Abstract
Metabolomics has become a powerful tool in chemical biology. Profiling the human sterolome has resulted in the discovery of noncanonical sterols, including oxysterols and meiosis-activating sterols. They are important to immune responses and development, and have been reviewed extensively. The triterpenoid metabolite fusidic acid has developed clinical relevance, and many steroidal metabolites from microbial sources possess varying bioactivities. Beyond the prospect of pharmacognostical agents, the profiling of minor metabolites can provide insight into an organism’s biosynthesis and phylogeny, as well as inform drug discovery about infectious diseases. This review aims to highlight recent discoveries from detailed sterolomic profiling in microorganisms and their phylogenic and pharmacological implications.
1. Introduction
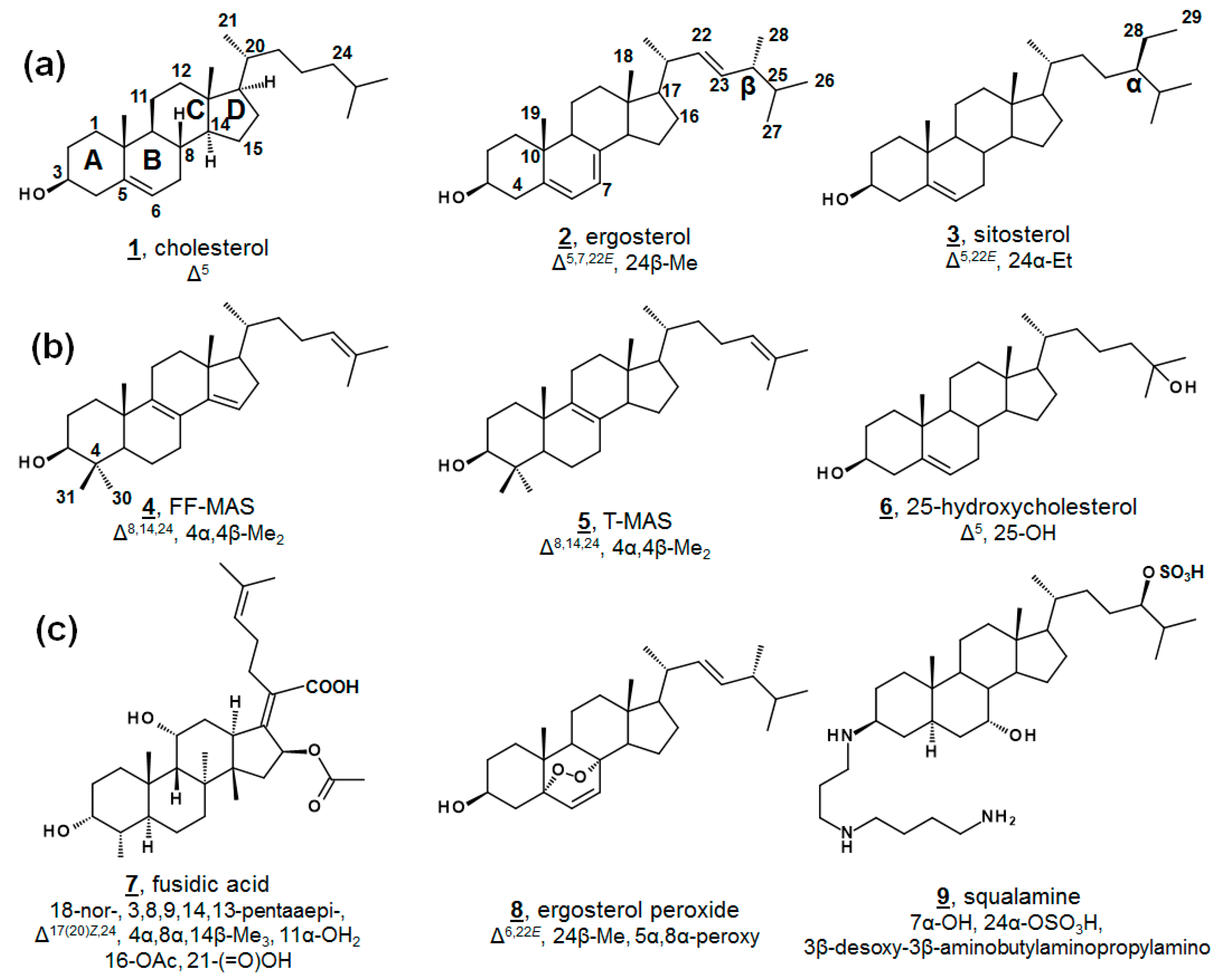
Sterols, like cholesterol 1, ergosterol 2, and sitosterol 3, as well as secondary metabolites, are amphipathic lipids that contain a 1,2-cyclopentanoperhydrophenanthrene ring nucleus (Figure 1). Sterols are ubiquitous molecules found in all eukaryotic life, serving a multitude of crucial biological functions [1]. Some prokaryotes synthesize sterols as well, and some prokaryotes contain enzymes with incomplete Δ5 sterol biosynthesis [1,2,3,4,5]. While sterol biosynthesis may predate eukaryotes [6], it is often hypothesized that aside from the protomitochondrial lineage, most bacteria have gained these genes via lateral gene transfer [3,4]. The end product of Δ5 sterols such as cholesterol 1 and ergosterol 2 (Figure 1a) contribute to cell membrane fluidity in their bulk insert role in mammals and fungi, respectively [1,7]. Steroidal secondary metabolites of the steroid hormone and bile acid classes serve well-known important roles in inflammation, sex characteristics, and lipid absorption [7].
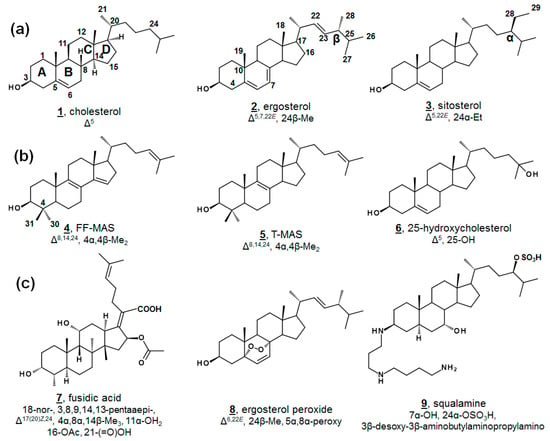
Figure 1.
Structure and numbering systems of sterols and steroids. (a) Δ5 end product inserts from mammals, fungi, and vascular plants, respectively, cholesterol 1, ergosterol 2, and sitosterol 3. (b) Examples of steroidal metabolites important in human biology for F-MAS 4, TT-MAS 5, 25-hydroxycholesterol 6. (c) Examples of steroidal metabolites from nonhuman sources with bioactivity, fusidic acid 7, ergosterol peroxide 8, and squalamine 9. The numbering system shown here, and used in this manuscript, is the conventional system [1]. Designations of α and β within the sterol nucleus signify below and above the plane. Unrelated to nucleus α and β, substituents on C24 are also designated α and β to reflect the C24 stereochemistries of sitosterol and ergosterol, respectively, as drawn above. Carbon numbering is provided on 1–4, and stereochemistries at C8, C9, C14, and C16 on structure 1 are hereafter implied on structures, unless otherwise annotated as in fusidic acid. Molecular features for each structure are provided relative to 5α-cholestanol for clarity. For a complete list of systematic names of compounds, see Table A1.
Minor components within the human sterol metabolome, which serve unusual but essential functions, have also been identified. For instance, the meiosis-activating sterols (MASs) 4,4-dimethylcholesta-8(9),14(15),24-trienol (follicular fluid meiosis-activating sterol; FF-MAS) 4 and 4,4-dimethylcholesta-8(9),24-dienol (testicular meiosis-activating sterol; T-MAS) 5 are biosynthetic intermediates in the cholesterol pathway that signal meiosis in mammalian oocytes and spermatozoa [7]. Various minor metabolites occurring both upstream and downstream of cholesterol have been demonstrated as ligands for nuclear hormone receptors and play critical roles in development and immunology, including 25-hydroxycholesterol 6 (Figure 1b) [8,9,10,11]. Recent advances in methodologies in lipidomics have expedited discoveries with regard to these necessary minor human sterols and steroids, as well as provided new diagnostic screens for patients with dysregulated sterol biosynthesis, as in Niemann-Pick and Smith-Lemli-Opitz syndrome. Contemporary discoveries in human sterolomics [11,12,13,14], as well as plant sterolomics [15], have been reviewed extensively elsewhere.
Metabolites can also be used to classify organisms and explore evolutionary relationships. Sterol distribution has long been used for chemotaxonomic purposes in plants [16], fungi [17,18,19], and other microorganisms [20,21,22]. There is also potential for sterols to serve as biomarkers, and sterol composition can play a role in feedstocks.
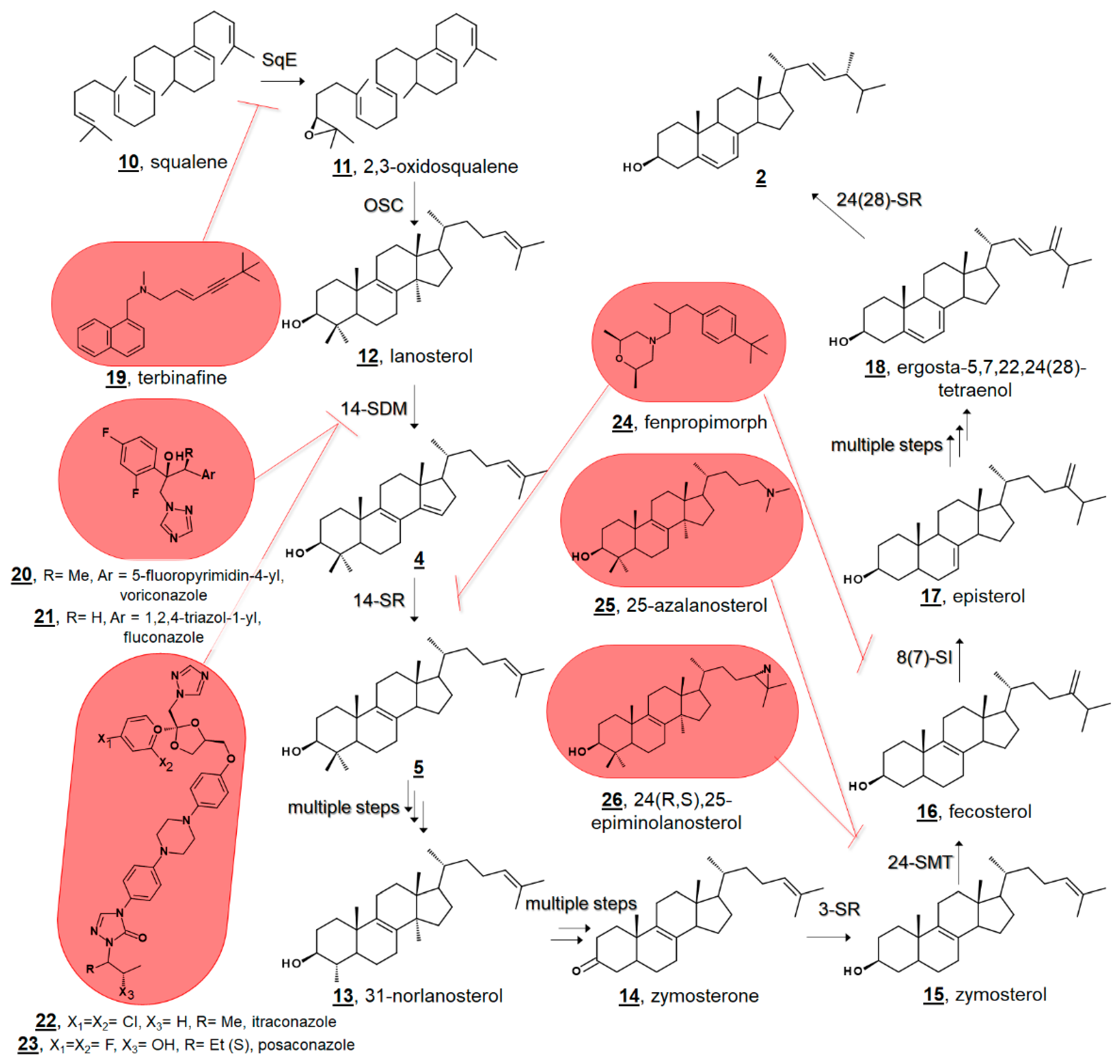
Small molecule ligands for ergosterol biosynthetic enzymes in fungi have long been clinically and agriculturally relevant [23,24,25]. Marketed antimycotics include molecules in such classes as allylamines, which target squalene epoxidase (SqE); azoles, which target sterol C14-demethylase (14-SDM = CYP51, =Erg11p in fungi); and morpholines, which target both sterol C14-reductase (14-SR, =Erg3p in fungi) and sterol C8(7)-isomerase (8(7)-SI, =Erg2p in fungi) (Figure 2) [24]. There is further interest in the design and discovery of inhibitors of other sterol enzymes, particularly sterol C24-methyltransferase (24-SMT, =Erg6p in fungi), which is absent from humans’ cholesterol biosynthesis [1,25,26]. Understanding sterol biosynthesis in non-fungal microbes may provide new insights for treating infections by eukaryotic pathogens.
