Medicinal Plants Used for the Traditional Management of Diabetes in the Eastern Cape, South Africa: Pharmacology and Toxicology
Abstract
:1. Introduction
1.1. Aetiology of Diabetes Mellitus
1.2. Prevalence
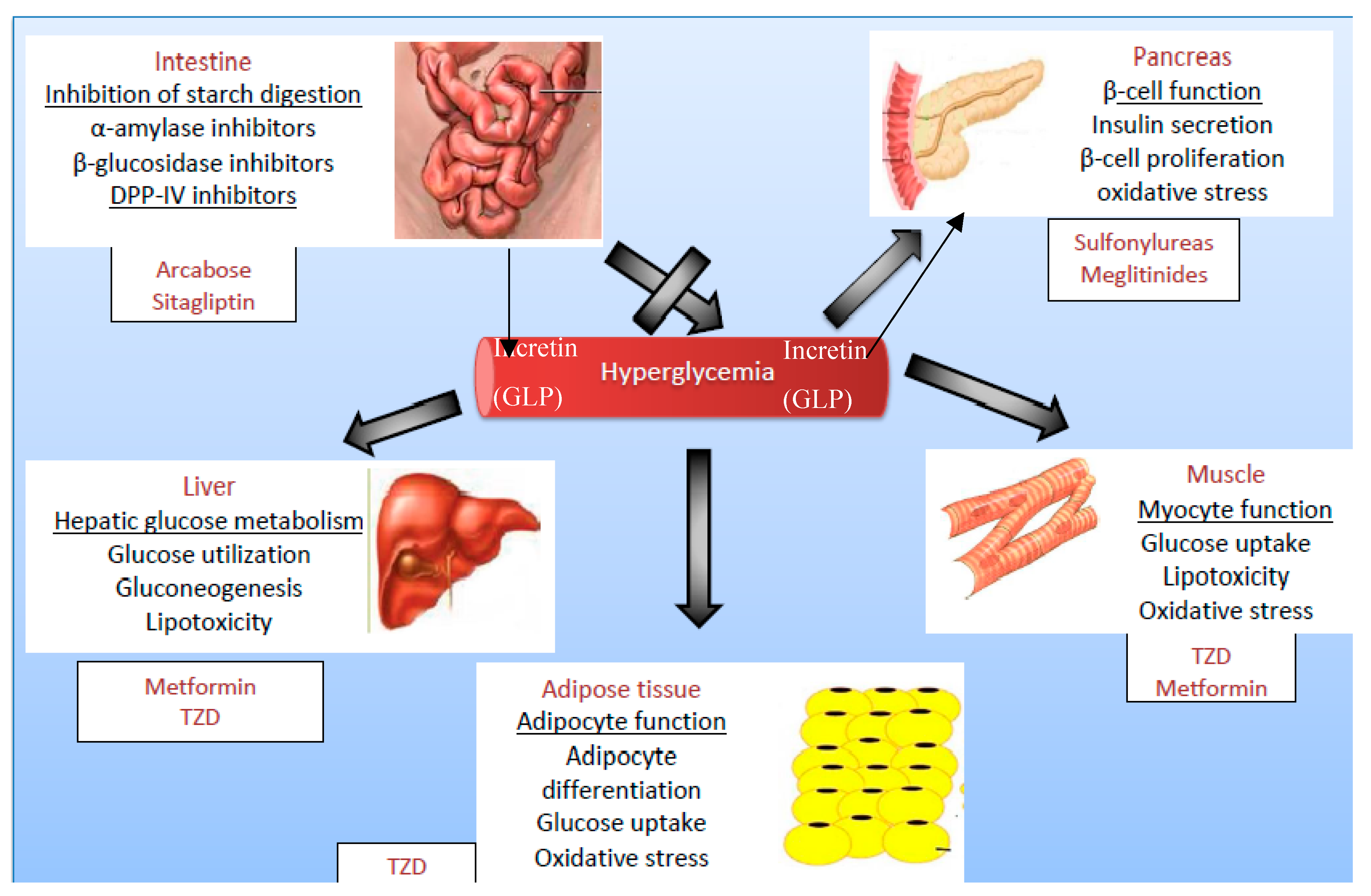
1.3. Target Organs in Diabetes Treatment
1.4. Ethnopharmacological Data
2. Results and Discussion
2.1. Ethno-Pharmacological Details of Plant Families with Documented Anti-Diabetic Activities
2.1.1. Alliaceae (Three)
2.1.2. Aloaceae (One)
2.1.3. Anacardiaceae (One)
2.1.4. Apiaceae (One)
2.1.5. Apocynaceae (Two)
2.1.6. Asphodelaceae (Five)
2.1.7. Asteraceae (Thirteen)
2.1.8. Buddlejaceae (One)
2.1.9. Cannabaceae (One)
2.1.10. Caryophyllaceae (One)
2.1.11. Celastraceae (Three)
2.1.12. Cucurbitaceae (Two)
2.1.13. Ebenaceae (One)
2.1.14. Fabaceae (One)
2.1.15. Gentianaceae (One)
2.1.16. Hyacinthaceae (Two)
2.1.17. Hypoxidaceae (Two)
2.1.18. Lamiaceae (One)
2.1.19. Loganiaceae (One)
2.1.20. Myrtaceae (One)
2.1.21. Menispermaceae (One)
2.1.22. Portulaceae (One)
2.1.23. Rutaceae (One)
2.1.24. Solanaceae (One)
2.1.25. Xanthorrhoeaceae (One)
2.2. Pharmacological Evidence
2.2.1. Bioactive Molecules
Phenolic Molecules
Terpenes
Saponins
Alkaloids
Hydroxylated Molecules Including Sugars
2.2.2. In Vitro Investigation of Hypoglycaemic Activity
2.2.3. In Vivo Investigation of Hypoglycaemic Activity
2.2.4. Dosages
2.3. Toxicological Evidence
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wadkar, K.; Magdum, C.; Patil, S.; Naikwade, N. Anti-diabetic potential and Indian medicinal plants. J. Herb. Med. Toxicol. 2008, 2, 45–50. [Google Scholar]
- Krishnakumar, K.; Augustii, K.; Vijayammal, P. Hypoglycaemic and anti-oxidant activity of salacia oblonga wall extract in streptozotocin-induced diabetic rats. Indian J. Physiol. Pharmacol. 1999, 43, 510–514. [Google Scholar] [PubMed]
- Ross, R. Atherosclerosis-an inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.L. Diabetes and dyslipidaemia. Diabetes Obes. Metab. 2006, 8, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Felig, P.; Marliss, E.; Ohman, J.L.; Cahill, C.F. Plasma amino acid levels in diabetic ketoacidosis. Diabetes 1970, 19, 727–728. [Google Scholar] [CrossRef] [PubMed]
- Knentz, A.; Nattras, M. Diabetic ketoacidosis, non-ketotic hyperosmolar coma and lactic acidosis. In Diabetes, 2nd ed.; Pickup, J.G.W., Ed.; Blackwell Science: Hoboken, NJ, USA, 1991. [Google Scholar]
- Kumar, P.; Clark, M. Clinical Medicine; Saunders: London, UK, 2002. [Google Scholar]
- Ge, K.; Niu, Y.; Xie, T.; Lin, W.; Tian, M.; Xu, B.; Cui, S.; Lu, S. Influence of advanced glycosylation end products on wound healing of burn rats with diabetes. Zhonghua Shao Shang Za Zhi 2009, 25, 433–436. [Google Scholar] [PubMed]
- Perkins, R.M.; Yuan, C.M.; Welch, P.G. Dipsogenic diabetes insipidus: Report of a novel treatment strategy and literature review. Clin. Exp. Nephrol. 2006, 10, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- IDF The global picture. In IDF Diabetes Atlas; Karuranga, S.; da Rocha Fernandes, J.; Huang, Y.; Malanda, B. (Eds.) International Diabetes Federation: Brussels, Belgium, 2017; pp. 40–65. ISBN 978-2-930229-87-4. [Google Scholar]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980: A pooled analysis of 751 population-based studies with 4·4 million participants. Lancet 2016, 387, 1513–1530. [Google Scholar] [CrossRef]
- Mbanya, J.C.; Boniface, F.; Nagan, K. Guidelines for the Management of NIDDM in Africa; A consensus document; Novo Nordisk A/S: Bagsværd, Denmark, 1996; pp. 1–35. [Google Scholar]
- Wilkinson, K. FACTSHEET: South Africa’s official poverty numbers. Africa Check, 2018. Available online: https://africacheck.org/factsheets/factsheet-south-africas-official-poverty-numbers/ (accessed on 7 August 2018).
- Erasto, P.; Adebola, P.; Grierson, D.; Afolayan, A.J. An ethnobotanical study of plants used for the treatment of diabetes in the Eastern Cape Province, South Africa. Afr. J. Biotechnol. 2005, 4, 1458–1460. [Google Scholar]
- Statistics South Africa. Mortality and Causes of Death in South Africa, 2016: Findings from Death Notification; P0309 Statistical Release; Statistics South Africa: Pretoria, South Africa, 2018.
- Bailey, C.J. Metformin: A historical overview. Diabetologia 2017, 60, 1566–1576. [Google Scholar] [CrossRef] [PubMed]
- Piédrola, G.; Novo, E.; Escobar, F.; García-Robles, R. White blood cell count and insulin resistance in patients with coronary artery disease. Ann. Endocrinol. (Paris) 2001, 62, 7–10. [Google Scholar] [PubMed]
- Calixto, J. Efficacy, safety, quality control, marketing and regulatory guidelines for herbal medicines (phytotherapeutic agents). Braz. J. Med. Biol. Res. 2000, 33, 179–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. WHO Traditional Medicine strategy. World Health Organ. 2002, 80, 610. [Google Scholar]
