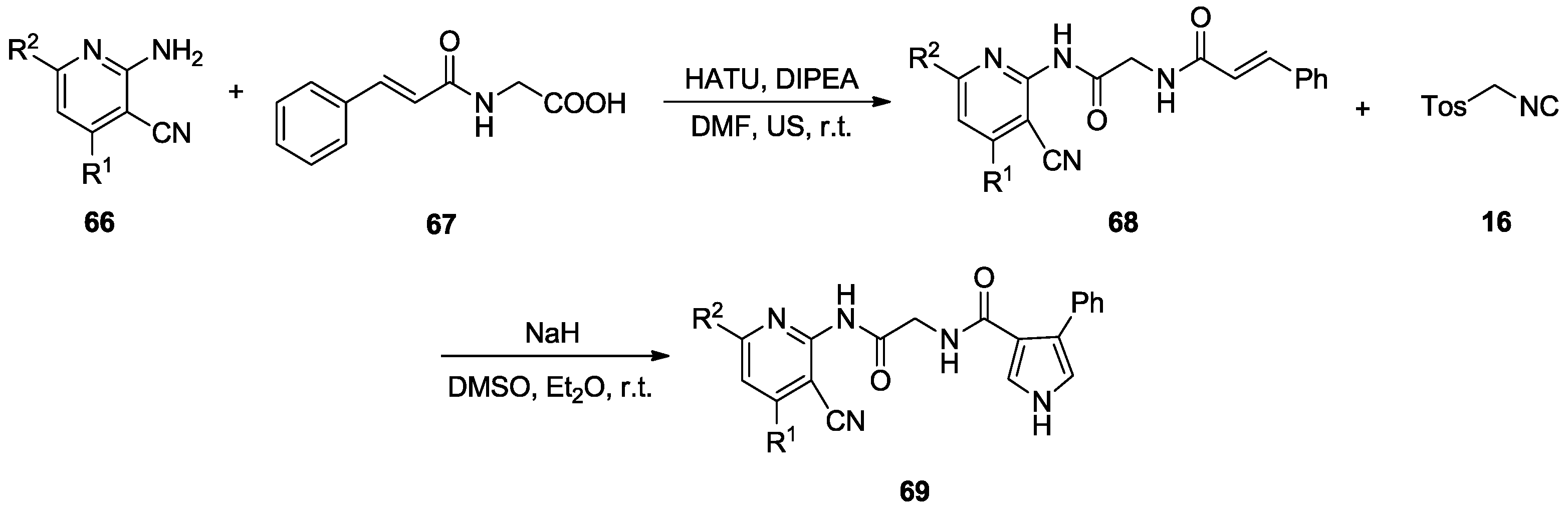
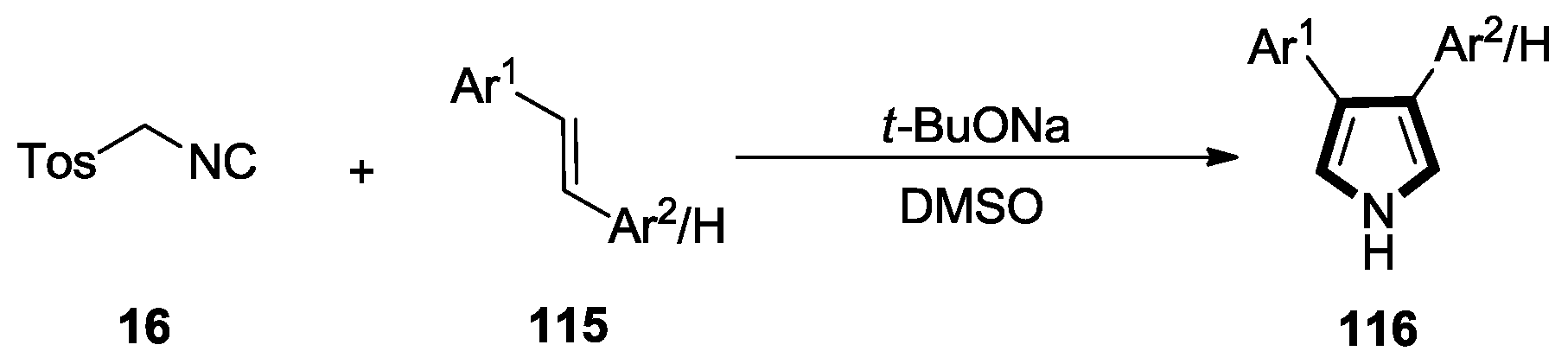
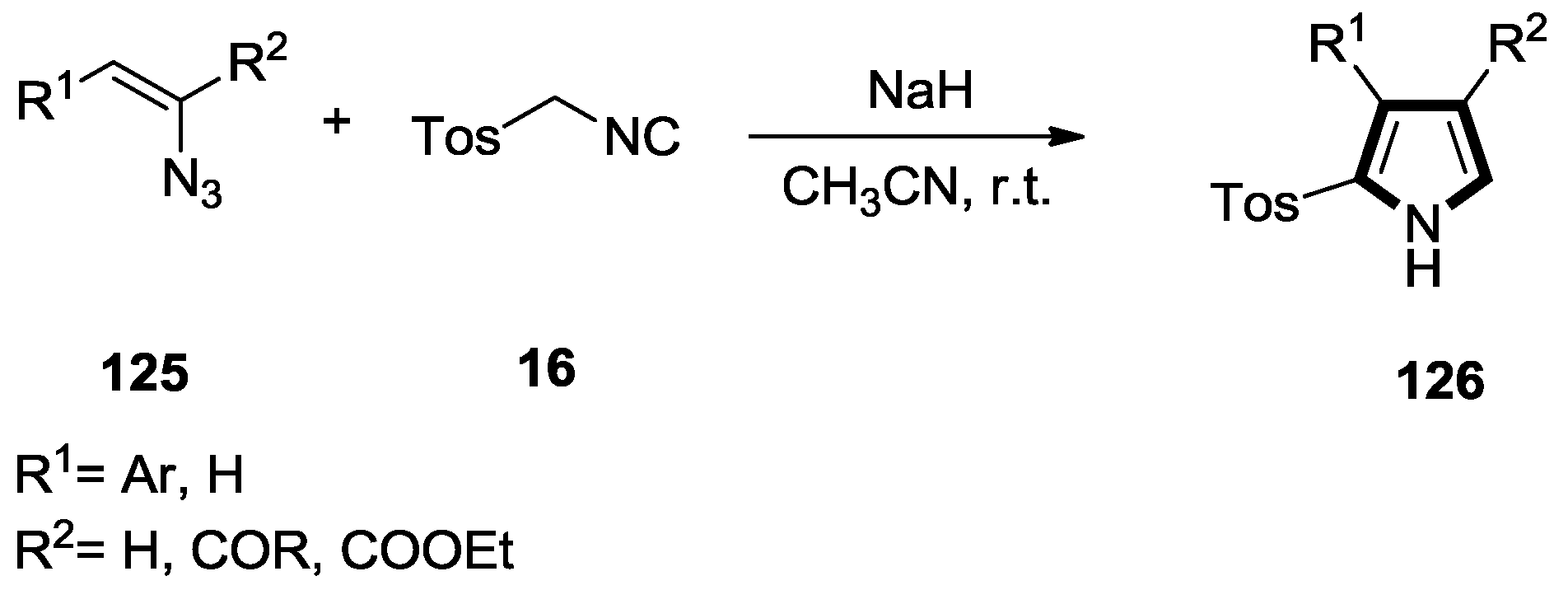
2.6. Alkenes with an Aryl Group
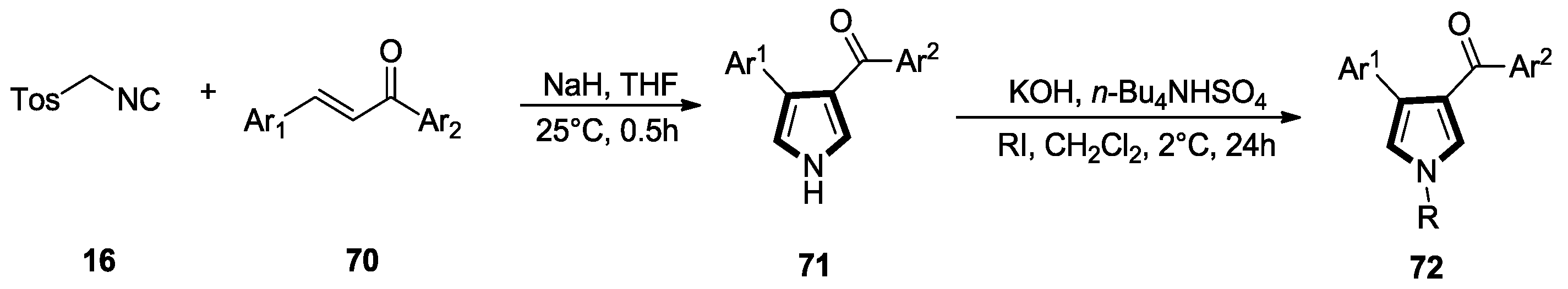
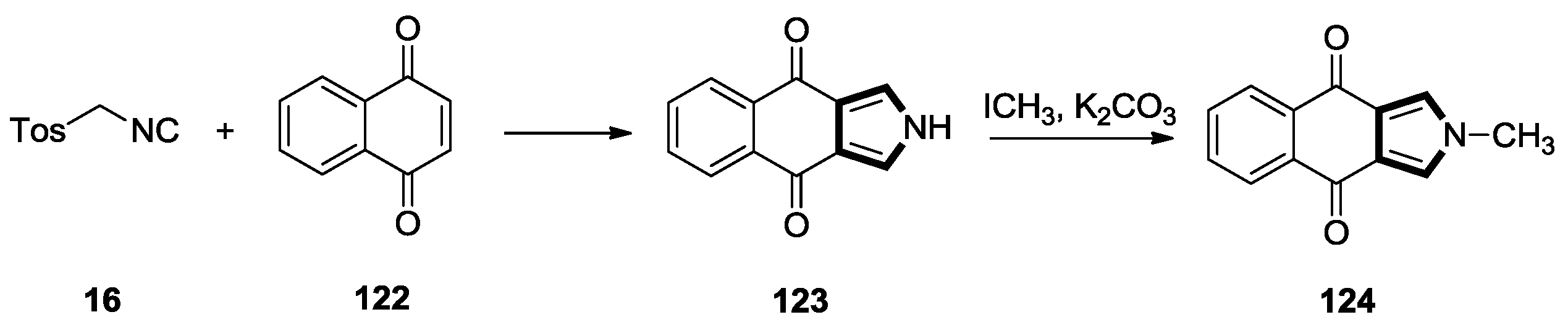
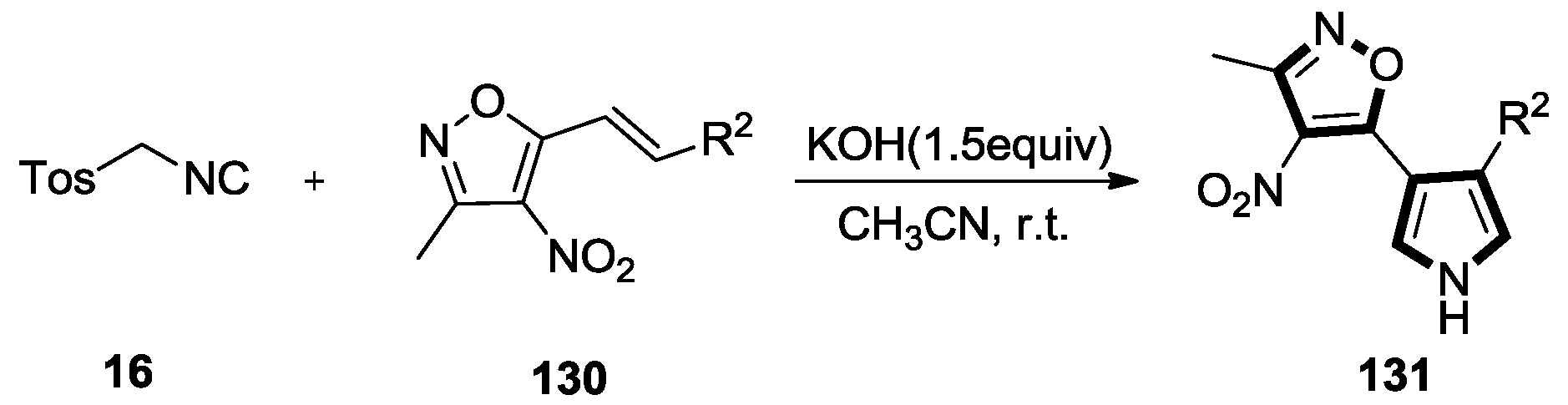
2.7. Other Alkene Synthons
3. Synthesis of Pyrrole Derivatives by [3+2] Cycloaddition of TosMICs with Alkynes
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Idhayadhulla, A.; Kumar, R.S.; Nasser, A.J.A. Synthesis, Characterization and Antimicrobial Activity of New Pyrrole Derivatives. J. Mex. Chem. Soc. 2011, 55, 218–223. [Google Scholar]
- Massa, S.; Artico, M.; Corelli, F.; Mai, A.; Di Santo, R.; Cortes, S.; Marongiu, M.E.; Pani, A.; La Colla, P. Synthesis and Antimicrobial and Cytotoxic Activities of Pyrrole-Containing Analogues of Trichostatin A. J. Med. Chem. 1990, 33, 2845–2849. [Google Scholar] [CrossRef] [PubMed]
- Gordee, R.S.; Matthews, T.R. Systemic Antifungal Activity of Pyrrolnitrini. Appl. Microbiol. 1969, 17, 690–694. [Google Scholar] [PubMed]
- Di Santo, R.; Tafi, A.; Costi, R.; Botta, N.; Artico, M.; Corelli, F.; Forte, M.; Caporuscio, F.; Angiolella, L.; Palamara, A.T. Antifungal Agents. 11. N-Substituted Derivatives of 1-[(Aryl)(4-aryl-1H-pyrrol-3-yl)methyl]-1H-imidazole: Synthesis, Anti-Candida Activity, and QSAR Studies. J. Med. Chem. 2005, 48, 5140–5153. [Google Scholar] [CrossRef] [PubMed]
- Hyneck, M.L.; Smitch, P.C.; Munafo, A.; Mcdonagh, A.F.; Benet, L.Z. Disposition and irreversible plasma protein binding of tolmetin in humans. Clin. Pharmacol. Ther. 1988, 44, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Wilkerson, W.W.; Copeland, R.A.; Covington, M.; Trzaskos, J.M. Antiinflammatory 4,5-Diarylpyrroles. 2. Activity as a Function of Cyclooxygenase-2 Inhibition. J. Med. Chem. 1995, 38, 3895–3901. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.B.; Lu, H.; Liu, S.W.; Zhao, Q.; He, Y.X.; Debnath, A.K. N-Substituted Pyrrole Derivatives as Novel Human Immunodeficiency Virus Type 1 Entry Inhibitors That Interfere with the gp41 Six-Helix Bundle Formation and Block Virus Fusion. Antimicrob. Agents Chemother. 2004, 48, 4349–4359. [Google Scholar] [CrossRef] [PubMed]