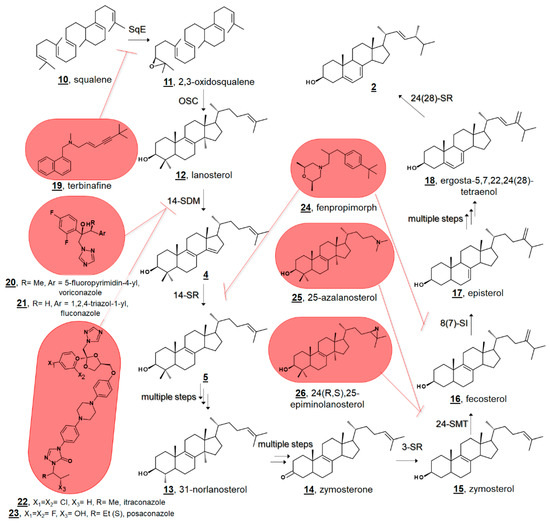
Figure 2.
Truncated hypothetical pathway of fungal ergosterol 2 biosynthesis from squalene 10. Inhibitor targets of squalene epoxidase (SqE) by allylamines, e.g., terbinafine 19, sterol C14-demethylase (14-SDM = CYP51) by azoles, e.g., voriconazole 20, fluconazole 21, itraconazole 22, and posaconazole 23, sterol C14-reductase (14-SR) and sterol C8(7)-isomerase (8(7)-SI) by morpholines, e.g., fenpropimorph 24, and sterol C24-methyltransferase (24-SMT) by 25-azalanosterol 25 or 24(R,S),25-epiminolanosterol 26 are highlighted at the biosynthetic steps they block. 3-SR; sterol C3 reductase, 24-SR, sterol C24 reductase.
Novel metabolites isolated from microbial sources are conversely often found to exhibit biological activity. Famously, fusidic acid 7 (Figure 1c), originally isolated from fungal Fusidium spp., is a tetracyclic triterpene antibacterial and has been used in the clinic for decades [27,28,29]. Fusidic acid inhibits growth by restricting protein synthesis via elongation factor G in Gram-positive bacteria, including Streptococcus spp., Clostridium spp., and penicillin-resistant strains of Staphylococcus spp. [28,29]. Structural analogues of fusidic acid, have shown varying antimicrobial, as well as anticholesterolemic and antineoplastic, characteristics [29]. Isolated from a variety of fungi and sponges, as well as vascular plants, ergosterol peroxide 8 possesses broad bioactivity, including anti-tumor, immunomodulatory, inhibitory hemolytic, anti-inflammatory, antioxidant, and antimicrobial properties. Several other endoperoxides of other phytosterols and of cholestenols have been reported to have similar properties, as well [30,31,32,33,34]. Squalamine 9 is a non-microbially derived natural steroidal, which has demonstrated antimicrobial and antiangiogenic properties and has led to interest in synthetic analogues for structure-activity improvement [35].
This short review aims to highlight new findings in microbial sterolomics, with respect to phylogeny, ecology, biosynthesis for drug discovery, and discovery of bioactive metabolites.
2. Phylogenic and Ecological Insights
2.1. Algal Phytosterol Biosynthesis
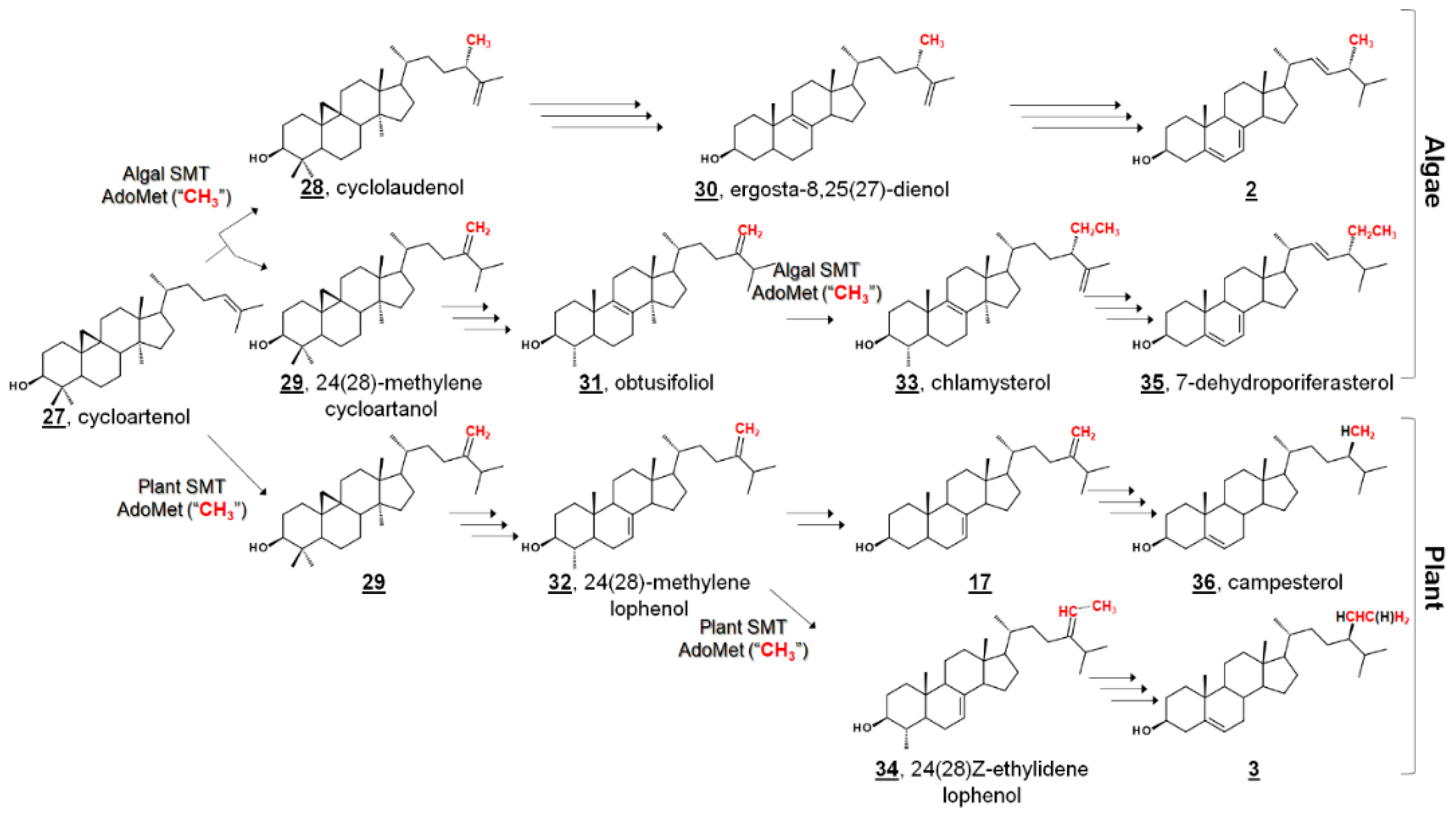
Ergosterol 2, having long been considered the “fungal sterol”, is nevertheless present in every major eukaryotic kingdom [1]. Ergosterol is present in amoebae [21,22,36,37] and trypanosomatids [38,39,40,41,42,43,44], and ergosterol is a major sterol of many taxa within green algae [20,45,46,47,48]. The unicellular green alga model organism Chlamydomonas reinhardtii uses ergosterol and its 24-ethyl analogue, 7-dehydroporiferasterol 35, as its main Δ5 sterols [45,46]. Vascular plants, on the other hand, chiefly use campesterol 36 and sitosterol 3 as Δ5 membrane inserts (Figure 3) [1,49]. Ergosterol and 7-dehydroporiferasterol differ from campesterol and sitosterol by units of unsaturation (double bonds) in the sterol nucleus and side chain, as well as stereochemistry at C24. While all four compounds possess 24R stereochemistry, 24-alkylation of ergosterol and 7-dehydroporiferasterol has β-stereochemistry (alkyl groups behind the plane, as drawn), while 24-alkylation of campesterol and sitosterol has α-stereochemistry (above the plane, as drawn) [1,45]. Conversely, the green alga synthesizes sterol from the photosynthetic protosterol. Fungi (nonphotosynthetic lineage) cyclize 2,3-oxidosqualene to lanosterol (Figure 2), while higher plants, and green algae, (photosynthetic lineage) cyclize 2,3-oxidosqualene to the plant protosterol cycloartenol [1,45].
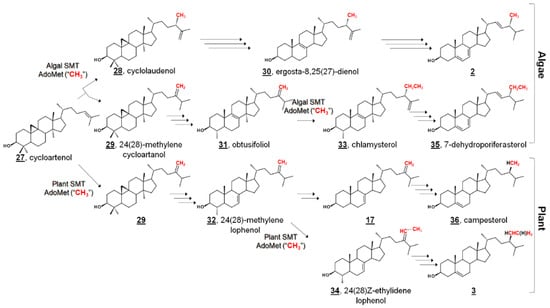
Figure 3.
Comparative phytosterol biosynthesis in the photosynthetic lineage from the protosterol cycloartenol 27. In algae, 24-methyl and 24-ethyl sterols arise from a bifurcation of products of biomethylation by sterol methyltransferase (SMT); In higher plants, they arise from alternate pathways from the intermediate 24(28)-methylene lophenol 30, which can be methylated again or metabolized to campesterol 36. Red methyl groups from SMT co-substrate S-adenosyl methionine (AdoMet) are annotated to show hypothetical labeling patterns of Δ5 sterols as discussed in [45,50]. An additional 15 algal sterols were reported in [45]. Truncated fungal phytosterol biosynthesis from protosterol lanosterol 12 is illustrated in Figure 2.
In C. reinhardtii, the biochemical pathway from the “plant” protosterol cycloartenol to the “fungal” Δ5 end product was investigated by sterolomic experiments of C. reinhardtii cultures. Sterol profiling of wild-type, mutant, and inhibitor-treated cultures revealed an additional 21 sterols beyond cycloartenol, ergosterol, and 7-dehydroporiferasterol 33 [45] (Figure 3). C. reinhardtii cultures that were not treated with a 24-SMT inhibitor contained only cycloartenol and 24-alkylsterols, indicating that bioalkylation and introduction of C28 by algal 24-SMT occurs upon cycloartenol itself early in the pathway. Further, 24-methylated cycloartenols were 24β-methylcycloart-25(27)-enol (cyclolaudenol) 28 and 24(28)-methylenecycloartanol 29, signifying a bifurcation of methylated products of algal 24-SMT [45]. The presence of C29 (i.e., a 24-ethyl group) on a 4α,14α-dimethyl sterol 33 led to the identification of obtusifoliol 31 as the substrate for the second biomethylation reaction of the algal sterol side chain, different from the substrate preference in higher plants (Figure 3). Furthermore, the alkylation product in plants has a 24-ethylene substituent, whereas the product in C. reinhardtii was found to bear a 24β-ethyl group with desaturation at C25 [45].
This pathway delineates algal biosynthesis of ergosterol disparate from the fungal pathway. In the former Δ25(27)-olefin pathway, C. reinhardtii alkylates sterols at C24 in a bifurcated manner to Δ25(27)-olefin and Δ24(28)-olefin products. Δ24(28)-Olefin products are further metabolized and later alkylated at C28 to only 24β-ethyl-Δ25(27)-olefin products. Conversely, fungal bioalkylation of C24 yields only Δ24(28)-olefin products, which are reduced to eventually yield ergosterol. That is, the stereochemistry of C24 in algal ergosterol arises from the methylation steps, whereas the stereochemistry of C24 in fungal ergosterol arises from a successive reduction step [45]. The Δ25(27)-olefin pathway was confirmed by sterol profiling of cultures incubated with isotopically labeled [methyl-2H3]methionine ([2H3]Met). These algal cultures incorporated three and five deuterium atoms into ergosterol and 7-dehydroporfierasterol, respectively [45].
The algal pathway was further corroborated by characterization of recombinant C. reinhardtii 24-SMT, found to catalyze the methylation of C24 by introduction of C28 and the methylation of C28 with C29. C. reinhardtii 24-SMT favored cycloartenol as a substrate, and a bifurcation of products to cyclolaudenol 28 and 24(28)-methylenecycloartanol 29 was found in ratios comparable to in vivo ratios of ergosterol and 7-dehydroporiferasterol [50]. A switch to Δ25(27)-olefin “algal” products of fungal or plant 24-SMT has been noted upon mutagenesis or incubation with electronically modified substrates [49,51]. In addition, obtusifoliol was found to be a substrate for the second methyltransfer of C. reinhardtii 24-SMT, 24β-methyl-Δ25(27)-sterols were not substrates, and incubation with [methyl-2H3]S-adenosyl methionine (2H3-AdoMet) produced labeled products with three and five deuterium atoms [50].