- Marles, R.J.; Farnsworth, N.R. Antidiabetic plants and their active constituents. Phytomedicine 1995, 2, 137–189. [Google Scholar] [CrossRef]
- Perez, G.R.M.; Zavala, S.M.A.; Perez, G.S.; Perez, G.C. Antidiabetic effect of compounds isolated from plants. Phytomedicine 1998, 5, 55–75. [Google Scholar] [CrossRef]
- Van Huyssteen, M.; Milne, P.J.; Campbell, E.E.; van de Venter, M. Antidiabetic and cytotoxicity screening of five medicinal plants used by traditional african health practitioners in the Nelson Mandela Metropole, South Africa. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 150–158. [Google Scholar] [PubMed]
- Afolayan, A.J.; Sunmonu, T.O. In vivo Studies on Antidiabetic Plants Used in South African Herbal Medicine. J. Clin. Biochem. Nutr. 2010, 47, 98–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oyedemi, S.; Bradley, G.; Afolayan, A.J. Ethnobotanical survey of medicinal plants used for the management of diabetes mellitus in the Nkonkobe municipality of South Africa. J. Med. Plants Res. 2009, 3, 1040–1044. [Google Scholar]
- Van de Venter, M.; Roux, S.; Bungu, L.C.; Louw, J.; Crouch, N.R.; Grace, O.M.; Maharaj, V.; Pillay, P.; Sewnarian, P.; Bhagwandin, N.; et al. Antidiabetic screening and scoring of 11 plants traditionally used in South Africa. J. Ethnopharmacol. 2008, 119, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Thring, T.S.A.; Weitz, F.M. Medicinal plant use in the Bredasdorp/Elim region of the Southern Overberg in the Western Cape Province of South Africa. J. Ethnopharmacol. 2006, 103, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Van Wyk, B.E.; de Wet, H.; Van Heerden, F.R. An ethnobotanical survey of medicinal plants in the southeastern Karoo, South Africa. S. Afr. J. Bot. 2008, 74, 696–704. [Google Scholar] [CrossRef]
- Deutschländer, M.S.S.; van de Venter, M.; Roux, S.; Louw, J.; Lall, N. Hypoglycaemic activity of four plant extracts traditionally used in South Africa for diabetes. J. Ethnopharmacol. 2009, 124, 619–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davison, C.; Frost, C.; Levendal, R.-A. A Biochemical Study of the Antidiabetic and Anticoagulant Effects of Tulbaghia violacea. Master’s Thsis, Nelson Mandela Metropolitan University, Port Elizabeth, South Africa, 2010. [Google Scholar]
- Aqil, F.; Ahmad, I.; Mehmood, Z. Antioxidant and Free Radical Scavenging Properties of Twelve Traditionally Used Indian Medicinal Plants. Turk. J. Biol. 2006, 30, 177–183. [Google Scholar]
- Ashraf, R.; Khan, R.A.; Ashraf, I. Garlic (Allium sativum) supplementation with standard antidiabetic agent provides better diabetic control in type 2 diabetes patients. Pak. J. Pharm. Sci. 2011, 24, 565–570. [Google Scholar] [PubMed]
- Choi, D.-J.; Lee, S.-J.; Kang, M.-J.; Cho, H.-S.; Sung, N.-J.; Shin, J.-H. Physicochemical Characteristics of Black Garlic (Allium sativum L.). J. Korean Soc. Food Sci. Nutr. 2008, 37, 465–471. [Google Scholar] [CrossRef]
- Eidi, A.; Eidi, M.; Esmaeili, E. Antidiabetic effect of garlic (Allium sativum L.) in normal and streptozotocin-induced diabetic rats. Phytomedicine 2006, 13, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Sudha, P.; Zinjarde, S.S.; Bhargava, S.Y.; Kumar, A.R.; P, S.; Zinjarde, S.S.; Bhargava, S.Y.; Kumar, A.R. Potent α-amylase inhibitory activity of Indian Ayurvedic medicinal plants. BMC Complement. Altern. Med. 2011, 11, 5. [Google Scholar] [CrossRef]
- Poonam, T.; Prakash, G.P.; Kumar, L.V. Influence of Allium sativum extract on the hypoglycemic activity of glibenclamide: An approach to possible herb-drug interaction. Drug Metabol. Drug Interact. 2013, 28, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Thomson, M.; Al-Amin, Z.M.; Al-Qattan, K.K.; Shaban, L.H.; Ali, M. Anti-diabetic and hypolipidaemic properties of garlic (Allium sativum) in streptozotocin-induced diabetic rats. Int. J. Diabetes Metab. 2007, 15, 108–115. [Google Scholar]
- Thomson, M.; Al-Qattan, K.K.; Bordia, T.; Ali, M. Including garlic in the diet may help lower blood glucose, cholesterol, and triglycerides. J. Nutr. 2006, 136, 800S–802S. [Google Scholar] [CrossRef] [PubMed]
- Loots, D.T.; Pieters, M.; Islam, M.S.; Botes, L. Antidiabetic effects of Aloe ferox and Aloe greatheadii var. davyana leaf gel extracts in a low-dose streptozotocin diabetes rat model. S. Afr. J. Sci. 2011, 107, 1–6. [Google Scholar] [CrossRef]
- Loots, D.T.; van der Westhuizen, F.H.; Botes, L. Aloe ferox leaf gel phytochemical content, antioxidant capacity, and possible health benefits. J. Agric. Food Chem. 2007, 55, 6891–6896. [Google Scholar] [CrossRef] [PubMed]
