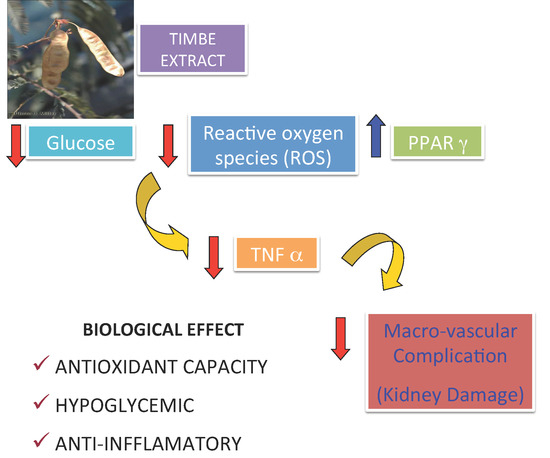
Timbe (Acaciella angustissima) Pods Extracts Reduce the Levels of Glucose, Insulin and Improved Physiological Parameters, Hypolipidemic Effect, Oxidative Stress and Renal Damage in Streptozotocin-Induced Diabetic Rats
Abstract
1. Introduction
2. Results and Discussion
2.1. Phenolic Compounds and Antioxidant Capacity of Methanolic Extract (MEA)
2.2. HPLC Analysis
2.3. Effect of A. angustissima in Serum Glucose and Insulin Levels
2.4. Physiological and Biochemical Evaluations
2.5. Determination of Serum Lipid Profile
2.6. Determination of Lipid Peroxidation and Protein Content in Kidney
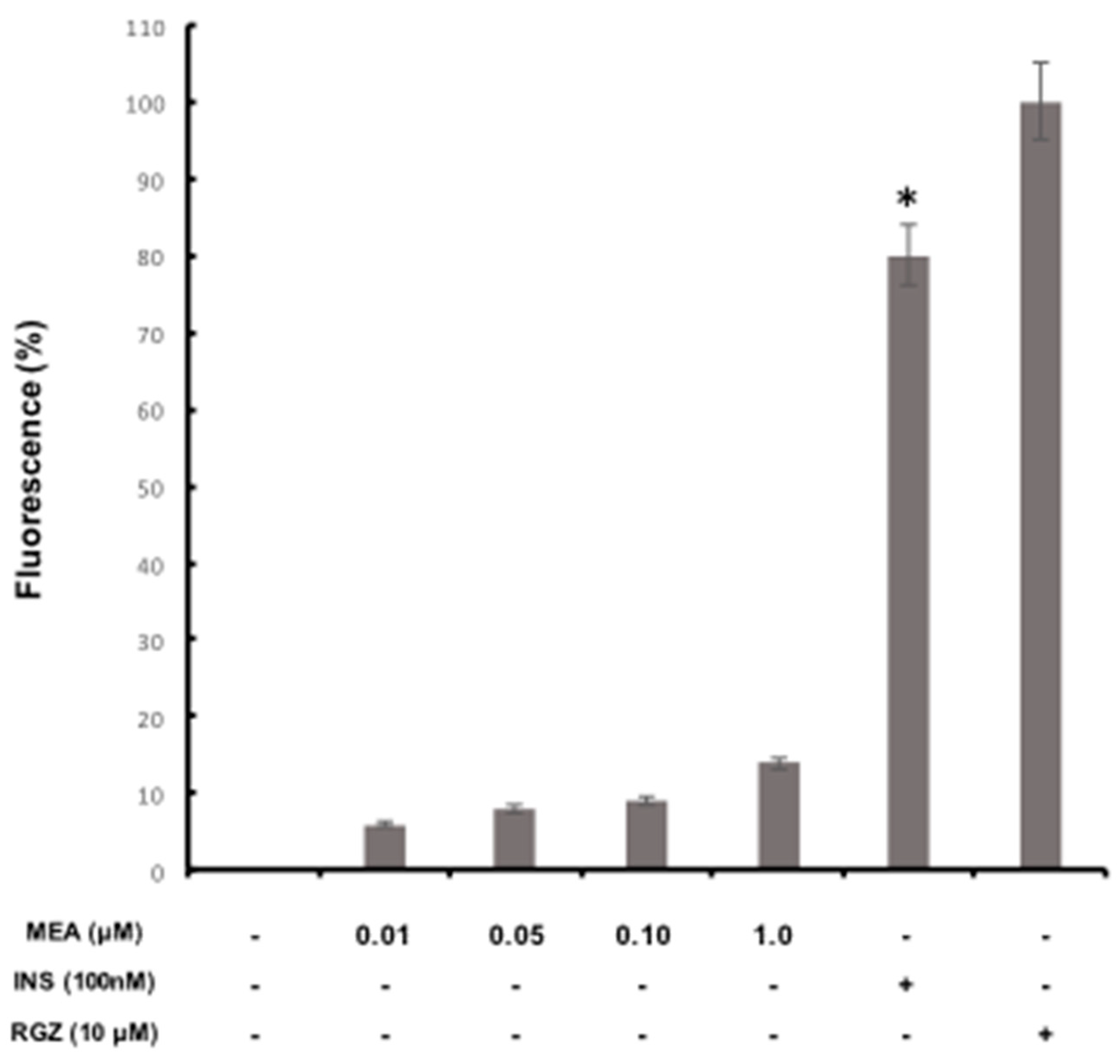
2.7. Glucose Incorporation Assay in Adipocyte Cells
3. Material and Methods
3.1. Collection of Material and Preparation of Extract
3.2. Phenolic Compounds and Antioxidant Capacity of Methanolic Extract (MEA)
3.3. HPLC Analysis
3.4. Experimental Protocol with Animals
3.5. Animal Grouping.
3.6. Collection of Blood and Tissue
3.7. Biochemical Evaluations.
3.8. Lipid Peroxidation and Protein Content in Kidney
3.9. Glucose Incorporation Assay in Adipocytic Cells
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability
Abbreviations
| mg | milligrams |
| kg | kilograms |
| DM | diabetes mellitus |
| ROS | Reactive oxygen species |
| MEA | methanolic extract of Acacciella angustissima |
| W | water extract of Acacciella angustissima |
| g | grams |
| dL | deciliters |
| nM | nanomolar |
| pH | hydrogen potential |
| TC | total cholesterol |
| TG | triglycerides |
| HDL | high-density lipoprotein |
| LDL | low-density lipoprotein |
| min | minutes |
| mL | milliliters |
| STZ | streptozotocin |
| CCr | creatinine clearance |
References
- Dada, F.A.; Oyeleye, S.I.; Ogunsuyi, O.B.; Olasehinde, T.A.; Adefegha, S.A.; Oboh, G.; Boligon, A.A. Phenolic constituents and modulatory effects of Raffia palm leaf (Raphia hookeri) extract on carbohydrate hydrolyzing enzymes linked to type-2 diabetes. J. Trad Comp. Med. 2017, 7, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Afolayan, A.J.; Sunmonu, T.O. Artemisia afra Jacq. ameliorates oxidative stress in the pancreas of streptozotocin-induced diabetic Wistar rats. Biosci. Biotechnol. Biochem. 2011, 75, 2083–2086. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Traditional Medicine Strategy; WHO Press: Geneva, Switzerland, 2013; pp. 2014–2023. [Google Scholar]