- Okanya, P.W.; Mohr, K.I.; Gerth, K.; Jansen, R.; Müller, R. Marinoquinolines A-F, Pyrroloquinolines from Ohtaekwangia kribbensis (Bacteroidetes). J. Nat. Prod. 2011, 74, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, J.; Lee, S.K.; Shin, Y.; Jung, W.; Kim, J.H.; Park, K.; Kim, K.; Cho, H.S.; Ro, S.; et al. A Novel Class of Highly Potent, Selective, and Non-Peptidic Inhibitor of Ras Farnesyltransferase (FTase). Bioorg. Med. Chem. Lett. 2001, 11, 3069–3072. [Google Scholar] [CrossRef]
- Paludetto, M.N.; Bijani, C.; Puisset, F.; Bernardes-Génisson, V.; Arellano, C.; Robert, A. Metalloporphyrin-Catalyzed Oxidation of Sunitinib and Pazopanib, Two Anticancer Tyrosine Kinase Inhibitors: Evidence for New Potentially Toxic Metabolites. J. Med. Chem. 2018, 61, 7849–7860. [Google Scholar] [CrossRef] [PubMed]
- Scala, F.; Fattorusso, E.; Menna, M.; Taglialatela-Scafati, O.; Tierney, M.; Kaiser, M.; Tasdemir, D. Bromopyrrole Alkaloids as Lead Compounds against Protozoan Parasites. Mar. Drugs 2010, 8, 2162–2174. [Google Scholar] [CrossRef] [PubMed]
- Ragno, R.; Simeoni, S.; Rotili, D.; Caroli, A.; Botta, G.; Brosch, G.; Massa, S.; Mai, A. Class II-selective histone deacetylase inhibitors. Part 2: Alignment-independent GRIND 3-D QSAR, homology and docking studies. Eur. J. Med. Chem. 2008, 43, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Di Sanro, R.; Tafi, A.; Costi, R.; Artico, M.; Miele, G.; Lavecchia, A.; Novellino, E.; Bergamini, A.; Cancio, R.; Maga, G. Arylthiopyrrole (AThP) Derivatives as Non-Nucleoside HIV-1 Reverse Transcriptase Inhibitors: Synthesis, Structure-Activity Relationships, and Docking Studies (Part 1). Chem. Med. Chem. 2006, 1, 1367–1378. [Google Scholar] [CrossRef] [PubMed]
- Ono, N.; Okujima, T. Synthesis of Pyrroles and Their Derivatives from Isocyanides. In Isocyanide Chemistry, 1st ed.; Nenajdenko, V.G., Ed.; Wiley-VCH: Weinheim, Germany, 2012; pp. 385–429. [Google Scholar]