- Dimo, T.; Rakotonirina, S.V.; Tan, P.V.; Azay, J.; Dongo, E.; Kamtchouing, P.; Cros, G. Effect of Sclerocarya birrea (Anacardiaceae) stem bark methylene chloride/methanol extract on streptozotocin-diabetic rats. J. Ethnopharmacol. 2007, 110, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Ojewole, J.A.O.; Mawoza, T.; Chiwororo, W.D.H.; Owira, P.M.O. Sclerocarya birrea (A. Rich) Hochst. [‘Marula’] (Anacardiaceae): A review of its phytochemistry, pharmacology and toxicology and its ethnomedicinal uses. Phytother. Res. 2010, 24, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Eloff, J.N. Antibacterial activity of Marula (Sclerocarya birrea (A. Rich.) Hochst. subsp. caffra (Sond.) Kokwaro) (Anacardiaceae) bark and leaves. J. Ethnopharmacol. 2001, 76, 305–308. [Google Scholar] [CrossRef]
- Makom Ndifossap, I.G.; Frigerio, F.; Casimir, M.; Ngueguim Tsofack, F.; Dongo, E.; Kamtchouing, P.; Dimo, T.; Maechler, P. Sclerocarya birrea (Anacardiaceae) stem-bark extract corrects glycaemia in diabetic rats and acts on -cells by enhancing glucose-stimulated insulin secretion. J. Endocrinol. 2010, 205, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Deutschländera, M.S.; Lalla, N.; van de Venter, M.; Hussein, A. Hypoglycemic evaluation of a new triterpene and other compounds isolated from Euclea undulata Thunb. var. myrtina (Ebenaceae) root bark. J. Ethnopharmacol. 2011, 133, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- Tiong, S.H.; Looi, C.Y.; Hazni, H.; Arya, A.; Paydar, M.; Wong, W.F.; Cheah, S.-C.C.; Mustafa, M.R.; Awang, K. Antidiabetic and antioxidant properties of alkaloids from Catharanthus roseus (L.) G. Don. Molecules 2013, 18, 9770–9784. [Google Scholar] [CrossRef] [PubMed]
- Pather, N.; Kramer, B. Bulbine Natalensis and Bulbine Frutescens promote cutaneous wound healing. J. Ethnopharmacol. 2012, 144, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Reddy, L. Apoptosis in the human laryngeal carcinoma (HEp-2) cell line by Bulbine natalensis and B. Frutescens fractions. Int. J. Biol. Pharm. Res. 2012, 3, 862–874. [Google Scholar]
- Odeyemi, S.W.; Afolayan, A.J. Identification of Antidiabetic compounds from Polyphenolic-rich Fractions of Bulbine abyssinica A. Rich Leaves. Pharmacogn. Res. 2018, 10, 72–80. [Google Scholar] [CrossRef]
- Musabayane, C.; Gondwe, M.; Shode, F.; Ojewole, J. Hypoglycaemic effects of Hypoxis hemerocallidea (Fisch. and C. A. Mey.) [Hypoxidaceae] corm ethanolic extract in rats. In Proceedings of the 196th Meeting of the Society for Endocrinology and Society for Endocrinology joint Endocrinology and Diabetes Day, London, UK, 7–9 November 2005; p. 34. [Google Scholar]
- Ojewole, J.A.O. Antinociceptive, anti-inflammatory and antidiabetic properties of Hypoxis hemerocallidea Fisch. & C.A. Mey. (Hypoxidaceae) corm [‘African Potato’] aqueous extract in mice and rats. J. Ethnopharmacol. 2006, 103, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Atangwho, I.J.; Egbung, G.E.; Ahmad, M.; Yam, M.F.; Asmawi, M.Z. Antioxidant versus anti-diabetic properties of leaves from Vernonia amygdalina Del. growing in Malaysia. Food Chem. 2013, 141, 3428–3434. [Google Scholar] [CrossRef] [PubMed]
- Atangwho, I.; Ebong, P.; Egbung, G.; Eteng, M.; Eyong, E. Effect of Vernonia amygdalina Del. on liver function in alloxan-induced hyperglycaemic rats. J. Pharm. Bioresour. 2007, 4, 27–31. [Google Scholar] [CrossRef]
- Osinubi, A. Effects of Vernonia amygdalina and chlorpropamide on blood glucose. Med. J. Islam. World Acad. Sci. 2008, 16, 115–119. [Google Scholar]
- Okolie, U.; Okeke, C.; Oli, J.; Ehiemere, I.O. Hypoglycemic indices of Vernonia amygdalina on post-prandial blood glucose concentration of healthy humans. Afr. J. Biotechnol. 2008, 7, 4581–4585. [Google Scholar]
- Sunmonu, T.O.; Afolayan, A.J. Evaluation of antidiabetic activity and associated toxicity of Artemisia afra aqueous extract in Wistar rats. Evid. Based Complement. Altern. Med. 2013, 2013, 929074. [Google Scholar] [CrossRef] [PubMed]
- Wintola, O.A.; Afolayan, A.J. Phytochemical constituents and antioxidant activities of the whole leaf extract of Aloe ferox Mill. Pharmacogn. Mag. 2011, 7, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Mellem, J.; Baijnath, H.; Odhav, B. Antidiabetic potential of Brachylaena discolor. African J. Tradit. Complement. Altern. Med. 2015, 12, 38. [Google Scholar] [CrossRef]
- Mellem, J. Effect of the methanolic extract of Brachylaena discolor in a streptozotocin-induced diabetic rat model. Afr. J. Pharm. Pharmacol. 2013, 7, 636–642. [Google Scholar] [CrossRef]
- Mellem, J.J. Isolation and Characterization of the Leaves of Brachylaena Discolor Extract as an Anti-Diabetic Agent. Ph.D. Thesis, University of Technology, Durban, South Africa, 2013. [Google Scholar]
- Deutschländer, M.S.; Lall, N.; Venter, M. van de Plant species used in the treatment of diabetes by South African traditional healers: An inventory. Pharm. Biol. 2009, 47, 348–365. [Google Scholar] [CrossRef]
- Gyang, S.S.; Nyam, D.; Sokomba, E. Hypo-glycaemic activity of Vernonia amygdalina (chloroform extract) in normoglycaemic and alloxan-induced hyper-glycaemic rats. J. Pharm. Bioresour. 2004, 1, 61–66. [Google Scholar]