- Aissaoui, A.; Zizi, S.; Israili, H.Z.; Lyoussi, B. Hypoglycemic and hypolipidemic effects of Coriandrum sativum L. in Meriones shawi rats. J. Ethnopharmacol. 2011, 137, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Loarca, G.; Mendoza, S.; Ramos, M.; Reynoso, R. Antioxidant, antimutagenic and antidiabetic activities of edible leaves from Cnidoscolus chayamansa. J. Food Sci. 2010, 75, 68. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.K.; Prasad, S.K.; Kumar, R.; Hemalata, S. An overview of antidiabetic plants having insulinomimetic activity. Asian Pacific J. Trop Med. 2012, 2, 320–330. [Google Scholar] [CrossRef]
- Roshetko, J.M. A compilation of the highlights and factsheets published by NFTA and FACT Net 1985-1999. In Agroforestry Species and Techologies; Winrock International: Morrilton, AR, USA, 2001; pp. 9–12. [Google Scholar]
- Terrones, R. Arbustivas Nativas de uso multiple en Guanajuato. In Libro técnico No.1; INIFAP: Gto, Mexio, 2006; Volume 1, pp. 1–99. [Google Scholar]
- Nabavi, S.M.; Ebrahimzadeha, M.A.; Nabavi, S.F.; Fazelian, M.; Eslami, B. In vitro antioxidant and free radical scavenging activity of Diospyros lotus and Pyrus boissieriana growing in Iran. Pharm. Mag. 2009, 5, 122–126. [Google Scholar]
- Subramanian, V.; Leelavinothan, P. Antioxidant effect of Phaseolus vulgaris in streptozotocin-induced diabetic rats. Asia Pacific J. Clin. Nutr. 2002, 11, 206–209. [Google Scholar]
- Choudhury, H.; Pandey, M.; Kui, H.Ch.; Shi, M.Ch.; Koh, J.J.; Kong, L.; Yee, E.L.; Ahmad, A.N.; Wai, K.S.; Sin, Y.T.; et al. An update on natural compounds in the remedy of diabetes mellitus: A systematic review. J. Trad. Comp. Med. 2017, 8, 361–376. [Google Scholar] [CrossRef] [PubMed]
- Udoamaka, E.F.; Prieto, J.M. The use of plants in the traditional management of diabetes in Nigeria: Pharmacological and toxicological considerations. J. Ethnopharm. 2014, 155, 857–924. [Google Scholar] [CrossRef]
- Feregrino, A.A.; Torres, I.; Vargas, M.; Munguia, P.; Loarca, G.; Mendoza, S.; Ocampo, R.; Rico, E.; Guevara, R. Antioxidant and antimutagenic activities of Acacia pennatula pods. J. Sci. Ind. Res. 2011, 70, 859–864. [Google Scholar]
- Vargas-Hernández, M.; Munguía-Fragozo, P.V.; Cruz-Hernández, A.; Guerrero, B.Z.; González-Chavira, M.M.; Feregrino-Pérez, A.A.; Mendoza-Díaz, S.O.; Loarca-Piña, G.; Torres-Pacheco, I.; Hernández-Salazar, M.; et al. Bioactivity and gene expression studies of an arbustive Mexican specie Acaciella angustissima (Timbe). Ind. Crops Prod. 2014, 52, 649–655. [Google Scholar] [CrossRef]
- Masella, R.; Santangelo, C.; D´Archivio, M.; LiVolti, G.; Giovannini, C.; Galvano, F. Protocatechuic acid and human disease prevention: Biological activities and molecular mechanisms. Curr. Med. Chem. 2012, 19, 2901–2917. [Google Scholar] [CrossRef] [PubMed]