- Knorr, L. Synthese von Pyrrolderivaten. Ber. Dtsch. Chem. Ges. 1884, 17, 1635–1642. [Google Scholar]
- Paal, C. Synthese von Thiophen- und Pyrrolderivaten. Ber. Dtsch. Chem. Ges. 1885, 18, 367–371. [Google Scholar] [CrossRef]
- Hantzsch, A. Neue Bildungsweise von Pyrrolderivaten. Ber. Dtsch. Chem. Ges. 1890, 23, 1474–1476. [Google Scholar] [CrossRef]
- Barton, D.H.R.; Kervagoret, J.; Zard, S.Z. A useful synthesis of pyrroles from nitroolefins. Tetrahedron 1990, 46, 7587–7598. [Google Scholar] [CrossRef]
- Van leusen, A.M.; Siderius, H.; Hoogenboom, B.E.; Van Leusen, D. A new and simple synthesis of the pyrrole ring system from Michael acceptors and tosylmethylisocyanides. Tetrahedron Lett. 1972, 13, 5337–5340. [Google Scholar] [CrossRef]
- Milgram, B.C.; Eskildsen, K.; Richter, S.M.; Scheidt, W.R.; Scheidt, K.A. Microwave-Assisted Piloty−Robinson Synthesis of 3,4-Disubstituted Pyrroles. J. Org. Chem. 2007, 72, 3941–3944. [Google Scholar] [CrossRef] [PubMed]
- Moskal, J.; Van Leusen, A.M. A New Synthesis of Indoles by Electrocyclic Ring Closure of Dialkenylpyrroles. Synthesis of Alkenylpyrroles from 1-Tosylalkenyl Isocyanides and Michael Acceptors. J. Org. Chem. 1986, 51, 4131–4139. [Google Scholar] [CrossRef]
- Ten Have, R.; Leusink, F.R.; Van Leusen, A.M. An Efficient Synthesis of Substituted 3(4)-Nitropyrroles from Nitroalkenes and Tosylmethyl Isocyanides. Synthesis 1996, 7, 871–876. [Google Scholar] [CrossRef]
- Tandon, V.K.; Rai, S. p-Toluenesulfonylmethyl isocyanide: A versatile synthon in organic chemistry. Sulfur Rep. 2003, 24, 307–385. [Google Scholar]
- Dijkstra, H.P.; Ten Have, R.; Van Leusen, A.M. A Direct Synthesis of 2-(Trimethylstannyl)pyrroles from Michael Acceptors and Stannylated Tosylmethyl Isocyanide. J. Org. Chem. 1998, 63, 5332–5338. [Google Scholar] [CrossRef]
- Pavri, N.P.; Trudell, M.L. An Efficient Method for the Synthesis of 3-Arylpyrroles. J. Org. Chem. 1997, 62, 2649–2651. [Google Scholar] [CrossRef] [PubMed]