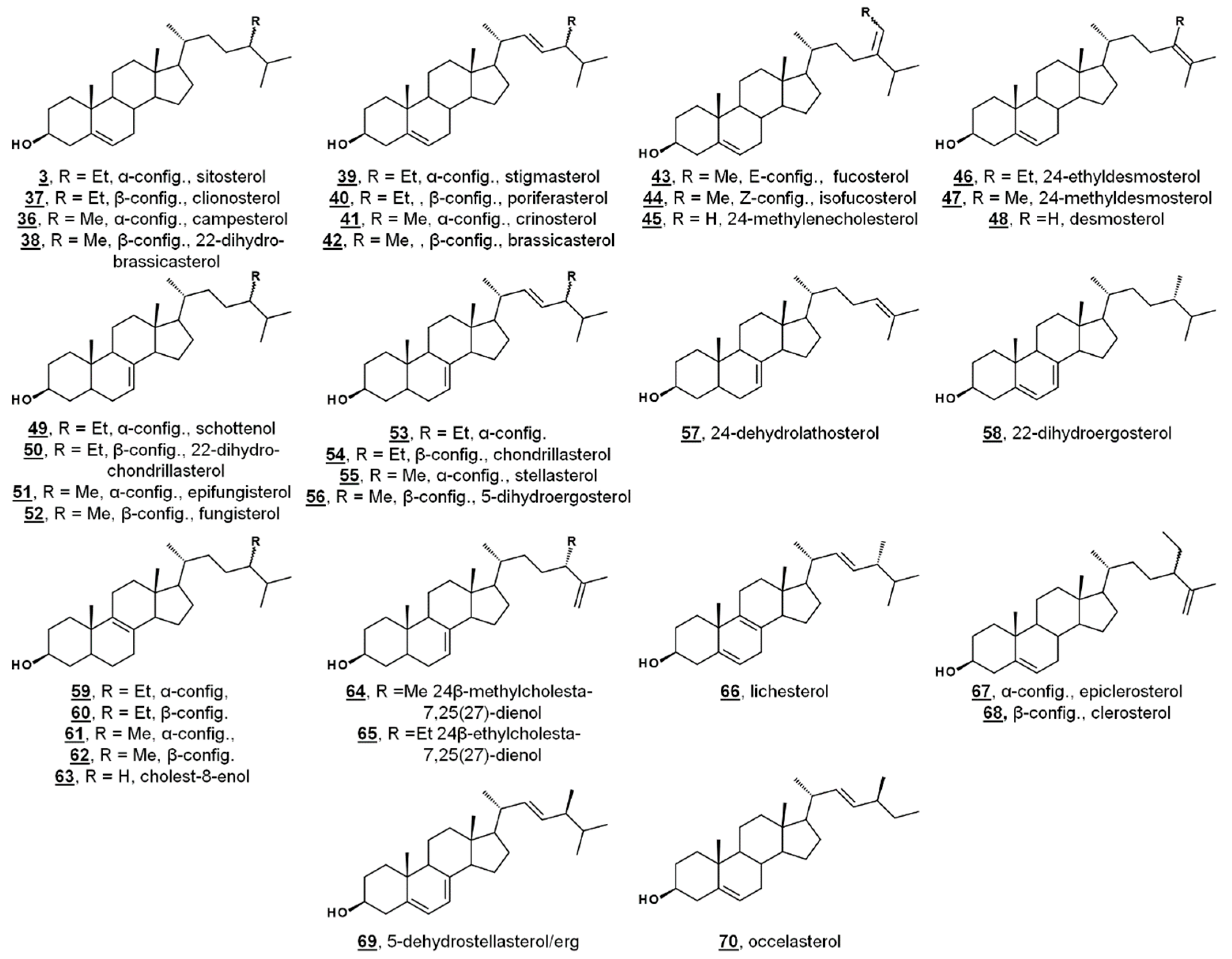
Green algae from the Acicularia spp. and Acetabularia spp. are macroscopic, yet unicellular. With a long and uninterrupted fossil record, they are often used to provide insight into the evolution of green algae and plants. Δ5-Bulk sterols of these genera lack Δ7 desaturations, in contrast to Chlamydomonas. Trimethylsilylated (TMS) sterols extracted from Acicularia schenckii and four species of Acetabularia revealed a principal Δ5 sterol (60–70%) of 24-ethylcholesterol (24α/24R = sitosterol 3, 24β/24S = clionosterol 37). Four other minor Δ5 sterols occurred in all five species: 24-methylcholesterol (24α/24R = campesterol 36, 24β/24S = 22-dihydrobrassicasterol 38), 24-ethylcholesta-5,22E-dienol (24α/24S = stigmasterol 39, 24β/24R = poriferasterol 40), 24-methylcholesta-5,22E-dienol (24α/24S = crinosterol 41, 24β/24R = brassicasterol 42), and 24-ethylidenecholesterol, which was tentatively assigned by the authors as the Δ24(28)Z isomer = isofucosterol 44. Among the TMS-derivatized sterols of Acetabularia caliculus, 24-ethylcholest-7-enol 46/47 was identified (Figure 4, Table 1) [52]. Prior studies had also identified cholesterol and 24-methylenecholesterol 45 in cultures of Acetabularia mediterranea, suggested by the authors to potentially be a result of differences in algal cultivation. Acetabularia caliculus also contained 24-ethylcholesterol in the sterol ester fraction, while the other Acetabularia species and Acicularia schenckii did not contain sterols in the ester fraction. These nearly identical sets of sterols from the five species, with a large separation in their geographical origin, illustrate a lack of divergence in sterol composition. It was thus hypothesized that these sterols represent an ancient biochemical trait within the photosynthetic lineage [52].
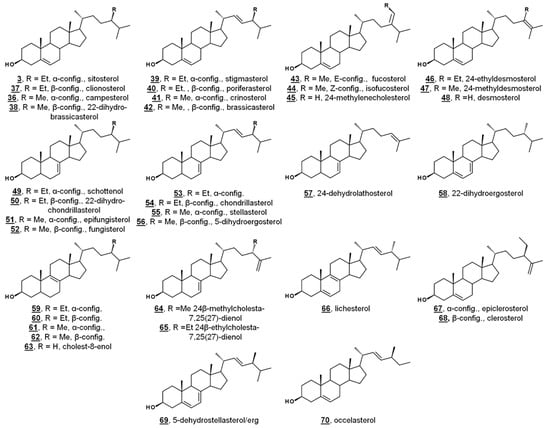
Figure 4.
Molecular structures of algal sterols.

Table 1.
Recently reported sterol profiles from algae across classes.
A study investigating the sterolome via free sterols and TMS derivatives from various classes of microalgae showed two species of the green algae Chlorella, C. vulgaris and C. luteoviridis, possessing different sterol profiles [53]. C. vulgaris contained chiefly ergosterol and fungisterol 52. In the past, C. vulgaris has been reported to also contain 7-dehydroporiferasterol. The reported minor components included 5-dihydroergosterol 56, 22-dihydroergosterol 58, 24β-methylcholesta-7,25(27)-dienol 64, 24β-methylcholest-8(9)-enol 62, and lichesterol 66. Conversely, the profile of Chlorella luteoviridis was dominated by poriferasterol 40 and 22-dihydrobrassicasterol 38, with minor composition by clionasterol 35, brassicasterol, and fungisterol [53]. The predominant sterol from Nanochloropsis limnetica was cholesterol, while its minor components were isofucosterol 44, 24-ethylcholesterol (3/37), 24-methylenecholesterol 45, and clerosterol 68 [53]. This report included the sterol profiling of several species of diatoms. The diatom Stephanodiscus hantzschii, whose sterols had not been studied prior to this report, had a composition of mostly 24-methylenecholesterol, with minor components of desmosterol 48, 24-methylenelathosterol (Δ7, rather than Δ5, termed episterol above) 17, and traces of two other sterols. Sterols from diatoms Cyclotella meneghiniana and Gomphonema parvulum were analyzed, with principal sterols of 24-methylenecholesterol and epibrassicasterol (called crinosterol, above; for list of trivial and systematic names, see Table A1) 41, respectively. C. meneghiniana also contained desmosterol 48, 24-methylenelathosterol, 24-dehydrolathosterol 57, and 24-ethyldesmosterol, and G. parvulum contained 5-dehydrostellasterol/ergosterol 69/2, 24α/β-ethylcholest-8(9)-enol 58/59, and campesterol/22-dihydrobrassicasterol 36/38 (C24 alkyl group was presumably α-oriented) [53]. A brief list of recently reported algal sterols by taxonomic class is presented in Figure 4 and Table 1; for more comprehensive and historical lists, see Refs. [47,48].
2.2. Trophic and Limnological Sterols
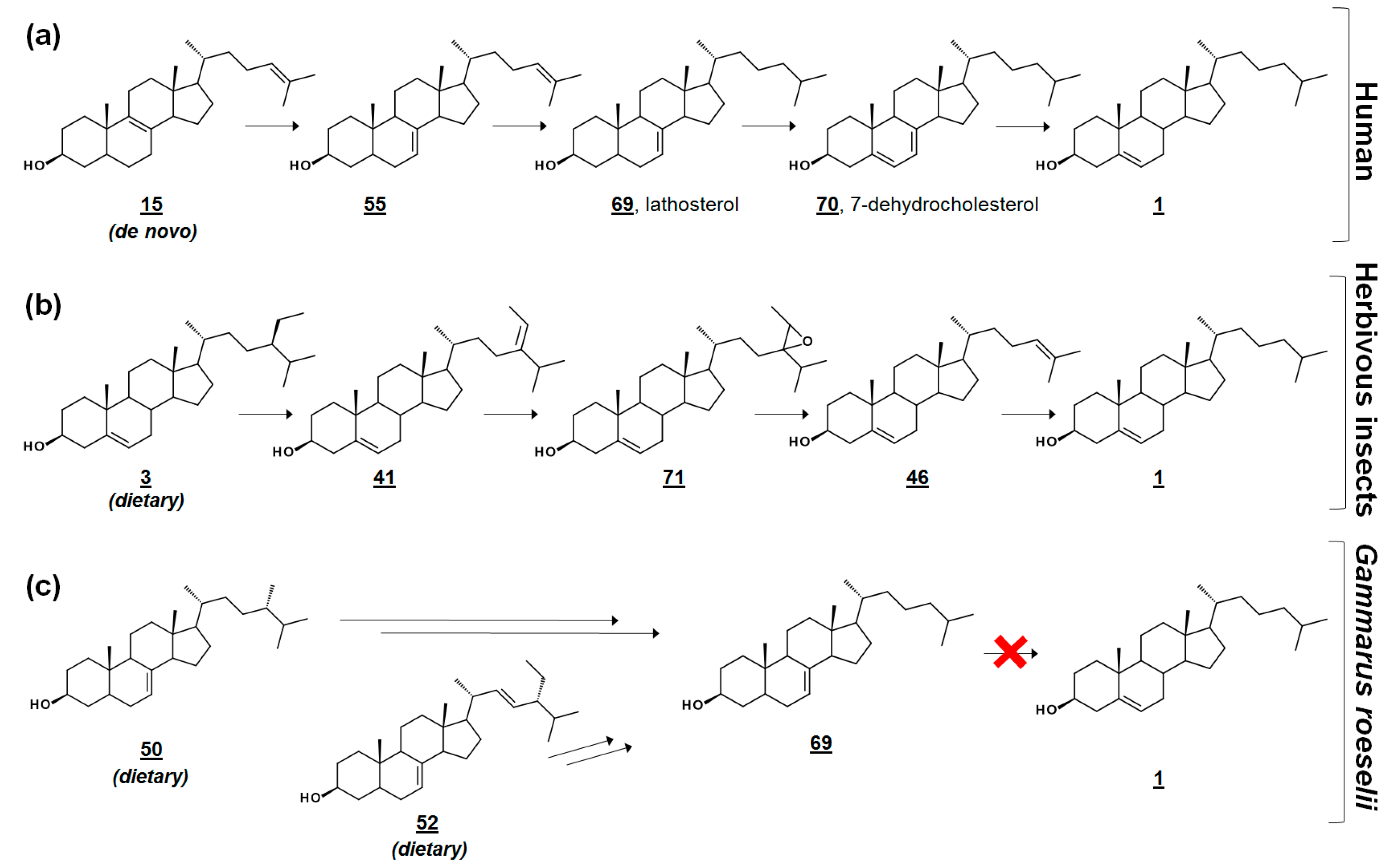
In the cross-class algal study [53], the researchers presented these profiles, along with their quantification, as references to the algal sterolome. As prey, Δ7 and Δ7,22 sterols are often nutritionally inadequate to invertebrate consumers [53,56,57]. Many invertebrates are auxotrophic for sterols and rely on diet to fulfill their sterol needs for cell membrane and hormonal requirements. Several of these specimens contain alternate enzymes, which dealkylate side chains of phytosterols, yet they lack the enzymes to desaturate C5–C6 or reduce C7–C8 (Figure 5) [57,58]. It has been proposed that these quantitated algal sterolome references can be used for studies involving the nutritional content of aquatic microorganisms for aquatic invertebrates [53]. Another study monitored the sterol profiles of an algal diet and the amphipod consumer Gammarus roeselii. Prey alga N. limnetica, rich in cholesterol, and alga S. obliquus, lacking cholesterol but rich in Δ7 sterols (See Table 1), were fed to G. roeselii. The sterol profile of S. obliquus-fed G. roeselii decreased in cholesterol, and increased in the Δ7 metabolite lathosterol 69, detectable when the diet was 50% S. obliquus (Figure 5) [56].