- Saliu, J.A.; Ademiluyi, A.O.; Akinyemi, A.J.; Oboh, G. In vitro antidiabetes and antihypertension properties of phenolic extracts from bitter leaf (Vernonia amigdalina Del.). J. Food Biochem. 2012, 36. [Google Scholar] [CrossRef]
- Robbins, R.J. Phenolic acids in foods: An overviewof analytical methodology. J. Agric. Food Chem. 2003, 51, 2866–2887. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.J.; Yin, M.C. Anti-glycative and anti-inflammatory effects of protocatechuic acid in brain of mice treated by dgalactose. Food Chem. Toxicol. 2012, 50, 3198–3205. [Google Scholar] [CrossRef] [PubMed]
- Semaming, Y.; Kukongviriyapan, U.; Kongyingyoes, B.; Thukhammee, W.; Pannangpetch, P. Protocatechuic acid resotres vascular responses in rats with chronic diabetes induced by streptoxotocin. Phytoher. Res. 2016, 30, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, W.Q.; Wang, H.Z.; Wu, Y.N. High performance liquid chromatographic determination of phenolic acids in fruits and vegetables. Biomed. Environ. Sci. 1993, 6, 389–398. [Google Scholar] [PubMed]
- Lin, H.-Y.; Chang, T.-C.; Chang, S.-T. A review of antioxidant and pharmacological properties of phenolic compounds in Acacia confusa. J. Trad. Comp. Med. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Saleem, F.; Sarkar, D.; Ankolekar, Ch.; Shetty, K. Phenolic bioactives and associated antioxidant and anti-hyperglycemic functions of select species of Apiaceae family targeting for type 2 diabetes relevant nutraceuticals. Ind. Crop. Prod. 2017, 107, 518–525. [Google Scholar] [CrossRef]
- Amalan, V.; Vijayakumar, N.; Indumathi, D.; Ramikrishnan, A. Antidiabetic and antihyperlipidemic activity of p-coumaric acid in diabetic rats, role of pancreatic GLUT 2: In vivo approach. Biomed. Pharmacotherapy 2016, 84, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Szkudelski, T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol. Res. 2001, 50, 536–546. [Google Scholar]
- Lomas, C.; Ramos, M.; Guevara, L.; Guevara, R.; Torres, I.; Gallegos, M.; Reynoso, R. Transcriptomic Analysis in Diabetic Nephropathy of Streptozotocin-Induced Diabetic Rats. Int. J. Mol. Sci. 2011, 12, 8431–8448. [Google Scholar] [CrossRef] [PubMed]
- Almuaigel, M.D.; Seif, M.; Albuali, H.W.; Alharbi, O.; Alhawash, A. Hypoglycemic and hypolipidemic effects of aqueous extract of phaseolus vulgaris pods in streptozotocin-diabetic rats Biomed. Pharmacotherapy 2017, 94, 742–746. [Google Scholar] [CrossRef] [PubMed]