- Di Santo, R.; Costi, R.; Massa, S.; Artico, M. Pyrrole-Annulated Heterocyclic Systems. Synthesis of 2H-Pyrrolo[3,4-b][1,5]benzothiazepine 4,4-Dioxide Derivatives. Synth. Commun. 1998, 28, 2517–2530. [Google Scholar] [CrossRef]
- Krishna, P.R.; Ramana Reddy, V.V.; Srinivas, R. A new synthetic route to oxazole and pyrrole 2-deoxy-C-ribosides. Tetrahedron 2007, 63, 9871–9880. [Google Scholar] [CrossRef]
- Chang, J.H.; Shin, H. Practical One-Pot Syntheses of Ethyl 4-Substituted-1H-Pyrrole-3-Carboxylates from Aldehydes. Org. Process Res. Dev. 2008, 12, 291–293. [Google Scholar] [CrossRef]
- Zhu, R.; Xing, L.X.; Liu, Y.T.; Deng, F.K.; Wang, X.Y.; Hu, Y.F. Practical one-pot sequential procedure for the preparation of N-arylated 3,4-disubstituted pyrroles from alkenes. J. Organomet. Chem. 2008, 693, 3897–3901. [Google Scholar] [CrossRef]
- Poulard, C.; Cornet, J.; Legoupy, S.; Dujardin, G.; Dhal, R.; Huet, F. Synthesis of Polysubstituted Pyrroles. Lett. Org. Chem. 2009, 6, 359–361. [Google Scholar] [CrossRef]
- Sánchez-García, D.; Borrell, J.I.; Nonell, S. One-Pot Synthesis of Substituted 2,2′-Bipyrroles. A Straightforward Route to Aryl Porphycenes. Org. Lett. 2009, 11, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Furlotti, G.; Alisi, M.A.; Apicella, C.; de Joannon, A.C.; Cazzolla, N.; Costi, R.; Crucitti, G.C.; Garrone, B.; Iacovo, A.; Magarò, G.; et al. Discovery and Pharmacological Profile of New 1H-Indazole-3-carboxamide and 2H-Pyrrolo[3,4-c]quinoline Derivatives as Selective Serotonin 4 Receptor Ligands. J. Med. Chem. 2012, 55, 9446–9466. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.M.; Li, J.; Cai, Z.J.; Wang, R.; Wang, S.Y.; Ji, S.J. One pot synthesis of pyrrolo[3,4-c]quinolinone/pyrrolo[3,4-c]quinoline derivatives from 2-aminoarylacrylates/2-aminochalcones and tosylmethyl isocyanide (TosMIC). Org. Biomol. Chem. 2014, 12, 9471–9477. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Feng, C.J.; Jiang, T.; Li, Y.F.; Pan, L.; Xu, X.X. Expedient and Divergent Tandem One-Pot Synthesis of Benz[e]indole and Spiro[indene-1,3′-pyrrole] Derivatives from Alkyne-Tethered Chalcones/Cinnamates and TosMIC. Org. Lett. 2015, 17, 3576–3579. [Google Scholar] [CrossRef] [PubMed]