- Ebong, P.; Atangwho, I.; Eyong, E.; Egbung, G. The antidiabetic efficacy of combined extracts from two continental plants: Azadirachta indica (A. Juss) (Neem) and Vernonia amygdalina (Del.) (African Bitter Leaf). Am. J. Biochem. Biotechnol. 2008, 4, 239–244. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Maiti, K.; Mukherjee, K.; Houghton, P.J. Leads from Indian medicinal plants with hypoglycemic potentials. J. Ethnopharmacol. 2006, 106, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.; Springfield, E. Tarchonanthus Camphoratus Herbal. Available online: www.plantzafrica.com/medmonographs/tarchonanthcamp.pdf (accessed on 1 October 2005).
- Wollenweber, E.; Mann, K.; Valant-Vetschera, K. External flavonoid aglycones in Artemisia and some further Anthemidae (Asteraceae). Fitoterapia 1989, 60, 460–463. [Google Scholar]
- Levendal, R.-A.; Frost, C. In vivo effects of Cannabis sativa L. extract on blood coagulation, fat and glucose metabolism in normal and streptozocin-induced diabetic rats. Afr. J. Tradit. Complement. Altern. Med. 2006, 3, 1–12. [Google Scholar] [CrossRef]
- Ali, E.M.; Sara, A.M.; Salwa, M.E.; Samia, H.; Abdelwahab, A.H.; Samia, A.H.; Abdelwahab, H. The Hypoglycemic and Hypocholesterolemic Effects of Aqueous Extract of Cannabis sativa in Albino Rats. J. Ethnobiol. Ethnopharmacol. 2012, 1, 10–12. [Google Scholar]
- Piero, N.M.; Joan, M.N.; Kibiti, M.C.; Ngeranwa, J.J.; Njue, M.W.; Maina, D.; Gathumbi, K.P.; Njagi, N.E. Hypoglycemic Activity of Some Kenyan Plants Traditionally used to Manage Diabetes Mellitus in Eastern Province. J. Diabetes Metab. 2011, 2, 1–6. [Google Scholar] [CrossRef]
- Dallak, M.A.; Bin-Jaliah, I.; Al-Khateeb, M.A.; Nwoye, L.O.; Shatoor, A.S.; Soliman, H.S.; Al-Hashem, F.H. In vivo acute effects of an orally administered hydro-ethanol extract of Catha edulis on blood glucose levels in normal, glucose-fed hyperglycemic, and alloxan-induced diabetic rats. Saudi Med. J. 2010, 31, 627–633. [Google Scholar] [PubMed]
- Saif-Ali, R.; Al-Qirbi, A.; Al-Geiry, A.; Al-Habori, M. Effect of Catha edulis on plasma glucose and C-peptide in both type 2 diabetics and non-diabetics. J. Ethnopharmacol. 2003, 86, 45–49. [Google Scholar] [CrossRef]
- Gruendel, S.; Otto, B.; Garcia, A.L.; Wagner, K.; Mueller, C.; Weickert, M.O.; Heldwein, W.; Koebnick, C. Carob pulp preparation rich in insoluble dietary fibre and polyphenols increases plasma glucose and serum insulin responses in combination with a glucose load in humans. Br. J. Nutr. 2007, 98, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Gorelik, S.; Ligumsky, M.; Kohen, R.; Kanner, J. A novel function of red wine polyphenols in humans: Prevention of absorption of cytotoxic lipid peroxidation products. FASEB J. 2008, 22, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Acquaviva, R.; Di Giacomo, C.; Vanella, L.; Santangelo, R.; Sorrenti, V.; Barbagallo, I.; Genovese, C.; Mastrojeni, S.; Ragusa, S.; Iauk, L. Antioxidant activity of extracts of Momordica Foetida Schumach. et Thonn. Molecules 2013, 18, 3241–3249. [Google Scholar] [CrossRef] [PubMed]
- Molehin, O.R.; Adefegha, S. Comparative study of the aqueous and ethanolic extract of Momordica foetida on the phenolic content and antioxidant properties. Int. Food Res. J. 2014, 21, 401–405. [Google Scholar]
- Gulati, V.; Gulati, P.; Harding, I.H.; Palombo, E.A. Exploring the anti-diabetic potential of Australian Aboriginal and Indian Ayurvedic plant extracts using cell-based assays. BMC Complement. Altern. Med. 2015, 15, 8. [Google Scholar] [CrossRef] [PubMed]
- Ojewole, J.A.O. Evaluation of the analgesic, anti-inflammatory and anti-diabetic properties of Sclerocarya birrea (A. Rich.) Hochst. stem-bark aqueous extract in mice and rats. Phyther. Res. 2004, 18, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Van de Venter, M.; Wilson, G.; Roux, S. An optimized method to screen for in vitro antidiabetic activity. In 9th International Conference on Ethnopharmacology; Haller, B.F., Ed.; International Society for Ethnopharmacology (ISE): Nanjing, China, 2006. [Google Scholar]
- Wilson, G.; Roux, S.; van de Venter, M. Optimisation of An In Vitro Model for Anti-Diabetic Screening. Ph.D. Thesis, Nelson Mandela Metropolitan University, Port Elizabeth, South Africa, 2006. [Google Scholar]
- Williams, S.; Roux, S.; Koekemoer, T.; van de Venter, M.; Dealtry, G. Sutherlandia frutescens prevents changes in diabetes-related gene expression in a fructose-induced insulin resistant cell model. J. Ethnopharmacol. 2013, 146, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, W.; Roux, S.; van de Venter, M.; Louw, J.; Oelofsen, W. Antidiabetic effects of Sutherlandia frutescens in Wistar rats fed a diabetogenic diet. J. Ethnopharmacol. 2007, 109, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Odeyemi, S.; Afolayan, A.; Bradley, G. In vitro anti-inflammatory and free radical scavenging activities of crude saponins extracted from Albuca bracteata Jacq. Bulb. African J. Tradit. Complement. Altern. Med. 2015, 12, 34–40. [Google Scholar] [CrossRef]