- Harini, R.; Pugalendi, K.V. Antihyperglycemic effect of protocatechuic acid on streptozotocin-diabetic rats. J. Basic Clin. Physiol. Pharmacol. 2010, 21, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Tsai, S.J.; Huand, C.S.; Yin, M.C. Antiglycative effects of protocatechuic acid in the kidneys of diabetic mice. J. Agric. Food Chem. 2011, 59, 5117–5124. [Google Scholar] [CrossRef] [PubMed]
- Scazzocchio, B.; Varì, R.; Filesi, C.; D′Archivio, M.; Santangelo, C.; Giovannini, C.; Iacovelli, A.; Silecchia, G.; Li Volti, G.; Galvano, F.; et al. Cyanidin-3-O-β-glucoside and protocatechuic acid exert insulin-like effects by upregulating PPARγ activity in human omental adipocytes. Diabetes 2011, 60, 2234–2244. [Google Scholar] [CrossRef] [PubMed]
- Yüksel, M.; Yildar, M.; Basbug, M.; Cavdar, F.; Cikman, O.; Aksit, H.; Aslan, F.; Aksit, D. Does protocatechuic acid, a natural antioxidant, reduce renal ischemia reperfusion injury in rats? Turkish J. Trau. Emerg. Surgery 2017, 23, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Safaeian, L.; Hajhashemi, V.; Haghjoo, J.S.; Sanaye, N.H. The effect of protocatechuic acid on bllod pressure and oxidative stress in glucocorticoid-induced hypertension in rat. Iran. J. Pharm. Res. 2016, 15, 83–91. [Google Scholar] [PubMed]
- Sabitha, V.; Ramachandran, S.; Naveen, K.R.; Panneerselvam, K. Antidiabetic and antihyperlipidemic potential of Abelmoschus esculentus (L.) Moench. in streptozotocin-induced diabetic rats. J. Pharm. Bioall. Sci. 2011, 3, 397–402. [Google Scholar] [CrossRef]
- Tsumbu, C.N.; Deby-Dupont, G.; Tits, M.; Angenot, L.; Franck, T.; Serteyn, D.; Mouithys-Mickalad, A. Antioxidant and antiradical activities of Manihot esculenta Crantz (Euphorbiaceae) leaves and other selected tropical green vegetables investigated on lipoperoxidation and phorbol-12-myristate-13-acetate (PMA) activated monocytes. Nutrients 2011, 3, 818–838. [Google Scholar] [CrossRef] [PubMed]
- Prakasam, A.; Sethupath, S.; Pugalendi, V. Influence of Casearia esculenta root extract on protein metabolism and marker enzymes in streptozotocin-induced diabetic rats. Pol. J. Pharmacol. 2004, 56, 587–593. [Google Scholar] [PubMed]
- Mansour, H.A.; Newairy, A.A. Amelioration of impaired renal function associated with diabetes by Balanites aegyptiaca fruits in streptozotocin-induced diabetic rats. J. Med. Res. Ins. 2000, 21, 115–125. [Google Scholar]
- Badr El-Din, N.K. Effect of Panax ginseng extract on the nephrotoxicity of streptozotocin-induced experimental diabetes. Egypt J. Biochem. 1997. 15, 29–52.