- Walter, H.; Lamberth, C.; Corsi, C. Synthesis of fungicidally active succinate dehydrogenase inhibitors with novel difluoromethylated heterocyclic acid moieties. Monatshefte Chem. 2018, 149, 791–799. [Google Scholar] [CrossRef]
- Donohoe, T.J.; Guyo, P.M.; Harji, R.R.; Heiliwell, M. The Birch reduction of 3-substituted pyrroles. Tetrahedron Lett. 1998, 39, 3075–3078. [Google Scholar] [CrossRef]
- Padmavathi, V.; Prema kumari, C.; Venkatesh, B.C.; Padmaja, A. Synthesis and antimicrobial activity of amido linked pyrrolyl and pyrazolyl-oxazoles, thiazoles and imidazoles. Eur. J. Med. Chem. 2011, 46, 5317–5326. [Google Scholar] [CrossRef] [PubMed]
- Gudi, Y.; Gundala, S.; Venkatapuram, P.; Adivireddy, P.; Chippada, A.R.; Allagadda, R. Synthesis and Antioxidant Activity of a New Class of Pyridinylcarbamoylmethyl Pyrrolyl/Pyrazolylcarboxamides. J. Heterocycl. Chem. 2017, 54, 3498–3509. [Google Scholar] [CrossRef]
- Dannhardt, G.; Kiefer, W.; Krämer, G.; Maehrlein, S.; Nowe, U.; Fiebich, B. The pyrrole moiety as a template for COX-1/COX-2 inhibitors. Eur. J. Med. Chem. 2000, 35, 499–510. [Google Scholar] [CrossRef]
- Rao, H.S.P.; Sivakumar, S. Aroylketene dithioacetal chemistry: Facile synthesis of 4-aroyl-3-methylsulfanyl-2-tosylpyrroles from aroylketene dithioacetals and TosMIC. Beilstein J. Org. Chem. 2007, 3, 31. [Google Scholar] [PubMed]
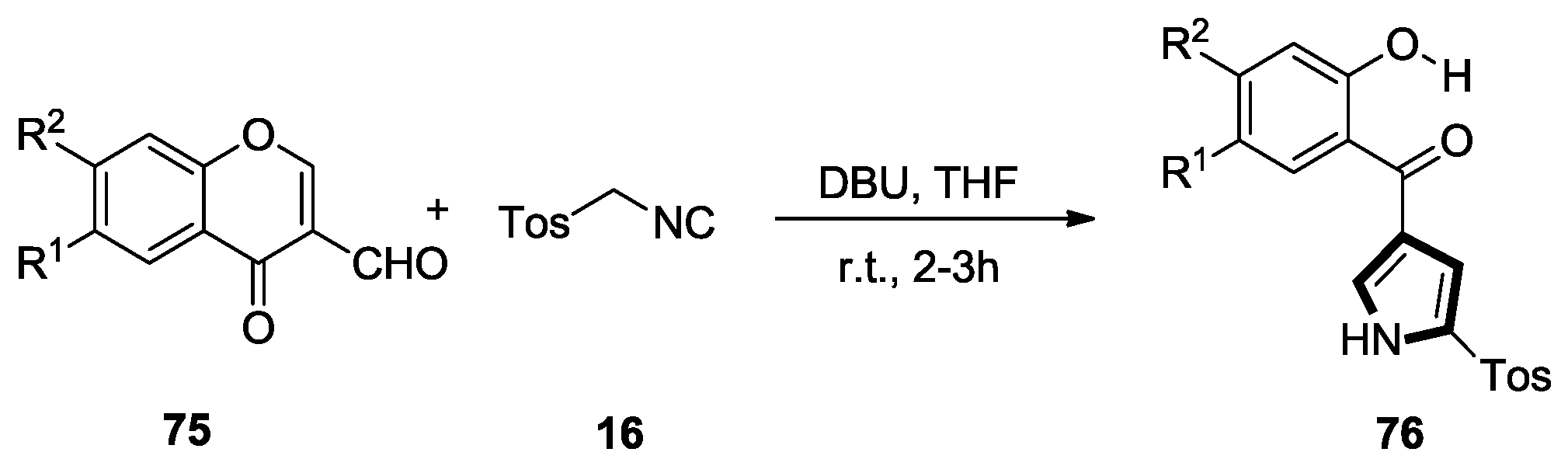
- Terzidis, M.; Tsoleridis, C.A.; Stephanidou-Stephanatou, J. Reaction of chromone-3-carboxaldehydes with TOSMIC: Synthesis of 4-(2-hydroxybenzoyl)pyrroles. Tetrahedron 2007, 63, 7828–7832. [Google Scholar] [CrossRef]
- Hormaza, A.; Pérez, O.F.A. Síntesis de una nueva serie de pirroles vía cicloadición. Rev. Soc. Quím. Perú 2009, 75, 12–16. [Google Scholar]