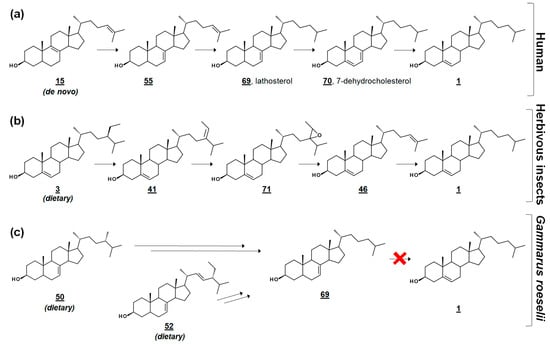
Figure 5.
Comparative cholesterol biosynthesis between humans and arthropods. (a) Late-stage cholesterol biosynthesis in humans from de novo zymosterol 15. (b) Proposed synthesis of cholesterol in herbivorous insects via dealkylation of dietary plant sterols (sitosterol) [58]. (c) Amphipod Gammarus roeselii can dealkylate the side chain of Δ7 algal sterols, such as fungisterol and chondrillasterol, but cannot produce cholesterol [56].
Isotopically labeled sterolomic experiments have been used to explore trophic modifications by the Northern Bay scallop Argopecten irradians irradians. Dietary alga Rhodomonas was supplemented with sterols enriched with 13C at the C22 position. The 13C-label was noted on new sterol metabolites, including those newly desaturated with Δ7 and those bearing an introduced 4α-methyl group. The mollusk’s ability to synthesize cholesterol from food was noted to correlate to Δ5 double bonds in the dietary sterols. They were more likely to dealkylate side chains possessing 24-ethyl groups. The only 24-methyl sterols dealkylated by A. irradians contained a Δ24(28) olefin (i.e., 24-methylene, rather than 24-methyl) [55].
A recent study investigated the lipid content of 37 strains within 10 classes of phytoplankton. Four classes, Cryptophyceae, Chlorophyceae, Treouciophyceae, and the diatoms are additionally represented in Table 1; this study additionally included dinoflagellates, euglenoids and the conjugatophyceae. Of the 37 strains, 29 sterols were detected, with notable variability of profile as a function of taxonomic class. The authors suggested Δ5,22 sterols as a potential biomarker for Chlorophyceae Sphaerocystis sp. and ergosterol as a potential biomarker for Chlamydomonas in habitats lacking other aquatic ergosterol-synthesizing microorganisms [59].
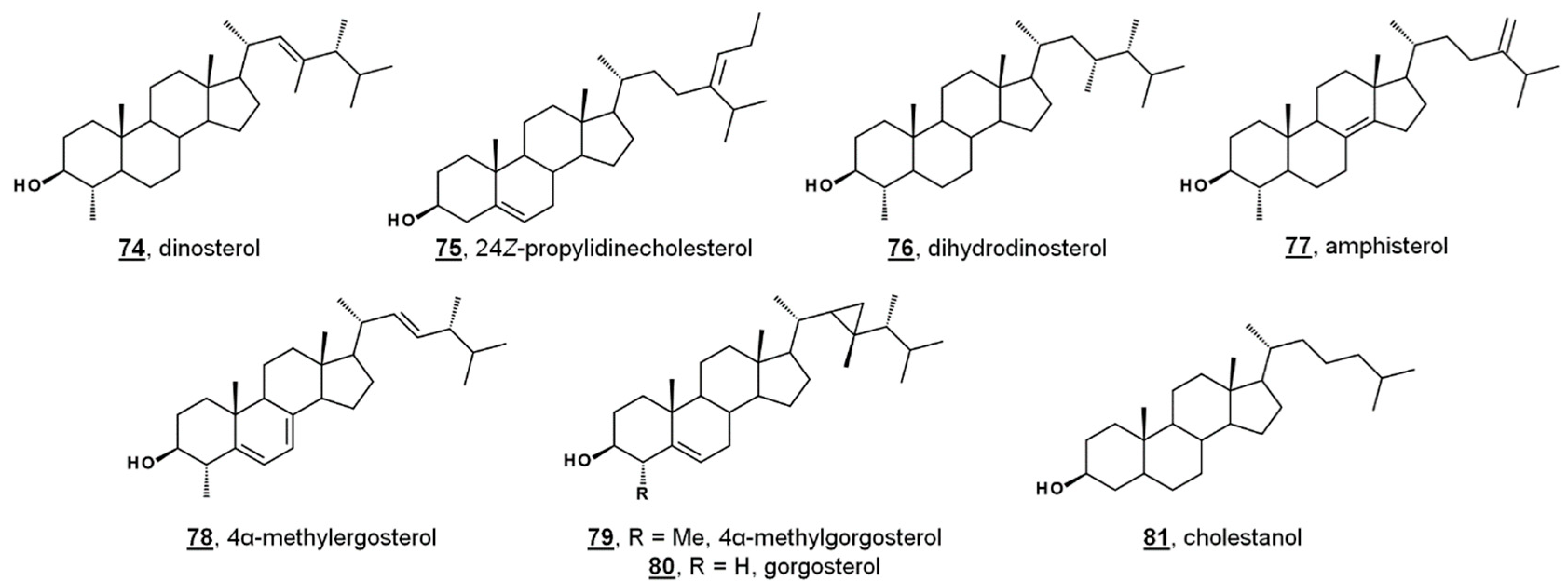
While sterol metabolites of toxic blooms are likely non-toxic to fish populations, these metabolites may have a stronger influence on marine invertebrates. Toxic bloom-causing algae Chloromorum toxicum, Chattonella marina, Heterosigma akashiwo, and Verrucophora farcimen [54] have sterol profiles given in Table 1. Verrucophora sp. were found to produce the rare 27-nor sterol occelasterol 68 (Figure 4) [54]. It has been proposed that isofucosterol 44 is a potential biomarker for the green-tide forming multicellular alga Ulva prolifera, and that dinosterol 74 and 24Z-propylidienecholesterol 75 are potential biomarkers for bloom-forming dinoflagellates [60] (Figure 6). Toxic bloom-causing dinoflagellate Cochlodinium polykrikoides had a sterol profile including prevalent sterols of dinosterol 74 (40%), dihydrodinosterol 76 (32%), and the rare 4α-methyl sterol amphisterol 77 (23%). Small amounts of 4-methylergost-24(28)-enol 78 (5%) were detected [61]. Two isolates of the bioluminescent dinoflagellate Pyrodinium bahamense had a sterol profile of largely cholesterol (74–75%), but also components of dinosterol 74 (13–14%) and 4α-methylgorgosterol 79 (11–13%), analyzed as their TMS derivatives. 4α-Methylgorgosterol is uncommon in dinoflagellates and has potential as a biomarker (Figure 6) [62].
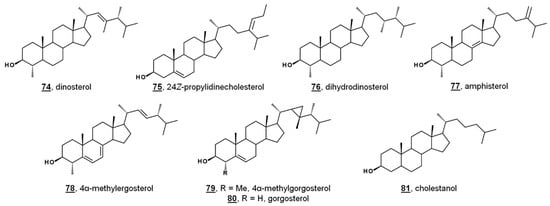
Figure 6.
Sterol structures from various dinoflagellates.
Lipidomic study of the coral Dendrophyllia cornigera revealed a geographical correlation to diet. D. cornigera analyzed from the Cantabrian Sea in the Northeast Atlantic reflected a productive environment, and the coral contained a high diversity of phytosterols. D. cornigera sampled from the Menorca Channel in the Mediterranean had a lower sterol content per dry weight and had less phytosterols. The Mediterranean coral had a higher relative abundance of occelasterol 70, brassicasterol 42, and cholestanol 81, or cholesterol and ergosterol, depending on the sample. The difference in the geographic profiles was attributed to a diet high in phytoplankton and herbivorous grazers in the Cantabrian coral, and a diet primarily consisting of dinoflagellates in the Mediterranean coral [63]. Specimens of the coral Agaricia spp. taken from shallow waters and deep waters were found to have markedly different sterol profiles from one another. From shallow Caribbean waters, Agaricia contained mostly cholesterol and 24-methylenecholesterol, with lower abundances of other phytosterols. Samples from deep waters contained mostly cholesterol and 24-ethylcholesterol. No gorgosterol was detected in either set. The Caribbean coral Montastraea cavernosa contained mostly 24-methylcholesterol, followed by cholesterol and gorgosterol, and variation in subsurface depth did not cause a significant change in sterol content. It was concluded that Agaricia spp. relies primarily on heterotrophy, even at greater depths [64].
3. Sterolome-Informed Antimicrobial Targets
3.1. Trypanosoma Brucei
Trypanosomatids are flagellated protozoa, all of which are parasitic. Some examples from this clade are Crithidia fasciculata, solely parasitic to insect hosts, Phytomonas serpens, soley phytopathogenic, and a number of human pathogens, including Trypanosoma cruzi, Leishmania spp., and Trypanosoma brucei, which are the etiological agents of the following human diseases: leishmaniasis, Chagas’ disease, and human African trypanosomiasis (also known as African sleeping sickness), respectively. Most of the species, C. fasciculata [38], P. serpens [40], T. cruzi [38,44], and Leishmania spp. [39] synthesize ergosterol and other 24β-methyl/24(28)-methylene-sterols (ergostenols) de novo as their Δ5 end products. In light of this de novo biosynthesis, there has been interest in using ergosterol biosynthesis inhibitors (EBIs) to treat Chagas’ disease and leishmaniasis, and some molecules have even progressed to the clinic [25,44]. Trypanosoma brucei, conversely, synthesizes ergostenols during its life cycle in the insect vector (procyclic form (PCF)), but uses largely cholesterol from the host’s blood as its Δ5 bulk sterol in the human host (bloodstream form (BSF)) [41,42,43].