- Dubey, G.P.; Dixit, S.P.; Singh, A. Alloxan-induced diabetes in rabbits and effect of a herbal formulation D-400. Ind. J. Pharmacol. 1994, 26, 225–226. [Google Scholar]
- Eidi, A.; Eidi, M.; Esmaeili, E. Antidiabetic effect of garlic (Allium sativum L.) in normal and streptozotocin-induced diabetic rats. Phytomedicine 2006, 13. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, E.; Haffner, S.M.; Mitchell, B.D.; Stern, M.P. Hyperinsulinemia: The key feature of a cardiovascular and metabolic syndrome. Diabetiologia 1991, 34, 416–422. [Google Scholar] [CrossRef]
- Hardman, J.G.; Limberd, L.E. Insulin, Oral Hypoglycemic Agents and the Pharmacology of the endocrine pancreas. In Goodman and Gilman’s: The Pharmacological Basis of Therapeutics; Pergamon Press: New York, NY, USA, 2001; p. 1383. [Google Scholar]
- Wang, D.; Zhao, S.; Liu, Y. Hipoglycemic and hypolipidemic affects of a polysaccharide from flower buds of Lonicera japonica in streptozotocin-induced diabetic rats. Int. J. Biol. Macromol. 2017, 102, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.A.; Rashid, M.; Wahed, M.I.I.; Islam, M.R.; Shaheen, S.H.; Islam, A.; Amran, M.S.; Ahmed, M. Comparison of Long-term Antihyperglycemic and Hypolipidemic Effects Between Coccinia cordifolia and Catharanthus roseus in Alloxan-induced Diabetic Rats. Res. J. Med. Med. Sci. 2007, 2, 29–34. [Google Scholar]
- Pothuraju, R.; Sharma, R.K.; Onteru, S.K.; Singh, S.; Hussain, S.A. Hypoglycemic and Hypolipidemic Effects of Aloe vera extract preparations: A review. Phyther. Res. 2016, 30. [Google Scholar] [CrossRef]
- Borate, R.A.; Suralkar, A.A.; Birje, S.S.; Malusare, V.P.; Bangale, A.P. Antihyperlipidemic effect of protocatechuic acid in fructose induced hiperlipidemia in rats. Int. J. Pharm. Bio. Sci. 2011, 2, 456–460. [Google Scholar]
- Ravi, K.; Ramachandran, B.; Subramanian, S. Effect of Eugenia jambolana seed kernel on antioxidant defense system in streptozotocin- induced diabetes in rats. Life Sci. 2004, 75, 2717–2731. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.B.; Xue-Qiao, Z.; Sacco, D.E.; Alberts, J.J. Lipid lowering and plaque regression: New insights into prevention events of plaque disruption and clinical in coronary disease. Circulation 1993, 87, 1781–1791. [Google Scholar] [CrossRef] [PubMed]
- Bagri, P.; Ali, M.; Aeri, V.; Bhowmik, M.; Sultana, S. Antidiabetic effect of Punica granate flowers: Effect on hyperlipidemia, pancreatic cells lipid peroxidation and antioxidant enzymes in experimental diabetes. Food Chem. Toxicol. 2009, 47, 50–52. [Google Scholar] [CrossRef] [PubMed]