- Odeyemi, S.; Afolayan, A.; Bradley, G. Phytochemical analysis and anti-oxidant activities of Albuca bracteata Jacq. and Albuca setosa Jacq bulb extracts used for the management of diabetes in the Eastern Cape, South Africa. Asian Pac. J. Trop. Biomed. 2017, 7, 577–584. [Google Scholar] [CrossRef]
- Zibula, S.M.X.; Ojewole, J.A. Hypoglycaemic effects of Hypoxis hemerocallidea corm ‘African Potato’ methanolic extract in rats. Med. J. Islam. Acad 2000, 13, 75–78. [Google Scholar]
- Mahomed, I.M.; Ojewole, J.A.O. Hypoglycemic effect of Hypoxis hemerocallidea corm (African potato) aqueous extract in rats. Methods Find. Exp. Clin. Pharmacol. 2003, 25, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Ncube, B.; Ndhlala, A.; Okem, A.; van Staden, J. Hypoxis (Hypoxidaceae) in African traditional medicine. J. Ethnopharmacol. 2013, 150, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Ojewole, J.A.O. Antinociceptive, anti-inflammatory and antidiabetic effects of Leonotis leonurus (L.) R. BR. [Lamiaceae] aqueous leaf extract in mice and rats. Methods Find. Exp. Clin. Pharmacol. 2005, 27, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Oyedemi, S.O.; Yakubu, M.T.; Afolayan, A.J.A. Antidiabetic activities of aqueous leaves extract of Leonotis leonurus in streptozotocin-induced diabetic rats. Acad. J. 2011, 5, 119–125. [Google Scholar]
- Oyedemi, S.O.; Bradley, G.; Afolayan, A.J. Beneficial effect of aqueous Stem Bark Extracts of Strychnos henningsii gilg in streptozotocin-nicotinamide induced type 2 diabetic Wistar rats. Int. J. Pharmacol. 2011, 7, 773–781. [Google Scholar] [CrossRef]
- Oyedemi, S.; Bradley, G.; Afolayan, A. Antidiabetic activities of aqueous stem bark extract of strychnoshenningsii Gilg in Streptozotocin-nicotinamide Type 2 Diabetic Rats. Iran. J. Pharm. Res. IJPR 2012, 11, 221–228. [Google Scholar] [PubMed]
- Oyedemi, S.; Koekemoer, T.; Bradley, G.; van de Venter, M.; Afolayan, A. In vitro anti-hyperglycemia properties of the aqueous stem bark extract from Strychnos henningsii (Gilg). Int. J. Diabetes Dev. Ctries. 2013, 33, 120–127. [Google Scholar] [CrossRef]
- Oyedemi, S.; Bradley, G.; Afolayan, A. In vitro and in vivo antioxidant activities of aqueous stem bark extract of Strychnos henningsii Gilg. Afr. J. Pharm. Pharmacol. 2010, 4, 70–78. [Google Scholar]
- Angenot, L.; Tits, M. Isolation of a New Alkaloid (O-Acetylretuline) and a Triterpenoid (Friedelin) from Strychnos henningsii of Zaïre. Planta Med. 1981, 41, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Babajide, J.O.; Mabusela, W.T.; Green, I.R. Some alkaloids and flavonoids from Cissampelos capensis. J. Med. Plants Res. 2015, 9, 16–29. [Google Scholar]
- Mukhtar, H.M.; Ansari, S.H.; Ali, M.; Naved, T.; Bhat, Z.A. Effect of water extract of Psidium guajava leaves on alloxan-induced diabetic rats. Pharmazie 2004, 59, 734–735. [Google Scholar] [PubMed]
- Mukhtar, H.M.; Ansari, S.H.; Bhat, Z.A.; Naved, T.; Singh, P. Antidiabetic activity of an ethanol extract obtained from the stem bark of Psidium guajava (Myrtaceae). Pharmazie 2006, 61, 725–727. [Google Scholar] [PubMed]
- Ojewole, J.A. Hypoglycemic and hypotensive effects of Psidium guajava Linn. (Myrtaceae) leaf aqueous extract. Methods Find. Exp. Clin. Pharmacol. 2005, 27, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.K.; Jaiswal, D.; Mehta, S.; Watal, G. Anti-hyperglycaemic potential of Psidium guajava raw fruit peel. Indian J. Med. Res. 2009, 129, 561–565. [Google Scholar] [PubMed]
- Rapaka, D.; Vennam, S. Evaluation and comparison of anti-diabetic activity of hydroalcoholic extracts of fresh and dry leaves of Psidium guajava in type-ii diabetes mellitus. Int. Res. J. Pharm. Appl. Sci. 2012, 2, 62–65. [Google Scholar]
- Manikandan, R.; Anand, A.V.; Muthumani, G.D. Phytochemical and in vitro anti-diabetic activity of methanolic extract of Psidium guajava leaves. Int. J. Curr. Microbiol. Appl. Sci. 2013, 2, 15–19. [Google Scholar]
- Wang, B.; Liu, H.-C.; Hong, J.-R.; Li, H.-G.; Huang, C.-Y. Effect of Psidium guajava leaf extract on alpha-glucosidase activity in small intestine of diabetic mouse. Sichuan Da Xue Xue Bao Yi Xue Ban 2007, 38, 298–301. [Google Scholar] [PubMed]
- Sanda, K.A.; Grema, H.A.; Geidam, Y.A.; Bukar-Kolo, Y.M. Pharmacological aspects of Psidium guajava: An Update. Int. J. Pharmacol. 2011, 7, 316–324. [Google Scholar] [CrossRef]