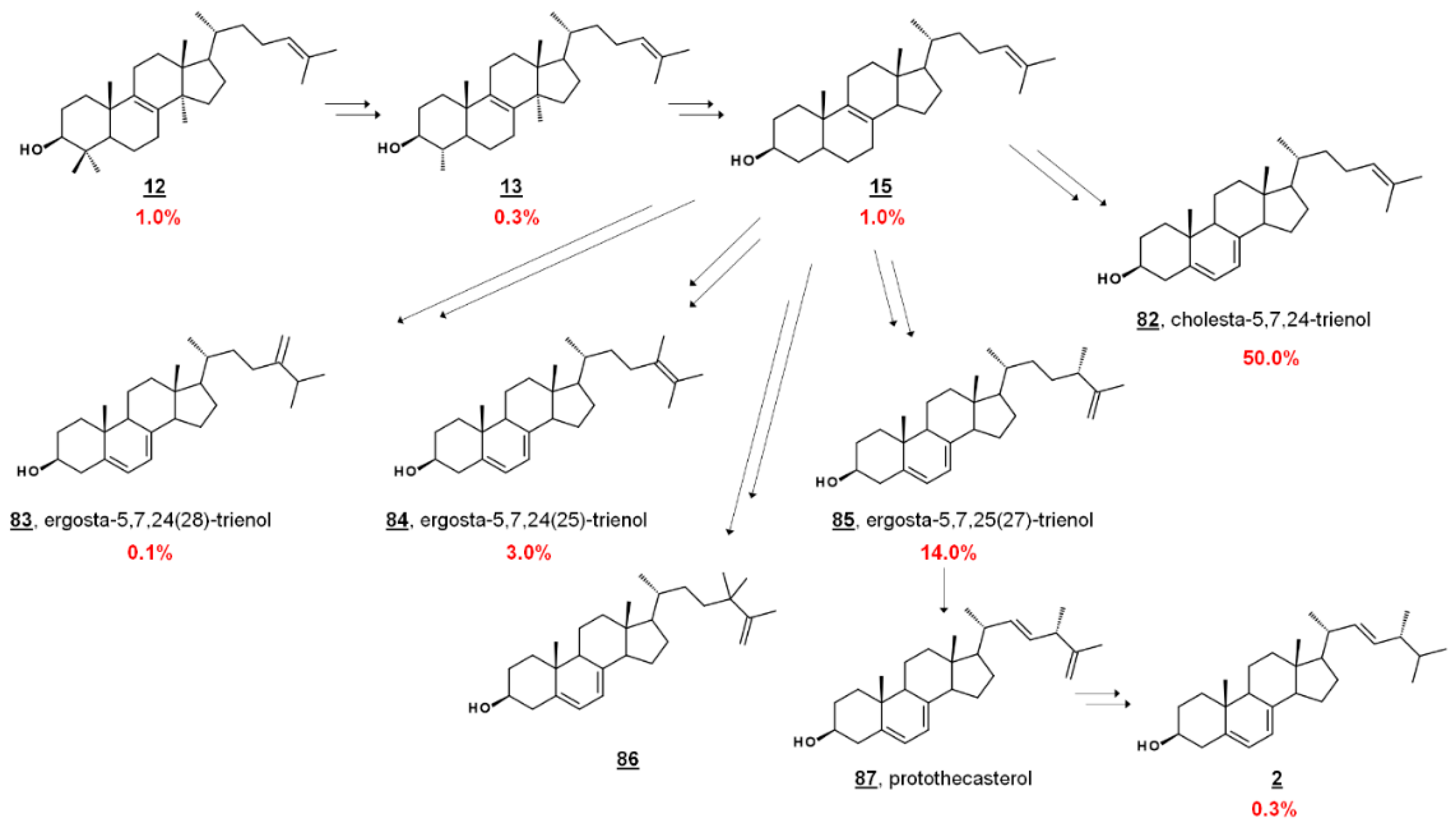
In the fly vector, cholesterol comprises a significant portion of the PCF sterol content. The profile contains sterols endogenous to PCF T. brucei, including prominent cholesta-5,7,24-trienol 82 and ergosta-5,7,25(27)-trienol 85. PCF synthesizes trace ergosterol 2; Ergosta-5,7,24(28)-trienol 85 and ergosta-5,7,24(25)-trienol 84 comprise some of the minor compounds present [41,42,43] (Figure 7). 24,24-Dimethylcholesta-5,7,25(27)-trienol 86 has also been detected in PCF profiles [42]. Culturing PCF in lipid-depleted media yields a higher composition of endogenous ergostenols and cholesta-5,7,24-trienol 82 relative to cholesterol [42,43]. Treatment of PCF cells with the 24-SMT inhibitor 25-azalanosterol 25 causes an increase in cholestenols in the profile [43].
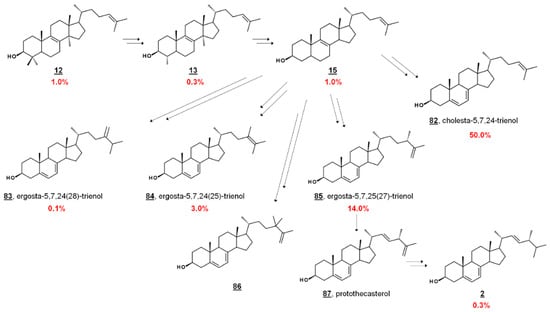
Figure 7.
Abbreviated biosynthetic sterol pathway and composition in T. brucei. In T. brucei, C4 is demethylated before C14, contrary to mammalian and fungal pathways (cf. Figure 2). Values are percentage sterol composition reported by Zhou et al. [43]. Dietary cholesterol 1 accounted for 20.0 %, and other components were 16 (0.1%), 30 (1.0%), 48 (1.0%), 57 (8.0%), and others (0.2%). 24,24-Dimethylcholesta-5,7,25(27)-trienol and 86 and protothecasterol 87 were not detected in this composition, but have been reported in subsequent studies [42,66], respectively.
Sterolomic analysis of PCF revealed a novel biosynthetic network. For instance, T. brucei demethylates protosterol lanosterol 12 at C4 initially (Figure 7), compared to mammalian and fungal pathways demethylating C14 first (cf. Figure 2) [42,43]. Moreover, the side chain methylation patterns of 24-SMT to yield Δ24(28), Δ25(27), and Δ24(25) products, as well as the Δ25(27) 24,24-dimethyl product 86, are unique [42,43,65]. Isotopic experiments with 13C-labeled carbon sources leucine, acetate, and glucose were shown to produce variable labeling of Δ5 endproducts and biosynthetic intermediates. No labelling was noted on cholesterol. Isotopic incorporation was higher with acetate and glucose. The variability of labeling was potentially attributed to the equilibrium of acetyl-CoA pools in the mitochondria and cytosol [42]. Trypanosomal sterols protothecasterol 87 (ergosta-5,7,22E,25(27)-tetraenol), cholesta-5,7,24-trienol 82, and ergosta-5,7,25(27)-trienol 83 have also been noted to incorporate isotope labeled from threonine [66].
In BSF T. brucei, however, the sterol content is overwhelmingly cholesterol, as well as dietary phytosterols, like sitosterol 3 and campesterol 36, present in the hosts’ blood [41,42,43]. Single trace 13C-labeled sterol was found in BSF cultures fed [1-13C]glucose [42]. Upon removal of the main sterol component cholesterol, detailed targeted sterolomics of BSF T. brucei cells revealed minor components of the sterol profile. Due to the S-cis double bond configuration in the B ring of ergosterol and compounds 81–87, UV absorbances of 282 nm can be monitored for the presence of endogenous Δ5,7 sterols, absent in serum. Endogenous cholesta-5,7,24-trienol and ergostenols were found at trace amounts, while they were undetectable when the presence of cholesterol was predominant. The ergosterol requirements for BSF was estimated to be 0.01 fg/cell, compared to the PCF requirement of 6 fg/cell [41]. Consequently, treatment with the EBIs itraconazole 22 and 25-azalanosterol 25 resulted in parasite death and an increased survival rate of infected mice. Correspondingly, the effects of EBIs on cultures were reversed upon supplementation of ergosterol [41].
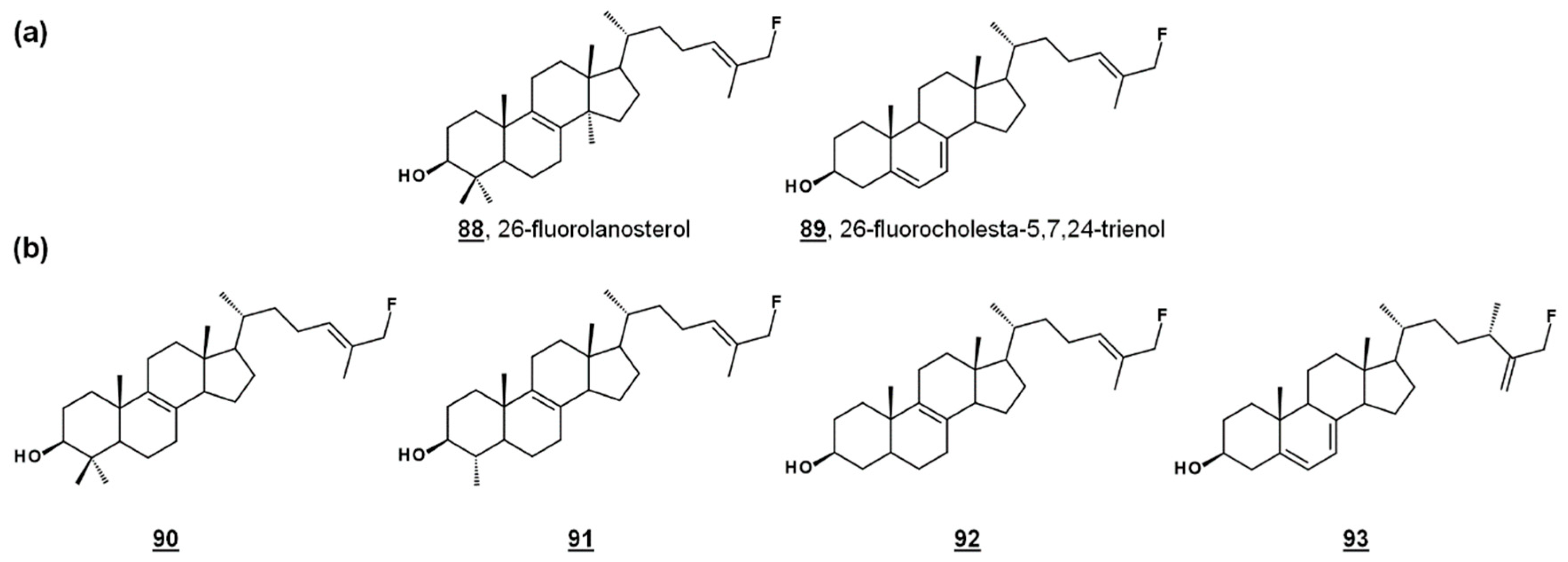
24-SMT substrate analogues substituted with fluorine at C26, 88 and 89 (Figure 8a), inhibited both PCF cultures and T. brucei 24-SMT in vitro. 26-Fluorolanosterol 88 inhibited trypanosome growth with an IC50 of about 3 µM, though it was not productively bound in T. brucei 24-SMT assays. 26-Fluorolanosterol is a reversible inhibitor of 24-SMT. Conversely, 26-fluorocholesta-5,7,24-trienol 89 is a substrate of 24-SMT, which can be turned over to 24-methylated products or bind irreversibly to the enzyme, with a kcat/kinact of 0.26 min−1/0.24 min−1. Sterol analysis of treated PCF revealed a loss of 24-alkylated sterols as well as a loss of 25(27)-desaturated sterols. Moreover, 26-fluorinated biosynthetic intermediates 90–93 downstream from lanosterol (Figure 8b) were detected in 26-fluorolanosterol-treated PCF and human epithelial kidney (HEK) cells. The activity of 26-fluorolanosterol on PCF was attributed to conversion to 26-fluorosterols lacking C4- and C14-methyl groups, capable of irreversibly binding to 24-SMT [67].
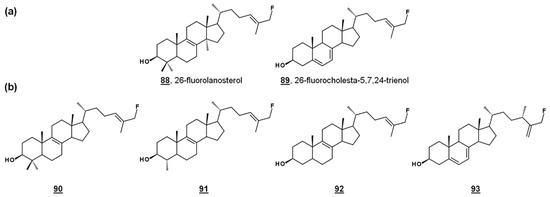
Figure 8.
26-Fluorinated sterol analogues. (a) Fluorinated inhibitors of T. brucei 24-SMT and growth. (b) Metabolites of 88 identified from T. brucei and HEK cells [67].
The importance of endogenous synthesis of ergostenols in BSF is accentuated by the effectiveness of other reported EBIs [41,43,67,68,69,70,71].
3.2. Acanthamoeba spp.
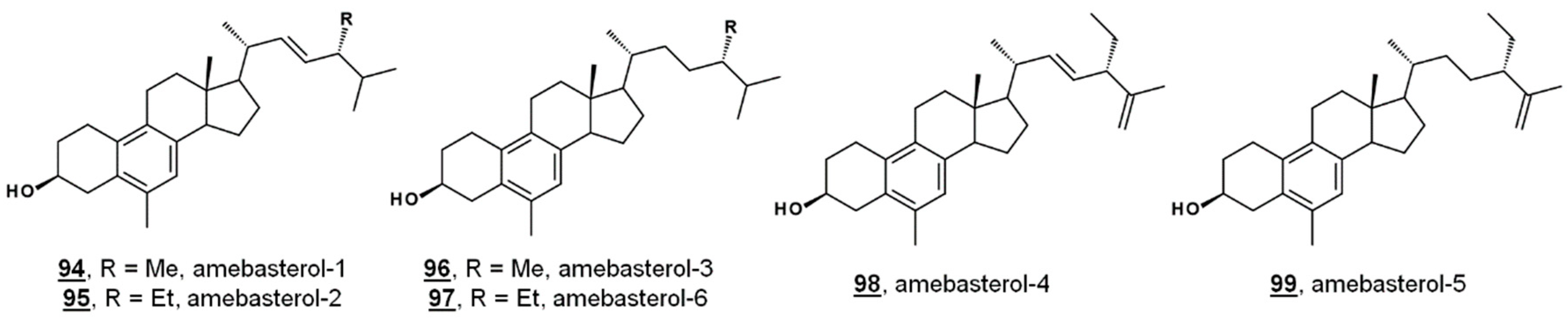
Ergosterol is a significant Δ5 bulk sterol in amoebae, as is 7-dehydroporiferasterol. Sterols are synthesized de novo in amoebae via a biosynthetic pathway involving the protosterol cycloartenol 25, as in green algae and higher plants. Amoebae also synthesize 19(10→6)-abeo-sterols containing aromatic B rings called the amebasterols [22,36]. Amebasterol-1 94, amebasterol-2 95, and amebasterol-4 98 have been described [22]; trace amebasterols-3 96, -5 99, and -6 97 have been identified as of late (Figure 9). These compounds can be selectively monitored at UV absorbances of 270 nm [36].