- Sroka, Z.; Cisowski, W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem. Toxicol. 2003, 41, 753–758. [Google Scholar] [CrossRef]
- Liu, F.; Kim, J.; Li, Y.; Liu, X.; Li, J.; Chen, X. An extract of Lagerstroemia speciosa L. has insulin-like glucose uptake stimulatory and adipocyte differentiation-inhibitory activities in 3T3-L1 cells. J. Nutrition. 2001, 131, 2242–2247. [Google Scholar] [CrossRef] [PubMed]
- Zainah, A.; Shafii, K.; Amin, I.; Muhajir, H. Ficus deltoidea: A Potential Alternative Medicine for Diabetes Mellitus. Evid. Based Compl. Alternat. Med. 2012, 12. [Google Scholar] [CrossRef]
- Gray, A.M.; Flatt, P.R. Insulin-releasing and insulin like activity of Agaricus campestris (mushroom). J. Endocrinol. 1998, 157, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.H.; Seol, H.J.; Jeon, S.J.; Son, K.H.; Lee, J.R. Insulin-sensitizing activities of tanshinones, diterpene compounds of the root of Salvia miltiorrhiza bunge. Phytomedicine 2009, 16, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Martineau, L.C.; Couture, A.; Spoor, D.; Benhaddou-Andaloussi, A.; Harris, C.; Meddah, B.; Leduc, C.; Burt, A.; Vuong, T.; Mai Le, P.; Prentki, M.; et al. Anti-diabetic properties of the Canadian low bush blueberry Vaccinium angustifolium ait. Phytomedicine 2006, 13, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Deshpande, S.S.; Cheryan, M. Evaluation of vanillin assay for tannin analysis of dry beans. J. Food Sci. 1985, 50, 905–910. [Google Scholar] [CrossRef]
- Liu, M.; Li, X.Q.; Weber, C. Antioxidant and antiproliferative activities of raspberries. J. Agric. Food Chem. 2002, 50, 2926–2930. [Google Scholar] [CrossRef] [PubMed]
- Van Den Berg, R.; Haenen, G.R.M.; Van Den Berg, H.B.A. Applicability of an improved trolox equivalent antioxidant capacity (TEAC) assay for evaluation of antioxidant capacity measurements of mixtures. Food Chem. 1999, 66, 511–517. [Google Scholar] [CrossRef]
- Barham, D.; Trinder, P. An improved colour reagent for the determination of blood glucose by the oxidase system. Analyst 1972, 97, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Leibovitz, B.E.; Tappel, A.L. Lipid peroxidation measured as thiobarbituric acid-reactive substances in tissue slices: Characterization and comparison with homogenates and microsomes. Free Radic Biol. Med. 1988, 4, 155–161. [Google Scholar] [CrossRef]
Sample Availability: Sample of the extracts of Acacciella angustissima pods are available from the authors. |