- Kelly, J.M.; Leeper, F.J. Synthesis of 3,4-fused cycloalkanopyrroles by 1,3-dipolar cycloaddition. Tetrahedron Lett. 2012, 53, 819–821. [Google Scholar] [CrossRef]
- Wang, R.; Wang, S.Y.; Ji, S.J. Chemoselective synthesis of 3H-pyrrolo[2,3-c]quinolin-4(5H)-one derivatives from 3-phenacylideneoxindoles and substituted tosylmethyl isocyanide (TosMIC). Tetrahedron 2013, 69, 10836–10841. [Google Scholar] [CrossRef]
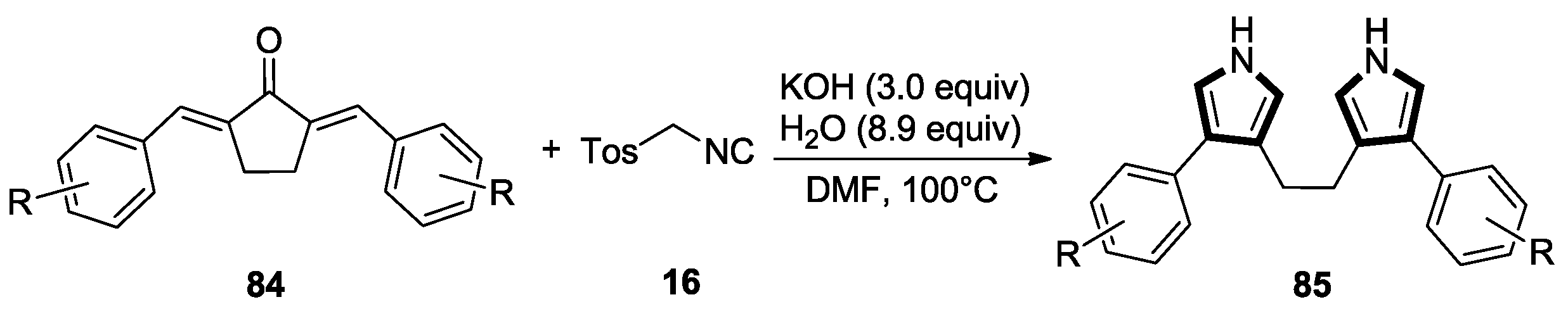
- Wang, R.; Wang, S.Y.; Ji, S.J. Water promoted C–C bond cleavage: Facile synthesis of 3,3-bipyrrole derivatives from dienones and tosylmethyl isocyanide (TosMIC). Org. Biomol. Chem. 2014, 12, 1735–1740. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.J.; Zhang, X.M.; Lu, Z.C.; Zhang, D.W.; Xu, X.X. Accessing benzo[f]indole-4,9-diones via a ring expansion strategy: Silver-catalyzed tandem reaction of tosylmethyl isocyanide (TosMIC) with 2-methyleneindene-1,3-diones. Tetrahedron 2016, 72, 7926–7930. [Google Scholar] [CrossRef]
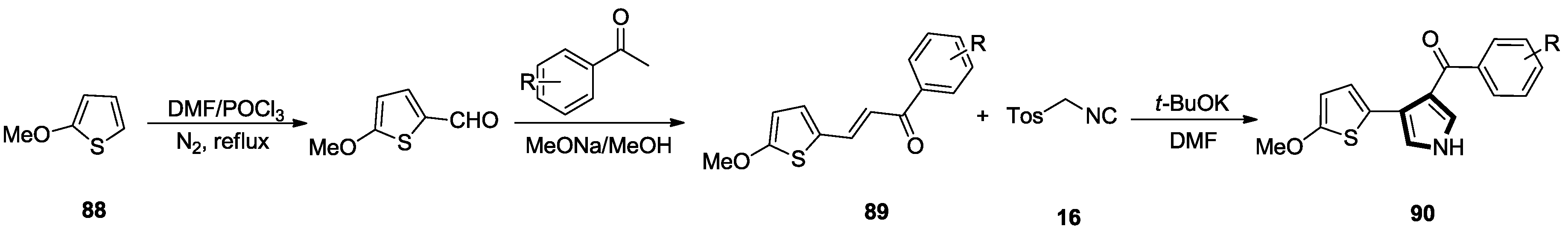
- Zhao, K.; Wang, S.; Zhan, X.Q.; Liu, Z.L.; Mao, Z.M. Synthesis and Anti-tumor Activity of 4-(Methoxyl thienyl)-3-(substituted benzoyl)pyrroles. Chin. J. Org. Chem. 2017, 37, 943–953. [Google Scholar] [CrossRef]
- Divakar, M.A.; Shanmugam, S. Live cell imaging of bacterial cells: Pyrenoylpyrrole-based fluorescence labeling. Chem. Biol. Drug Des. 2017, 90, 554–560. [Google Scholar]
- Sharma, R.; Kumar, K.; Chouhan, M.; Grover, V.; Nair, V.A. Lithium hydroxide mediated synthesis of 3,4-disubstituted pyrroles. RSC Adv. 2013, 3, 14521–14527. [Google Scholar] [CrossRef]
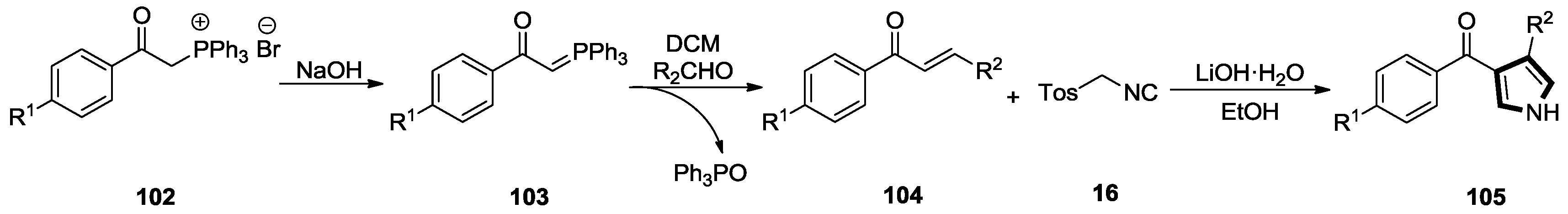
- Kumar, K.; More, S.S.; Goyal, S.; Gangar, M.; Khatik, G.L.; Rawal, R.K.; Nair, V.A. A convenient synthesis of 4-alkyl-3-benzoylpyrroles from α,β-unsaturated ketones and tosylmethyl isocyanide. Tetrahedron Lett. 2016, 57, 2315–2319. [Google Scholar] [CrossRef]
- Dhanalakshmi, P.; Shanmugam, S. Convenient one-pot multicomponent strategy for the synthesis of 6-pyrrolylpyrimidines. RSC Adv. 2014, 4, 29493–29501. [Google Scholar]