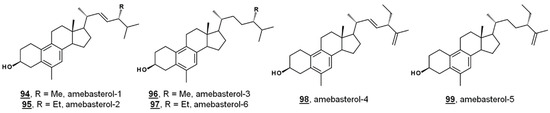
Figure 9.
Structures of amebasterols.
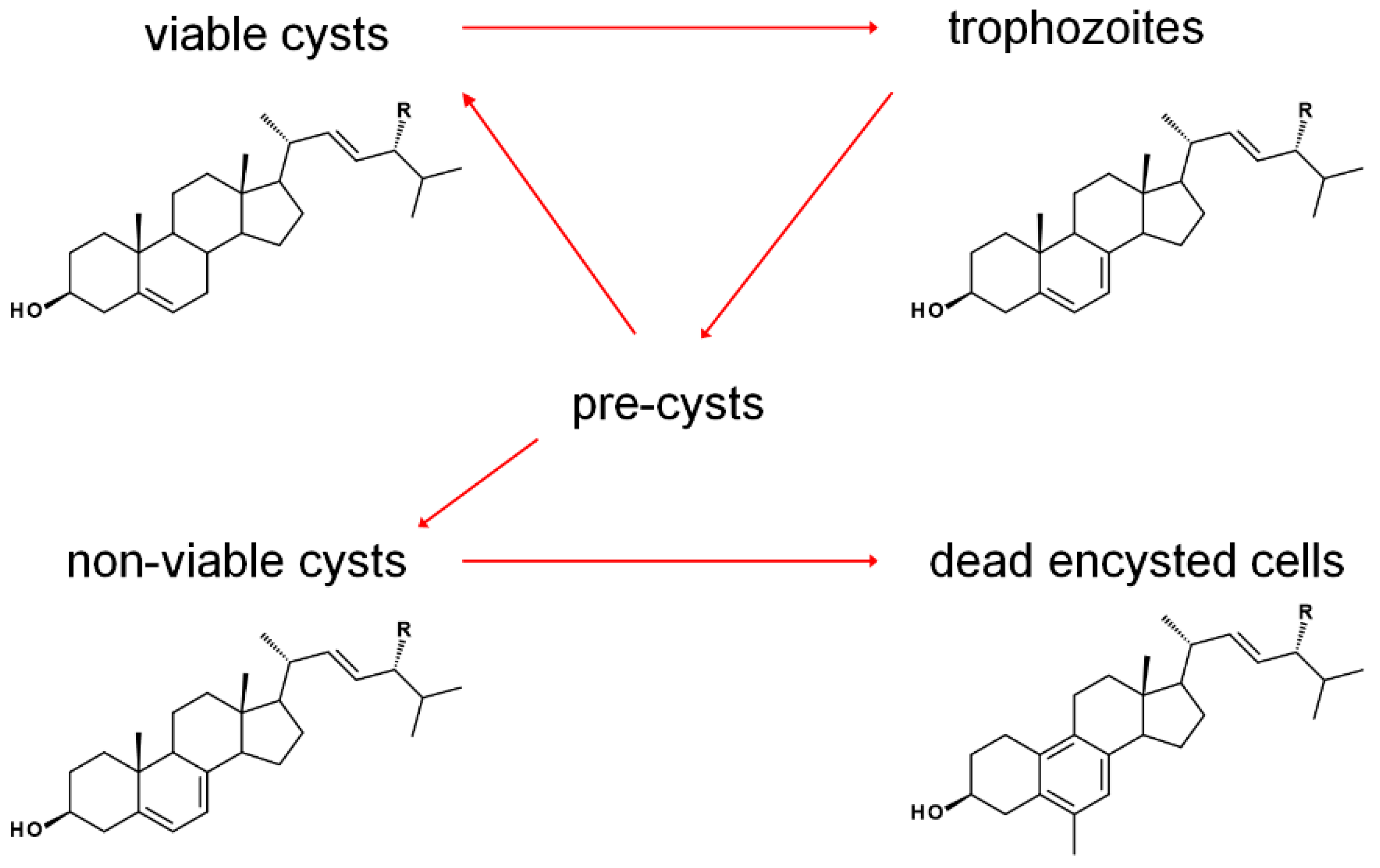
The sterol profile of was found to be variable as a function of growth and encystment phases. Analysis of the Acanthamoeba castellanii sterolome throughout the first week and one month after inoculation revealed a variable composition with changes to cell morphology and viability. At the beginning of the excystment-trophozoite-encystment cycle, in early log phase of growth, an accumulation of protosterol cycloartenol 27 and 24-methylenated cycloartanol 29 was noted. As the cells replicated, trophozoites contained mostly the Δ5,7 products ergosterol and 7-dehydroporiferasterol, whereas, in the stationary growth phase, with a mixture of trophozoites and cysts, sterols shifted to the Δ5 products brassicasterol and poriferasterol. Supplementation of trophozoite cultures with cholesterol had only a minor stimulation effect on their growth. After one-month incubation, dead cells were mostly comprised of amebasterols, amebasterol-1 94 and amebasterol-2 95 (Figure 9). The shift from Δ5,7 products in non-viable encysted cells to the amebasterols was attributed to turnover from stress and a sterol composition associated with altered membrane fluidity affording lysis (Figure 10) [36].
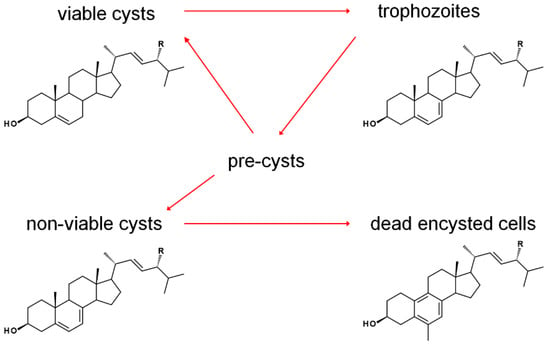
Figure 10.
Growth-phase dependence of predominant sterols in A. castellanii. R = Me and Et. Adapted from [36].
Beyond the protosterols, ergosterol/poriferasterol pairs, brassicasterol/poriferasterol pairs, and amebasterol-1/amebasterol-2 pairs, this study identified an additional 13 minor sterols in the metabolome of A. castellanii. Labeled experiments with [2H3]Met elucidated labeling patterns of dideuterated ergosterol and pentadeuterated 7-dehydroporfierasterol, consistent with a Δ24(28) product in its first biomethylation by SMT and a Δ25(27) product in the second biomethylation (Vs. Section 2.1) [36]. Labeling outcomes are supported by in vitro mechanisms with recombinant Acanthamoebic SMTs yielding a single Δ24(28) product in the first biomethylation (introduction of C28) [72]. While recombinant SMT yielded both Δ25(27) and Δ25(27) products for the second biomethylation (introduction of C29) [72], the authors concluded the labeling pattern of sterols from [2H3]Met-fed cultures, indicating that 24(28)-ethyidene sterols are not incorporated into 7-dehydroporiferasterol under physiological conditions [36].
The noted pairs of cycloartenol and 24(28)-methylenecycloartanol (24-H/24-Me), and pairs of ergosterol/poriferasterol, brassicasterol/poriferasterol, and amebasterol-1/amebasterol-2 (each 24-Me/24-Et) in the various portions of the Acanthamoebic life cycle [36], along with product outcomes being largely determined by biomethylation patterns of A. castellanii SMTs [36,72], underscores the crucial nature of SMT function in the pathogen. Subtle alterations in substrate selectivity were noted to have a profound impact on the balance of 24-methyl and 24-ethyl sterols [36]. After treatment with the 24-SMT inhibitor 24(R,S),25-epiminolatnosterol 26 and the azole 14-SDM inhibitor voriconazole 20 (See Figure 2 for structures), and small increase in amounts of cycloartenol and obtusifoliol were noted [72,73]. Upon treatment with EBIs, trohpozoites were stimulated to encyst, while excystment was insensitive to treatment. The correlation between stage-specific sterol compositions and the physiological effects of EBIs provide insight on opportunities for therapeutics (Figure 10). It is imagined that EBIs targeting the enzyme that reduces the Δ7 olefin of ergosterol/7-dehydroproferasterol to brassicasterol/poriferasterol could be used to modulate Acanthamoeba growth phases and prevent recurrence of the disease [36].
Azole inhibitors of 14-SDM have been reported to restrict Acanthamoeba growth in the nanomolar to micromolar range [36,37,73,74,75,76], and inhibitors of 24-SMT have been reported with nanomolar activity against Acanthamoeba cultures [36,72]. Treatments of 14-SDM- and 24-SMT-inhibitors in combination led to complete eradication of the amoeba parasite at concentrations as low as their respective IC50s [36].
3.3. Fungal Sterol Profiles in Drug-Treated Cultures
EBIs are a staple of antimycotic drug discovery [23,24,25]. A general hypothetical biosynthetic pathway, as well as popular block points for EBIs, are presented in Figure 2. Sterolomics can be used to confirm the inhibition of ergosterol biosynthesis upon treatment with new small molecules with antifungal properties.
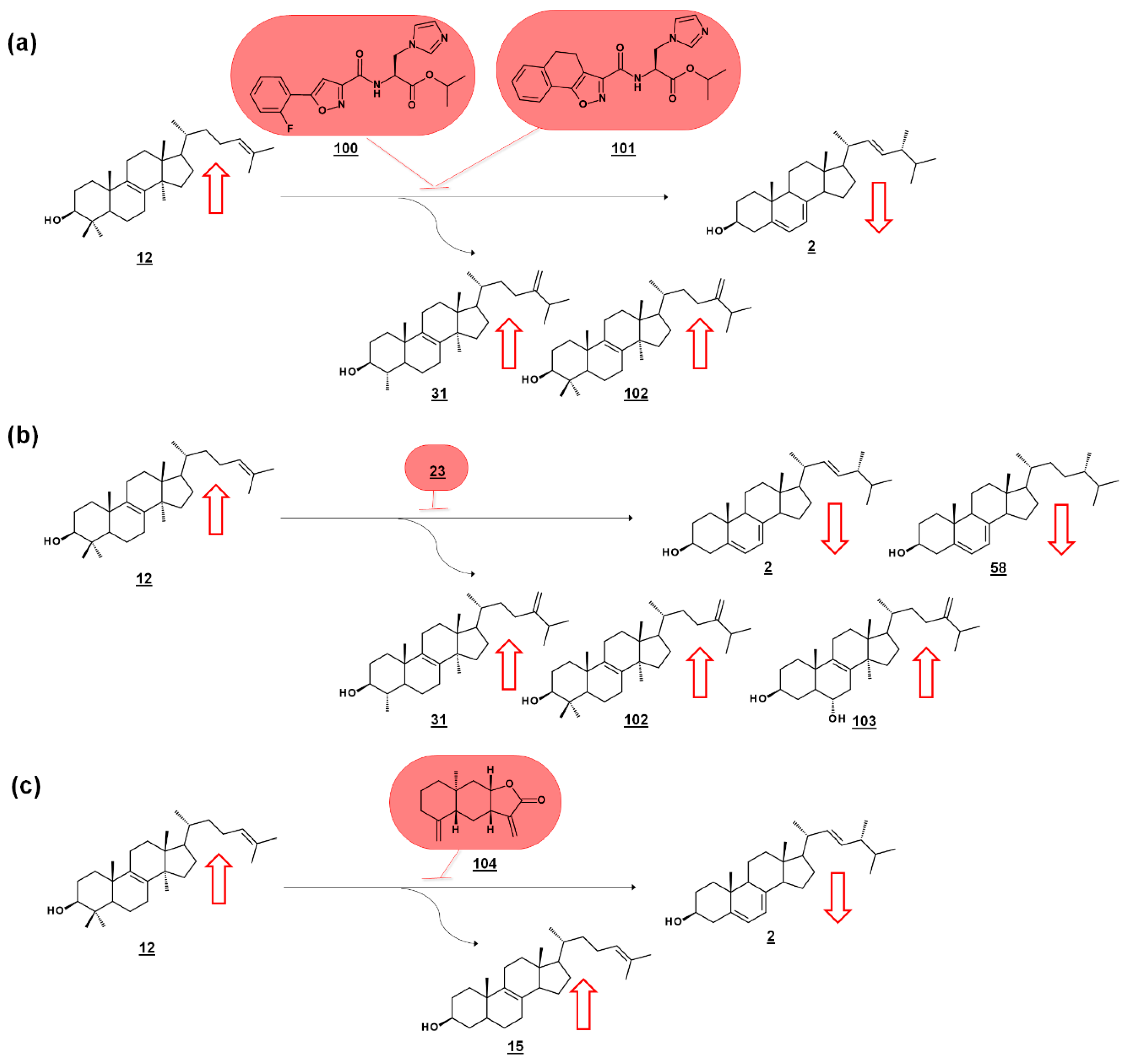
Series of amidoesters substituted with imidazolylmethyl groups were reported to have bioactivity against opportunistic fungal pathogens Candida albicans, Candida tropicalis, Cryptococcus neoformans, and Aspergillus fumigatus [77,78]. Some of these compounds, including 100 [77] and 101 [78] (Figure 11a) displayed better antifungal properties than fluconazole 21 (cf. Figure 2). The sterols of C. albicans administered with these compounds were analyzed to confirm a mechanism of disrupting ergosterol biosynthesis. Ergosterol normally comprises of the vast majority of the sterol profile in C. albicans (>98%), and treatment with 100 [77] or 101 [78] reduced ergosterol in a dose dependent manner. Dose-dependent increases in lanosterol 12 were noted, as well as increases in 14α-methylsterol by-products eburicol 102 and obtusifoliol 31 (Figure 11a). The increase in lanosterol (substrate for C. albicans 14-SDM), the increase in 14-methylsterols, and a commensurate decrease in ergosterol itself, suggested 14-SDM as a target for these molecules [77,78].