| Sample | Extraction Solvent | Total Phenols 1 | Condensed Tannins 2 | Total Flavonoids 3 | ABTS 4 |
|---|---|---|---|---|---|
| Acacciella angustissima | Methanol (MEA) | 121.75 ± 2.46 a | 0.616 ± 0.008 a | 4.60 ± 0.14 a | 70.34 ± 13.96 a |
| Water (W) | 36.68 ± 0.70 b | 0.170 ± 0.04 b | 0.31± 0.001 b | 15.88 ± 1.94 b |
| Groups | Glucose (mg/dL) | Insulin (µU/mL) |
|---|---|---|
| Healthy control | 86.8 ± 2.05 a | 28.2 ± 0.1 a |
| 25 mg/kg MEA | 85.6 ± 1.27 a | 29.03 ± 0.3 a |
| 50 mg/kg MEA | 87.47 ± 1.87 a | 29.45 ± 0.6 a |
| 100 mg/kg MEA | 92.6 ± 2.35 a | 27.8 ± 0.7 a |
| Diabetic control | 372.6 ± 6.05 b | 7.0 ± 0.01 b |
| Diabetic + 25 mg/kg MEA | 318.9 ±3.35 b | 7.3 ± 0.06 b |
| Diabetic + 50 mg/kg MEA | 324.6 ±2.28 b | 8.7 ± 0.1 b |
| Diabetic + 100 mg/kg MEA | 146.2 ±4.03 a | 18.1 ± 0.02 a |
| Physiological | Urine | ||||||
|---|---|---|---|---|---|---|---|
| Groups | Body Weight (g) | Kindney Weight (g/100g B.W.) | Food Consumption (g/rat/day) | Water Consumption (ml/rat/day) | Urea (mg/dL) | Creatinine Clearance (ml/min) | Protein (mg/24 h) |
| Healthy Control | 304 ± 20 a | 0.73 ± 0.1 a | 23 ± 5 a | 95 ± 19 a | 7.2 ± 4.0 a | 0.57 ± 0.01 a | 0.52 ± 0.03 a |
| 25 mg/Kg MEA | 300 ± 12 a | 0.70 ± 0.3 a | 21.5 ± 4 a | 105 ± 17 a | 10.2 ± 7.0 a | 0.54 ± 0.04 a | 0.50 ± 0.06 a |
| 50 mg/Kg MEA | 305 ± 09 a | 0.72 ± 0.6 a | 28 ± 6 a | 99 ± 09 a | 12.8 ± 4.0 a | 0.49 ± 0.02 a | 0.55 ± 0.02 a |
| 100 mg/kg MEA | 297 ± 15 a | 0.75 ± 0.5 a | 22 ± 7 a | 117 ± 20 a | 13.1 ± 4.0 a | 0.50 ± 0.03 a | 0.52 ± 0.07 a |
| Diabetic Control | 272 ± 15 b | 1.01 ± 0.4 b | 46 ± 3b | 237 ± 22b | 50.4 ± 22.7 b | 0.70 ± 0.18b | 1.05 ± 0.01 b |
| Diabetic + 25 mg/Kg MEA | 270 ± 15 b | 1.02 ± 0.6 b | 39 ± 5 b | 226 ± 23 b | 29.0 ± 0.4 b | 0.62 ± 0.06b | 0.69 ± 0.05 ab |
| Diabetic + 50 mg/Kg MEA | 240 ± 17 b | 1.06 ± 0.5 b | 37 ± 4 b | 246 ± 18 b | 25.2 ± 16.4 b | 0.63 ± 0.09b | 0.62 ± 0.09 ab |
| Diabetic + 100 mg/Kg MEA | 281 ± 8 b | 0.93 ± 0.4 b | 30 ± 3.1 a | 141 ± 11 a | 18.1 ± 6.5 a | 0.46 ± 0.09a | 0.54 ± 0.05 a |
| Determinations | ||||
|---|---|---|---|---|
| Groups | TC 1 | TG 2 | HDL 3 | LDL 4 |
| Healthy control | 65.6 ± 4.0 a | 47.5 ± 2.1 a | 59.4 ± 6.3 a | 15.5 ± 1.4 a |
| 25 mg/Kg MEA | 66.7 ± 1.9 a | 50.6 ± 2.9 a | 58.4 ± 5.4 a | 14.7 ± 2.5 a |
| 50 mg/Kg MEA | 64.6 ± 2.1 a | 55.3 ± 3.8 a | 56.6 ± 2.3 a | 16.9 ± 2.1 a |
| 100 mg/Kg MEA | 68.7 ± 3.4 a | 58.8 ± 3.4 a | 50.7 ± 3.7 a | 13.12 ± 1.9 a |
| Diabetic control | 104.2 ± 2.1 b | 72.4 ± 4.8 b | 47.9 ± 2.3 b | 51.9 ± 4.3 b |