- Gutiérrez, R.M.P.; Mitchell, S.; Solis, R.V. Psidium guajava: A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2008, 117, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.K.; Lee, C.H.; Lee, M.S.; Bae, E.Y.; Sohn, C.B.; Oh, H.; Kim, B.Y.; Ahn, J.S. Antidiabetic effects of extracts from Psidium guajava. J. Ethnopharmacol. 2005, 96, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Chang, W. Studies on Active Principles of Hypoglycemic Effect from Psidium grajava (I). Master’s Thesis, Taipei Medical College, Taipei, Taiwan, 1982. [Google Scholar]
- Ahmed, M.O.; Moneim, A.A.; Yazid, I.A.; Mahmoud, A.M. Antihyperglycemic, antihyperlipidemic and Antioxidant effects and the probable mechanisms of action of Ruta graveolens infusion and rutin in Nicotinamide-streptozotocin-induced diabetic rats. Diabetol. Croat. 2010, 39, 15–35. [Google Scholar]
- Odeyemi, S.; Afolayan, A.; Bradley, G. A Comparative Study of the In Vitro Antidiabetic Properties, Cytotoxicity and Mechanism of Action of Albuca Bracteata and Albuca Setosa Bulb Extracts. Ph.D. Thesis, University of Fort Hare, Alice, South Africa, 2015. [Google Scholar]
- Huzaifa, U.; Labaran, I.; Bello, A.B.; Olatunde, A. Phytochemical Screening of Aqueous Extract of Garlic (Alliumsativum) bulbs. Rep. Opin. 2014, 6, 1–4. [Google Scholar]
- Wintola, O.A.; Sunmonu, T.O.; Afolayan, A.J. The effect of Aloe ferox Mill. in the treatment of loperamide-induced constipation in Wistar rats. BMC Gastroenterol. 2010, 10, 95. [Google Scholar] [CrossRef] [PubMed]
- Mousinho, N.M.; van Tonder, J.J.; Steenkamp, V. In vitro anti-diabetic activity of Sclerocarya birrea and Ziziphus mucronata. Nat. Prod. Commun. 2013, 8, 1279–1284. [Google Scholar]
- Ojewole, J.A.O. Analgesic, anti-inflammatory and hypoglycemic effects of Sutherlandia frutescens R. BR. (variety Incana E. MEY.) [Fabaceae] shoot aqueous extract. Methods Find. Exp. Clin. Pharmacol. 2004, 26, 409–416. [Google Scholar] [PubMed]
- Ojewole, J.A.O. Hypoglycemic effect of Sclerocarya birrea [(A. Rich.) Hochst.] [Anacardiaceae] stem-bark aqueous extract in rats. Phytomedicine 2003, 10, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.N.; Vats, P.; Suri, S.; Shyam, R.; Kumria, M.M.L.; Ranganathan, S.; Sridharan, K. Effect of an antidiabetic extract of Catharanthus roseus on enzymic activities in streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2001, 76, 269–277. [Google Scholar] [CrossRef]
- Aguilar, M.I.; Delgado, G.; Hernández, M.D.L.; Villarreal, M.L. Bioactive compounds from Iostephane heterophylla (Asteraceae). Nat. Prod. Lett. 2001, 15, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Chávez, J.L.; Egas, V.; Linares, E.; Bye, R.; Hernández, T.; Espinosa-García, F.J.; Delgado, G. Mexican Arnica (Heterotheca inuloides Cass. Asteraceae: Astereae): Ethnomedical uses, chemical constituents and biological properties. J. Ethnopharmacol. 2017, 195, 39–63. [Google Scholar] [CrossRef] [PubMed]
- Adewusi, E.A.; Fouche, G.; Steenkamp, V. In Vitro Effect of Selected Medicinal Plants on β-amyloid-Induced Toxicity in Neuroblastoma Cells. Ph.D. Thesis, University of Pretoria, Pretoria, Africa, 2012. [Google Scholar]
- Aslan, M.; Orhan, N.; Orhan, D.; Ergun, F. Hypoglycemic activity and antioxidant potential of some medicinal plants traditionally used in Turkey for diabetes. J. Ethnopharmacol. 2010, 128, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Murakami, T.; Yashiro, K.; Yamahara, J.; Yoshikawa, M. Antidiabetic Principles of Natural Medicines. IV. Aldose Reductase and α-Glucosidase Inhibitors from the Roots of Salacia oblonga Wall. (Celastraceae): Structure of a New Friedelane-Type Triterpene, Kotalagenin 16-Acetate. Chem. Pharm. Bull. (Tokyo) 1999, 47, 1725–1729. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Murakami, T.; Yashiro, K.; Matsuda, H. Kotalanol, a Potent α-Glucosidase Inhibitor with Thiosugar Sulfonium Sulfate Structure, from Antidiabetic Ayurvedic Medicine Salacia reticulata. Chem. Pharm. Bull. (Tokyo) 1998, 46, 1339–1340. [Google Scholar] [CrossRef] [PubMed]
- Raman, A.; Lau, C. Anti-diabetic properties and phytochemistry of Momordica charantia L. (Cucurbitaceae). Phytomedicine 1996, 2, 349–362. [Google Scholar] [CrossRef]
- Teugwa, C.M.; Boudjeko, T.; Tchinda, B.T.; Mejiato, P.C.; Zofou, D. Anti-hyperglycaemic globulins from selected Cucurbitaceae seeds used as antidiabetic medicinal plants in Africa. BMC Complement. Altern. Med. 2013, 13, 63. [Google Scholar] [CrossRef] [PubMed]
- Harinantenaina, L.; Tanaka, M.; Takaoka, S.; Oda, M.; Mogami, O.; Uchida, M.; Asakawa, Y. Momordica charantia constituents and antidiabetic screening of the isolated major compounds. Chem. Pharm. Bull. (Tokyo) 2006, 54, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- Ferreres, F.; Gil-Izquierdo, A.; Vinholes, J.; Silva, S.T.; Valentão, P.; Andrade, P.B. Bauhinia forficata Link authenticity using flavonoids profile: Relation with their biological properties. Food Chem. 2012, 134, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Plančić, M.; Božin, B.; Kladar, N.; Rat, M.; Srđenović, B. Phytochemical profile and biological activities of the genus Ornithogalum L. (Hyacinthaceae). Biol. Serbica 2015, 36, 1–2. [Google Scholar]