- Qin, J.; Zhang, J.; Wu, B.; Zheng, Z.G.; Yang, M.; Yu, X.Q. Efficient and Mild Protocol for the Synthesis of 4(3)-Substituted 3(4)-Nitro-1H-pyrroles and 3-Substituted 4-Methyl-2-tosyl-1H-pyrroles from Nitroolefins and Tosylmethyl Isocyanide in Ionic Liquids. Chin. J. Chem. 2009, 27, 1782–1788. [Google Scholar] [CrossRef]
- Qiu, F.L.; Wu, J.W.; Zhang, Y.H.; Hu, M.; Yu, F.; Zhang, G.L.; Yu, Y.P. A Novel Synthesis of Multisubstituted Pyrroles via Trisubstituted Olefins and TosMIC Derivatives. Lett. Org. Chem. 2012, 9, 305–308. [Google Scholar]
- Qiu, F.L.; Wu, J.W.; Zhang, Y.H.; Hu, M.; Yu, Y.P. One-pot cascade approach to 1,3’-bipyrrole derivatives from trisubstituted olefins with tosylmethyl-isocyanide (TosMIC). Tetrahedron Lett. 2012, 53, 446–448. [Google Scholar] [CrossRef]
- Smith, N.D.; Huang, D.; Cosford, N.D.P. One-Step Synthesis of 3-Aryl- and 3,4-Diaryl-(1H)-Pyrroles Using Tosylmethyl Isocyanide (TOSMIC). Org. Lett. 2002, 4, 3537–3539. [Google Scholar] [CrossRef] [PubMed]
- Magnus, P.; Gallagher, T.; Schultz, J.; Or, Y.S.; Ananthanarayan, T.P. Studies on the synthesis of the antitumor agent CC-1065. Synthesis of the unprotected cyclopropapyrroloindole A portion using the 3,3′-bipyrrole strategy. J. Am. Chem. Soc. 1987, 109, 2706–2711. [Google Scholar] [CrossRef]
- De Leon, C.Y.; Ganem, B. A New Approach to Porphobilinogen and its Analogs. Tetrahedron 1997, 53, 7731–7752. [Google Scholar] [CrossRef]
- Di Santo, R.; Costi, R.; Massa, S.; Artico, M. Synthesis of Pyrrolo [3,4-c][1] benzazepine-4,10(2H, 5H)-dione, a Model System Useful for the Design of 11-Oxosibiromycinone Analogues. Synth. Commun. 1996, 26, 1839–1847. [Google Scholar] [CrossRef]
- Chen, W.T.; Shao, J.A.; Li, Z.; Giulianotti, M.A.; Yu, Y.P. Synthesis of 2,3,4-trisubstituted pyrroles via a facile reaction of vinyl azides and tosylmethyl isocyanide. Can. J. Chem. 2012, 90, 214–221. [Google Scholar] [CrossRef]
- Zhang, X.M.; Xu, X.X.; Zhang, D.W. [3+2] Cycloaddition of Tosylmethyl Isocyanide with Styrylisoxazoles: Facile Access to Polysubstituted (isoxazol-5-yl)pyrroles. Molecules 2017, 22, 1131. [Google Scholar] [CrossRef] [PubMed]