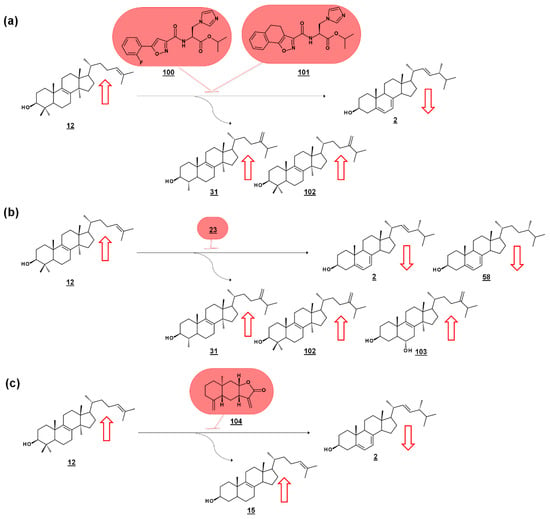
Figure 11.
Sterolomic identification of ergosterol biosynthesis inhibitors (EBIs) in fungi. Red arrows signify increase or decrease in sterols within the profile of inhibited cultures relative to non-inhibited cultures. (a) Oxazole amidoester-treated cultures of C. albicans decrease in ergosterol and increase in lanosterol and by-products obtusifoliol and eburicol, indicating disruption of 14-SDM activity [77,78]. (b) Posaconazole-treated cultures of Rhizopus arrhizus decrease in ergosterol and ergosta-5,7-dienol and increase in lanosterol, obtusifoliol, and eburicol, and produce toxic 14-methylergosta-8,24(28)-dien-3β,6α-diol [79]. (c) ent-Isoalantolactone-treated cultures of C. albicans decrease in ergosterol and increase in lanosterol and zymosterol, indicating disruption of 24-SMT activity [80].
Many molds, like clinically relevant Mucorales, methylate the side chain of protosterol lanosterol 12, before demethylating the sterol nucleus, to produce eburicol 102 as a normal intermediate (Figure 11b). Sterols were examined from six pathogenic molds from the order Mucorales, as well as sterols from cultures treated with the azole drug posaconazole 23 (cf. Figure 2). The untreated molds were reported to contain ergosterol, with prominent composition by ergosta-5,7-dienol 58. An additional 12 sterols from untreated cultures were reported. Rhizopus arrhizus contained 76.3% ergosterol and 10.6% ergosta-5,7-dienol within its sterol fraction. Upon administering sub-lethal concentration of 0.5 µg/mL posaconazole 23, these percentages were reduced to 58.5% and 5.1%, respectively. Correspondingly, lanosterol and eburicol 102 increased with azole, and other 14-methylsterols were noted. Moreover, non-physiological and toxic 14-methylergosta-8,24(28)-dien-3β,6α-diol 103, which had only been found prior in azole-dosed yeasts, was detected at 0.7% in treated cells (Figure 11b) [79].
Of a set of sesquiterpenes isolated from Chinese liverwort Tritomaria quinquedentata (Huds.) Buch., 5 exhibited activity against strains of C. albicans. The most potent of these compounds, ent-isoalantolactone 104 suppressed hyphal formation of the yeast and was further investigated for its antifungal mechanism. An increase in lanosterol 12 and zymosterol 15 was noted in C. albicans sterol composition when applied with MIC80 concentrations of ent-isoalantolactone (Figure 11c). The accumulation of zymosterol connotes inhibition of Erg6p (=24-SMT). Subsequent transcriptional analysis of treated C. albicans revealed increased expression of the Erg6 gene 9.3-fold and the Erg11 (=14-SDM) gene 2.7-fold, supporting the sterolomic findings [80].
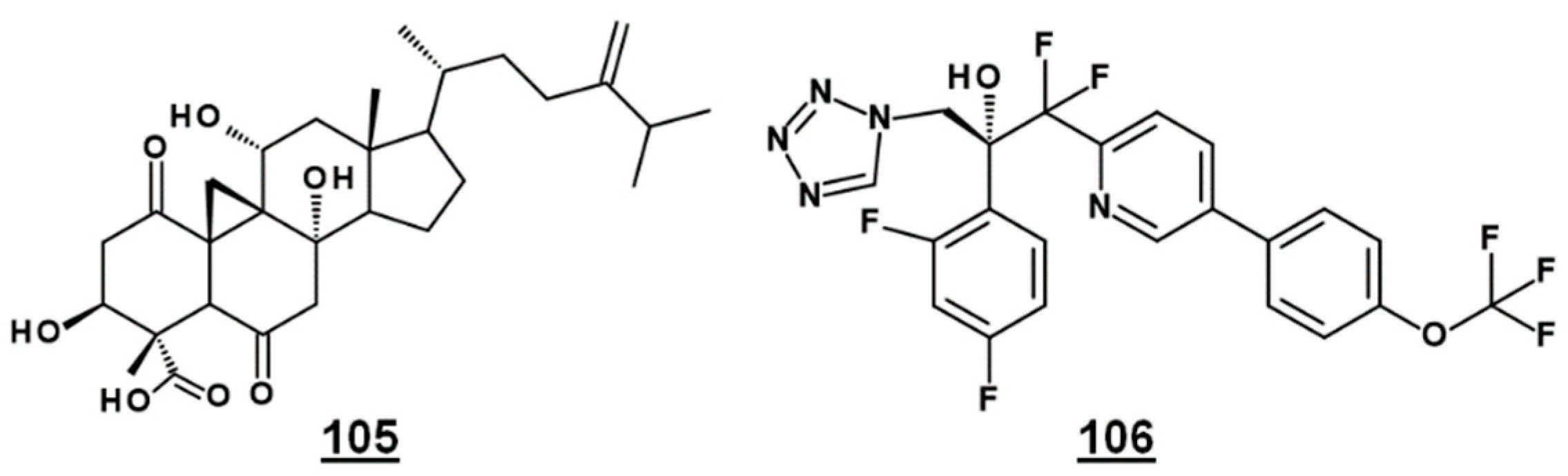
Bioactive natural product FR171456 105 (Figure 12) was shown to inhibit ergosterol biosynthesis of C. albicans, by a dose-dependent decrease in labeled zymosterol 15 and ergosterol and increase in labeled lanosterol, upon co-incubation with 13C-glucose, 13C-acetate. Similarly, fluconazole 21 –treated cultures also decreased in zymosterol and ergosterol [81]. Likewise, investigative drug VT-1129 106 (Figure 12) caused an increase in lanosterol, eburicol, obtusifoliol, and its 3-ketone analogue, as well as reduction in ergosterol and fungisterol, in Cryptococcus sp. [82].
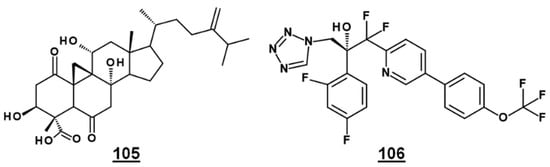
Figure 12.
EBIs FR171456 105 and VT-1129 106, confirmed by sterolomic analysis.
4. Bioactive Steroidal Metabolites
Endogenous oxysterols play essential roles in human biology, including signaling, development, and immunology [8,9,10,11]. Similarly, several oxysterols isolated from microbial sources have been reported to exhibit therapeutic properties. Many of these compounds from microbes are oxyphytosterols, i.e., unlike human endogenous sterols, they possess alkyl groups at C24 and therefore do not occur in human biology. Bioactivities include those against cancer cell lines, as well as ligands for nuclear receptors, antioxidants, anti-inflammatory agents, and inhibitors of amyloid-β (Aβ) aggregation.
Minor steroidal metabolites often possess bioactivity against other microbes, like fusidic acid, as discussed above. Study of these natural products can further lead to semi-synthetic analogues for structure-activity relationship studies and improvement of antimicrobial agents. For instance, squalamine 9 (Figure 1c), isolated from dogfish shark, is a steroid with polyamine substitution. The cationic polyamine moiety and its polyvalence have been attributed to much of its antimicrobial and anticancer properties [35], and, as a result, a class of synthetic and semi-synthetic analogues, collectively termed cationic steroid antibiotics, have been developed [35,83,84]. For the purposes of this review, only isolated compounds are discussed, though these compounds can inform synthetic and semisynthetic analogues for increased bioactivity. Likewise, steroidal metabolites with a compromised cyclopentanoperhydrophenanthrene nucleus are omitted here.
4.1. Peroxides
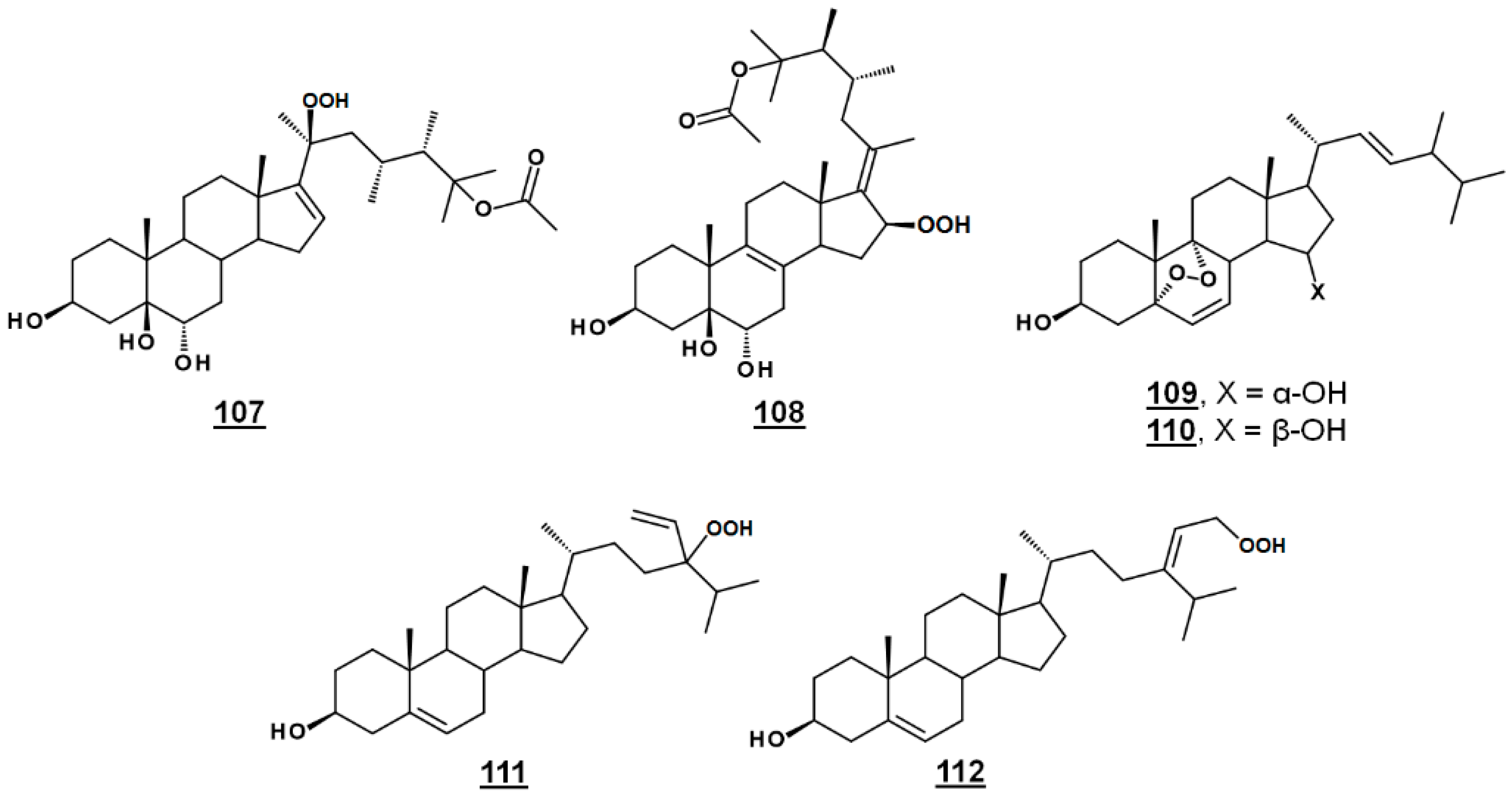
Michosterol A 107 (Figure 13) is a newly described polyoxygenated sterol with a C20 hydroperoxyl group and a C25 acetoxyl group, isolated by the ethyl acetate extract of the soft coral Lobophytum michaelae. Michosterol A demonstrated moderate cytotoxic effects against A549 cells, with an IC50 of 14.9 µg/mL, and was not cytotoxic (IC50s > 20 µg/mL) to DLD-1 and LNCap cell lines. Its anti-inflammatory activity was examined by assaying against superoxide formation in human neutrophils and against elastase release. Michosterol A had IC50s of 7.1 µM and 4.5 µM for superoxide anion generation and elastase release, respectively. A second hydroperoxyl polyoxygenated sterol (C15 hydroperoxyl, and Δ17(20)), named michosterol B 108 (Figure 13) was discovered in this extract. Michosterol B did not display cytotoxicity against the cell lines tested, but inhibited superoxide anion generation 14.7% and elastase release 31.8% each at 10 µM michosterol B [85].