- Ezuruike, U.F.; Prieto, J.M. The use of plants in the traditional management of diabetes in Nigeria: Pharmacological and toxicological considerations. J. Ethnopharmacol. 2014, 155, 857–924. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, R.; Nikfar, S.; Larijani, B.; Abdollahi, M. A review on the role of antioxidants in the management of diabetes and its complications. Biomed. Pharmacother. 2005, 59, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Adebajo, A.C.; Ayoola, M.D.; Odediran, S.A.; Aladesanmi, A.J.; Schmidt, T.J.; Verspohl, E.J. Evaluation of ethnomedical claim III: Anti-hyperglycemic activities of Gongronema latifolium root and stem. J. Diabetes 2013, 5, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Perez Gutierrez, R.M. Inhibition of Advanced Glycation End-Product Formation by Origanum majorana L. In Vitro and in Streptozotocin-Induced Diabetic Rats. Evid. Based Complement. Altern. Med. 2012, 2012, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Juma, K.K.; Abdirahman, Y.A.; Mukundi, M.J.; Gitahi, S.M.; Agyirifo, D.S.; Ngugi, M.P.; Gathumbi, P.K.; Ngeranwa, J.J.N.; Njagi, E.N.M. In-vivo antidiabetic activity and safety of the aqueous stem bark extract of Kleinia squarrosa. J. Diabetes Metab. 2015, 9, 601–611. [Google Scholar] [CrossRef]
- Gurley, B. Pharmacokinetic Herb-Drug Interactions (Part 1): Origins, Mechanisms, and the Impact of Botanical Dietary Supplements. Planta Med. 2012, 78, 1478–1489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borrelli, F.; Capasso, R.; Izzo, A.A. Garlic (Allium sativum L.): Adverse effects and drug interactions in humans. Mol. Nutr. Food Res. 2007, 51, 1386–1397. [Google Scholar] [CrossRef] [PubMed]
| S/N | Family | Plants | References |
|---|---|---|---|
| 1 | Alliaceae | Allium sativum | [25] |
| Tulbaghia alliacea | [25] | ||
| Tulbaghia violacea. Harv. | [23] | ||
| 2 | Aloaceae | Aloe ferox Mill | [25] |
| 3 | Anacardiaceae | Sclerocarya birrea (A. Rich.) Hochst. subsp. caffra (Sond.) Kokwaro | [26] |
| 4 | Apiaceae | Heteromorphica arborescens. Hochst. Ex A. Rich. | [15,24] |
| 5 | Apocynaceae | Catharanthus roseus (L.) G. Don. | [15,26] |
| Vinca major L. | [26] | ||
| 6 | Asphodelacea | Bulbine abyssinica | [25] |
| Bulbine natalensis | [25] | ||
| Hypoxis colchicifolia Bak. | [15] | ||
| 7 | Asteraceae | Artemisia afra Jacq. | [15,27] |
| Brachylaena discolor DC. | [15,24] | ||
| Brachylaena elliptica (Thunb.) DC | [28] | ||
| Brachylaena ilicifolia | [15] | ||
| Conyza scabrida DC. | [24,27] | ||
| Helichrysum gymnocomum | [25] | ||
| Herichrysum nudifolium L. | [15] | ||
| Herichrysum odoratissimum L. | [15] | ||
| Herichrysum petiolare H & B.L. | [15,24] | ||
| Tarchonanthus camphoratus L. | [23] | ||
| Vernonia amygdalina DeL. | [15] | ||
| Vernonia oligocephala Sch. Bip. | [15,27] | ||
| 8 | Buddlejaceae | Chilianthus olearaceus Burch. | [15] |
| 9 | Cannabaceae | Cannabis sativa L. | [26] |
| 10 | Caryophyllaceae | Dianthus thunbergii | [25] |
| 11 | Celastraceae | Catha edulis (Vahl) Forrsk. ex EndL. | [26] |
| Lauridia tetragonia | [25] | ||
| 12 | Cucurbitaceae | Momordica balsamina L. | [26] |
| Momordica foetida Schumach. | [26] | ||
| 13 | Ebenaceae | Euclea undulata Thunb. | [29] |
| 14 | Fabaceae | Sutherlandia frutescens L. | [24,28] |
| 15 | Gentianaceae | Chironia baccifera L. | [26] |
| 16 | Hyacinthaceae | Albuca setosa | [25] |
| Ornithogalum longibracteatum (Jacq) | [23] | ||
| 17 | Hypoxidaceae | Hypoxis argentae | [24,25] |
| Hypoxis hemerocallidea Fisch. and C. A | [15] | ||
| 18 | Lamiaceae | Leonotis leonorus | [24,25,27] |
| 19 | Loganiaceae | Strychnos henningsii | [25] |
| 20 | Menispermaceae | Cissampelos capensis L.f. | [25,26] |
| 21 | Myrtaceae | Psidium guajava L. | [26] |
| 22 | Portulaceae | Anacampseros ustulata | [25] |
| 23 | Rutaceae | Ruta graveolens L. | [23,27,28] |
| 24 | Solanaceae | Solanum aculeastrum | [25] |
| 25 | Xanthorrhoeaceae | Bulbine frutescens L. (Willd) | [23] |
| Bulbine natalensis (Syn. B. latifolia) MilL. | [15] |
| Family | Bioactive Molecules | Toxicity | Mechanism of Action | References | |
|---|---|---|---|---|---|
| 1 | Alliaceae | Allicin, tannins, cardiac glycosides, saponins, alkaloids | Some fatalities including abdominal pain, gastroenteritis, cessation of gastrointestinal peristalsis, contraction of the pupils and sloughing of the intestinal mucosa have been implicated in some members | Pancreatic secretion of insulin | [34,36,37,108] |
| 2 | Aloaceae | Phenolic acids/polyphenols, sterols, alkaloids, fatty acids, and indoles | Not known | Antioxidant | [39,40,57,109] |