| Diabetic + 25 mg/Kg MEA | 84.1 ± 4.4 b | 84.1 ± 3.6 b | 46.8 ± 1.9 b | 30.5 ± 4.3 b |
| Diabetic + 50 mg/Kg MEA | 84.9 ± 3.1 b | 85.1 ± 4.3 b | 38.3 ± 3.7 ab | 47.8 ± 3.9 b |
| Diabetic + 100 mg/Kg MEA | 72.9 ± 2.8 a | 64.5 ± 2.1 a | 51.2 ± 1.8 b | 17.6 ± 3.9 a |
| Healthy Rats | Diabetic Rats | |||||||
|---|---|---|---|---|---|---|---|---|
| Healthy Control (HC) | 25 * | 50 * | 100 * | Diabetic Control (DC) | 25 * | 50 * | 100 * | |
| Base diet and water | √ | √ | √ | √ | √ | √ | √ | √ |
| Oral MEA | √ | √ | √ | √ | √ | √ | ||
| Oral Water | √ | √ | ||||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Méndez, A.J.; Carmen-Sandoval, W.; Lomas-Soria, C.; Guevara-González, R.G.; Reynoso-Camacho, R.; Villagran-Herrera, M.E.; Salazar-Olivo, L.; Torres-Pacheco, I.; Feregrino-Pérez, A.A. Timbe (Acaciella angustissima) Pods Extracts Reduce the Levels of Glucose, Insulin and Improved Physiological Parameters, Hypolipidemic Effect, Oxidative Stress and Renal Damage in Streptozotocin-Induced Diabetic Rats. Molecules 2018, 23, 2812. https://doi.org/10.3390/molecules23112812
Rodríguez-Méndez AJ, Carmen-Sandoval W, Lomas-Soria C, Guevara-González RG, Reynoso-Camacho R, Villagran-Herrera ME, Salazar-Olivo L, Torres-Pacheco I, Feregrino-Pérez AA. Timbe (Acaciella angustissima) Pods Extracts Reduce the Levels of Glucose, Insulin and Improved Physiological Parameters, Hypolipidemic Effect, Oxidative Stress and Renal Damage in Streptozotocin-Induced Diabetic Rats. Molecules. 2018; 23(11):2812. https://doi.org/10.3390/molecules23112812
Chicago/Turabian StyleRodríguez-Méndez, Adriana Jheny, Wendy Carmen-Sandoval, Consuelo Lomas-Soria, Ramón G. Guevara-González, Rosalía Reynoso-Camacho, María Elena Villagran-Herrera, Luis Salazar-Olivo, Irineo Torres-Pacheco, and Ana A. Feregrino-Pérez. 2018. "Timbe (Acaciella angustissima) Pods Extracts Reduce the Levels of Glucose, Insulin and Improved Physiological Parameters, Hypolipidemic Effect, Oxidative Stress and Renal Damage in Streptozotocin-Induced Diabetic Rats" Molecules 23, no. 11: 2812. https://doi.org/10.3390/molecules23112812
APA StyleRodríguez-Méndez, A. J., Carmen-Sandoval, W., Lomas-Soria, C., Guevara-González, R. G., Reynoso-Camacho, R., Villagran-Herrera, M. E., Salazar-Olivo, L., Torres-Pacheco, I., & Feregrino-Pérez, A. A. (2018). Timbe (Acaciella angustissima) Pods Extracts Reduce the Levels of Glucose, Insulin and Improved Physiological Parameters, Hypolipidemic Effect, Oxidative Stress and Renal Damage in Streptozotocin-Induced Diabetic Rats. Molecules, 23(11), 2812. https://doi.org/10.3390/molecules23112812