- Saikachi, H.; Kitagawa, T.; Sasaki, H. Reaction of Tosylmethyl Isocyanide with Methyl 3-Substituted Propiolates as Acetylenic Michael Acceptors. Chem. Pharm. Bull. 1979, 27, 2857–2861. [Google Scholar] [CrossRef]
- Alizadeh, A.; Masrouri, H.; Rostamnia, S.; Movahedi, F. One-Step Synthesis of Dialkyl 2-[(4-Methylphenyl)sulfonyl]-1H-pyrrole-3,4-dicarboxylates by Reaction of Acetylenedicarboxylates with ‘Tosylmethyl Isocyanide’ (TsMIC) and Triphenylphosphine. Helv. Chim. Acta 2006, 89, 923–926. [Google Scholar] [CrossRef]
- Adib, M.; Mohammadi, B.; Sheikhi, E.; Bijanzadeh, H.R. 1-Methylimidazole-catalyzed reaction between tosylmethyl isocyanide and dialkyl acetylenedicarboxylates: An efficient synthesis of functionalized pyrroles. Chin. Chem. Lett. 2011, 22, 314–317. [Google Scholar] [CrossRef]




















© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Z.; Ma, Z.; Zhang, D. Synthesis of Multi-Substituted Pyrrole Derivatives Through [3+2] Cycloaddition with Tosylmethyl Isocyanides (TosMICs) and Electron-Deficient Compounds. Molecules 2018, 23, 2666. https://doi.org/10.3390/molecules23102666
Ma Z, Ma Z, Zhang D. Synthesis of Multi-Substituted Pyrrole Derivatives Through [3+2] Cycloaddition with Tosylmethyl Isocyanides (TosMICs) and Electron-Deficient Compounds. Molecules. 2018; 23(10):2666. https://doi.org/10.3390/molecules23102666
Chicago/Turabian StyleMa, Zhengning, Zicheng Ma, and Dawei Zhang. 2018. "Synthesis of Multi-Substituted Pyrrole Derivatives Through [3+2] Cycloaddition with Tosylmethyl Isocyanides (TosMICs) and Electron-Deficient Compounds" Molecules 23, no. 10: 2666. https://doi.org/10.3390/molecules23102666
APA StyleMa, Z., Ma, Z., & Zhang, D. (2018). Synthesis of Multi-Substituted Pyrrole Derivatives Through [3+2] Cycloaddition with Tosylmethyl Isocyanides (TosMICs) and Electron-Deficient Compounds. Molecules, 23(10), 2666. https://doi.org/10.3390/molecules23102666