| 3 | Anacardiaceae | Polyphenols, flavonoids, saponins /saponides, triterpenes, tannins, alkaloids, steroids and cardiac glycosides. | Mixed results for toxicity, not cytotoxic to the C2C12, 3T3-L1 and HepG2 cells and in rat models. Serious concern from the in vitro toxicity results for Sclerocarya birrea. | Increase glucose absorption, possesses insulin-mimetic properties, inhibition of α-amylase and α -glucosidase and interactions with the insulin receptor that lead to the activation of biochemical cascades (PI3K and MAPK) | [110,111,112] |
| 4 | Apiaceae | Not known | Not known | Not known | |
| 5 | Apocynaceae | Alkaloids | Catharanthus roseus and Vinca major are cytotoxic in vitro | Enhance glucose utilization and PTP-1B inhibition, activation of PPARγ, PPARα and PPARδ. Good antioxidants | [26,46,113] |
| 6 | Asphodelacea | Phenolics and aloe emodin | Not known | Decrease hepatic glucose production similar to metformin | [50] |
| 7 | Asteraceae | Saponins, flavanones, tannins, flavonoids (aglycones), aesquiterpenoids, sesquiterpene lactones, alkaloids and polysaccharide, bisabolene | Cytotoxicities at higher concentrations have been reported | Insulin release, repair of pancreatic β-cells, inhibition of carbohydrate digesting enzymes and oxidative stress | [23,56,64,65,66,114,115] |
| 8 | Buddlejaceae | Not known | Toxic molecules have been isolated from plants in this family | No scientific information about the anti-diabetic properties | [24,116] |
| 9 | Cannabaceae | Not known | Not known | Insulin release | [26,67,68,117] |
| 10 | Caryophyllaceae | Not known | Not known | Not known | |
| 11 | Celastraceae | Phenolic molecules, elaeocyanidin, allotannins, ouratea proanthocyanidin A and triterpenes | Not known | Insulinomimetic properties and inhibits carbohydrate digesting enzymes | [26,29,69,72,73,118,119] |
| 12 | Cucurbitaceae | Glycosides, globulins, alkaloids, triterpenoids and phenolic molecules | Cytotoxic to cell lines | Insulinomimetic properties; inhibit carbohydrate digesting enzymes and prevention of oxidative stress | [26,120,121,122] |
| 13 | Ebenaceae | α-amyrin-3O-β-(5-hydroxy) ferulic acid, betulin, lupeol and epicatechin | Not known | Insulin dependent glucose uptake and inhibition of α-glucosidase | [45,76] |
| 14 | Fabaceae | Phenolic, flavonoids | Not known | Normalizes insulin levels, glucose uptake in peripheral tissues suppresses intestinal glucose uptake, prevents insulin resistance and significantly reversed the effects of fructose and insulin on lipid accumulation | [51,78,123] |
| 15 | Gentianaceae | Not known | Not known | Not known | [26] |
| 16 | Hyacinthaceae | Alkaloids, saponins, polyhydroxylated pyrrolidines, piperidines, (2R,5R)-bis(dihydroxymethyl)-(3R,4R)-dihydroxypyrrolidine (DMDP) and 1,4-dideoxy-1,4-imino-d-arabinitol (d-AB1) | Some members are highly toxic | Glucose uptake in cell lines and inhibition of carbohydrate digesting enzymes | [23,82,107,124] |
| 17 | Hypoxidaceae | Phytosterols and sterolin | Reported to be toxic only at high doses (≥1800 mg/kg) | Stimulating insulin release | [85] |
| 18 | Lamiaceae | Tetracyclic triterpenoid, carbohydrates, alkaloids, flavonoids, | L. leonurus has been reported to be toxic in rats | Insulin secretion | [92] |
| tannins, steroids, terpenes/triterpenes and saponins | |||||
| 19 | Loganiaceae | Phenols and alkaloid (O-acetylretuline) | Some of the genus in this family e.g., Strychnos are extremely toxic, producing the poisin strychnine | Potentiate insulin secretion | [25,26,90,91,94] |
| 20 | Menispermaceae | Alkaloids and flavonoids | Not cytotoxic | Glucose uptake in adipocytes | [25,26,94] |
| 21 | Myrtaceae | Polyphenolics, ursolic acid, oleanolic acid, arjunolic acid and glucuronic acid | Not known | Free radical scavenging, alpha-glucosidase inhibitory activity | [103,104,105] |
| 22 | Portulaceae | Not known | Not known | Not known | |
| 23 | Rutaceae | Not known | Not known | Insulin action, inhibition of intestinal glucose uptake | [106] |
| 24 | Solanaceae | Not known | Not known | Not known | |
| 25 | Xanthorrhoeaceae | Not known | Cytotoxicity reported | Increase glucose utilization in Chang cells | [15,23] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Odeyemi, S.; Bradley, G. Medicinal Plants Used for the Traditional Management of Diabetes in the Eastern Cape, South Africa: Pharmacology and Toxicology. Molecules 2018, 23, 2759. https://doi.org/10.3390/molecules23112759
Odeyemi S, Bradley G. Medicinal Plants Used for the Traditional Management of Diabetes in the Eastern Cape, South Africa: Pharmacology and Toxicology. Molecules. 2018; 23(11):2759. https://doi.org/10.3390/molecules23112759
Chicago/Turabian StyleOdeyemi, Samuel, and Graeme Bradley. 2018. "Medicinal Plants Used for the Traditional Management of Diabetes in the Eastern Cape, South Africa: Pharmacology and Toxicology" Molecules 23, no. 11: 2759. https://doi.org/10.3390/molecules23112759