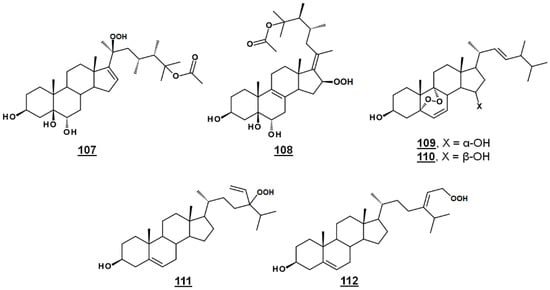
Figure 13.
Steryl peroxides discussed in text.
Nigerasterol A 109 and nigerasterol B 110 (Figure 13) are C15 epimers of 3,15-diols containing a 5,α,9α-peroxide obtained from Aspergillus niger MA-132, an endophytic fungus isolated from the mangrove plant Avicennia marina. Nigerasterol A and nigerasterol B inhibited cell growth in cancer cell lines HL-60 (IC50s 0.3 µM and 1.50 µM, respectively) and A549 (IC50s 1.82 µM and 5.41 µM, respectively) [32].
24-Vinyl-24-hydroperoxycholesterol 111 (Figure 13) has been isolated from Xestponsgia sp. [33,86]. It had an IC50 in an NF-κB-luciferase assay of 31.3 µg/mL [33] and restricted growth of various human cell lines, including A549 (IC50 29.0 µM) and WI-38 (IC50 43.4 µM) [86]. From Xestospongia, the 29-hydroperoxyl derivative 112 (Figure 13) of isofucosterol has also been reported, with broad activity against such targets as NF-κB-luciferase (IC50 12.6 µg/mL), 3-hydroxy-3-methylglutaryl CoA reductase (HMGR)-green fluorescent protein (IC50 3.8 µg/mL) and protein tyrosine phosphatase 1B (IC50 5.8 µg/mL) [33].
4.2. Acetates
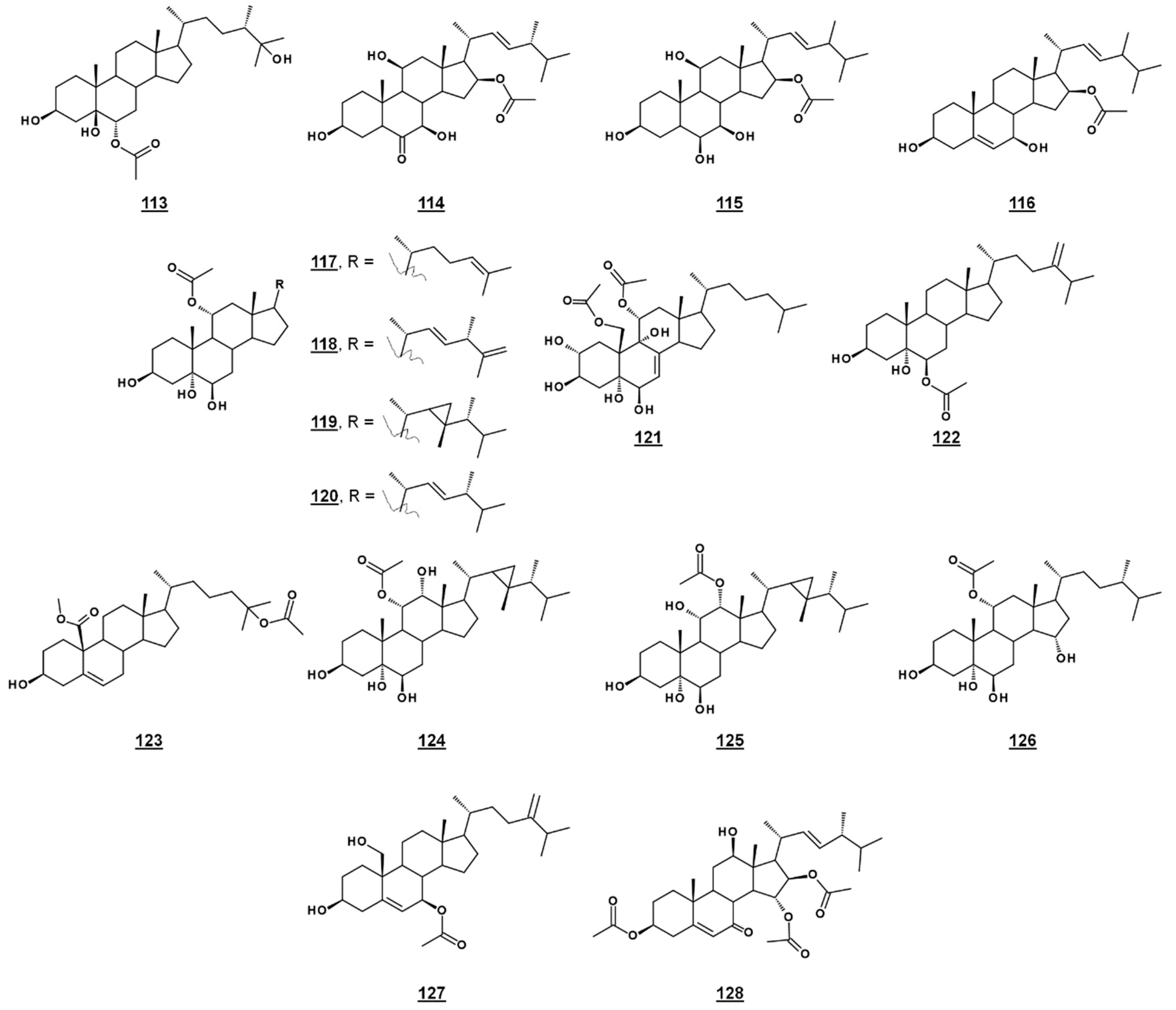
A third michosterol, michosterol C 113 (Figure 14), isolated from the soft coral Lobophytum michaelae (Vs. 4.1. peroxides) lacked a peroxyl moiety, but contained a 6α-acetoxyl group. Michosterol C was not cytotoxic on cell lines tested, but inhibited superoxide anion generation 17.8% at 10 µM and had an IC50 for elastase release of 0.9 µM [85].
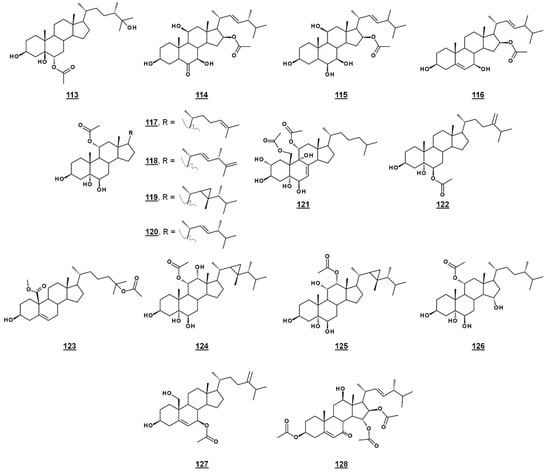
Figure 14.
Steryl acetates discussed in text.
Anicequol 114 (Figure 14), also known as NGA0187, is a polyhydroxylated ergost-6-one first described in 2002. Originally isolated from the fungi Penicillium aurantiogriseum Dierckx TP-F0213 [87] and Acremonium sp. TF-0356 [88], Anicequol inhibited anchorage-dependent growth of human colon cancer DLD-1 cells with an IC50 of 1.2 µM [87]. Anicequol was found to induce anoikis, or apoptosis by loss of cell adhesion to the extracellular matrix. Induction of anoikis by anicequol, as well as 25-hydroxycholesterol, was additionally found to involve p38 mitogen-activated protein kinase (p38MAPK) and Rho-associated, coiled-coil containing kinase (ROCK), suggesting new therapeutic strategies against cancer [89]. Anicequol has neurotrophic activity and induced significant neurite outgrowth at 30 µg/mL in PC12 cells [88]. Aniceuquol has also been isolated from Aspergillus terreus (No. GX7-3B) [90] and Penicillum chrysogeum QEN-24S [91], and supplementary activities against α-acetylcholinesterase (AchE) with an IC50 of 1.89 µM [90] and against other fungi, with a zone of inhibition (ZOI) of cultures of the pathogen Alternaria brassicae of 6 mm compared to 16 mm by amphotericin B [91].
Penicisteroid A 115 (Figure 14) is an analogue of anicequol bearing a 7α-hydroxyl rather than a 7-oxo-group. Extracted from Penicillium chrysogenum QEN-24S, an endophytic fungus isolated from a red alga of the genus Laurencia, penicisteroid A exhibited both antimycotic and cytotoxic effects. Against the pathogenic fungi Aspergillus niger and Alternaria brassicae, penicisteroid A gave ZOIs (20 µg) of 18 mm and 9 mm, respectively, compared to 24 mm and 16 mm for control amphotericin B. Penicisteroid A also inhibited HeLa, SW1990, and NCI-H460 cancer cell lines with IC50s of 15 µg/mL, 31 µg/mL, and 40 µg/mL, showing selectivity variable from the anicequol parent compound [91]. Penicisteroid C 116 (Figure 14) also has a C16 acetate, but is less oxygenated than penicisteroid A. It was isolated from a co-cultivation of bacteria Streptomyces piomogenus AS63D and fungus Aspergillus niger using solid-state fermentation on rice medium. Penicisteroid C displayed selective antimicrobial activity against tested organisms. ZOIs for penicisteroid C were 7 mm, 9 mm, and 10 mm for bacterial cultures Staphylococcus aureus, Bacillus cereus, and Bacillus subtilis, respectively, and were 8 mm and 12 mm for fungal cultures Candida albicans and Saccharomyces cerevisiae, respectively [92].
A study of the oxysterols from the marine sponge Haliclona crassiloba (Figure 14) identified two steryl acetates with antibacterial properties. Newly identified halicrasterol D 120 had minimum inhibition constants (MICs) against tested Gram-positive bacteria ranging from 4 µg/mL against Enterococcus faecalis to 128 µg/mL against S. aureus. The known diacetate compound 121, additionally isolated from H. crassiloba, had MICs ranging from 8 µg/mL against S. aureus to 32 µg/mL E. faecalis, in the bacteria tested [93]. A newly identified phytosterol acetate 122 from the soft coral Sinularia conferta exhibited low micromolar IC50s against cell lines PANC-1 (1.78 µM), A549 (IC50 3.64 µM), and HeLa (19.34 µM) [94]. From Xestospongia, 25-acetoxyl sterol with an oxidized C19 (carboxylate substitution on C10), 123, was identified and exhibited an IC50 against AMP activated protein kinase of 8.5 µg/mL [33]. Acetates 117–119 and 124–126 (Figure 14) isolated from the coral Sacrophyton sp. inhibited Gram-positive and Gram-negative bacteria, with ZOIs ranging from 7.0–14.5 mm for Escherichia coli and from 7.5–12.0 mm for Bacillus megaterium. They also displayed antifungal properties, inhibiting Septoria tritci growth 4.5–10.5 mm [95]. Acetate 127 from the coral Nephthea erecta stimulated cytC release and inhibited Akt and mTOR phosphorylation in small cell lung cancer cells, as well as inhibiting tumor growth in the mouse xenograft model [96]. Halymeniaol 128, an triacetoxyl steroid from the rhodophyte Halymenia floresii, was recently reported to have antiplasmodial activity with an IC50 of 3.0 µM [97].
4.3. Cyclopropanes
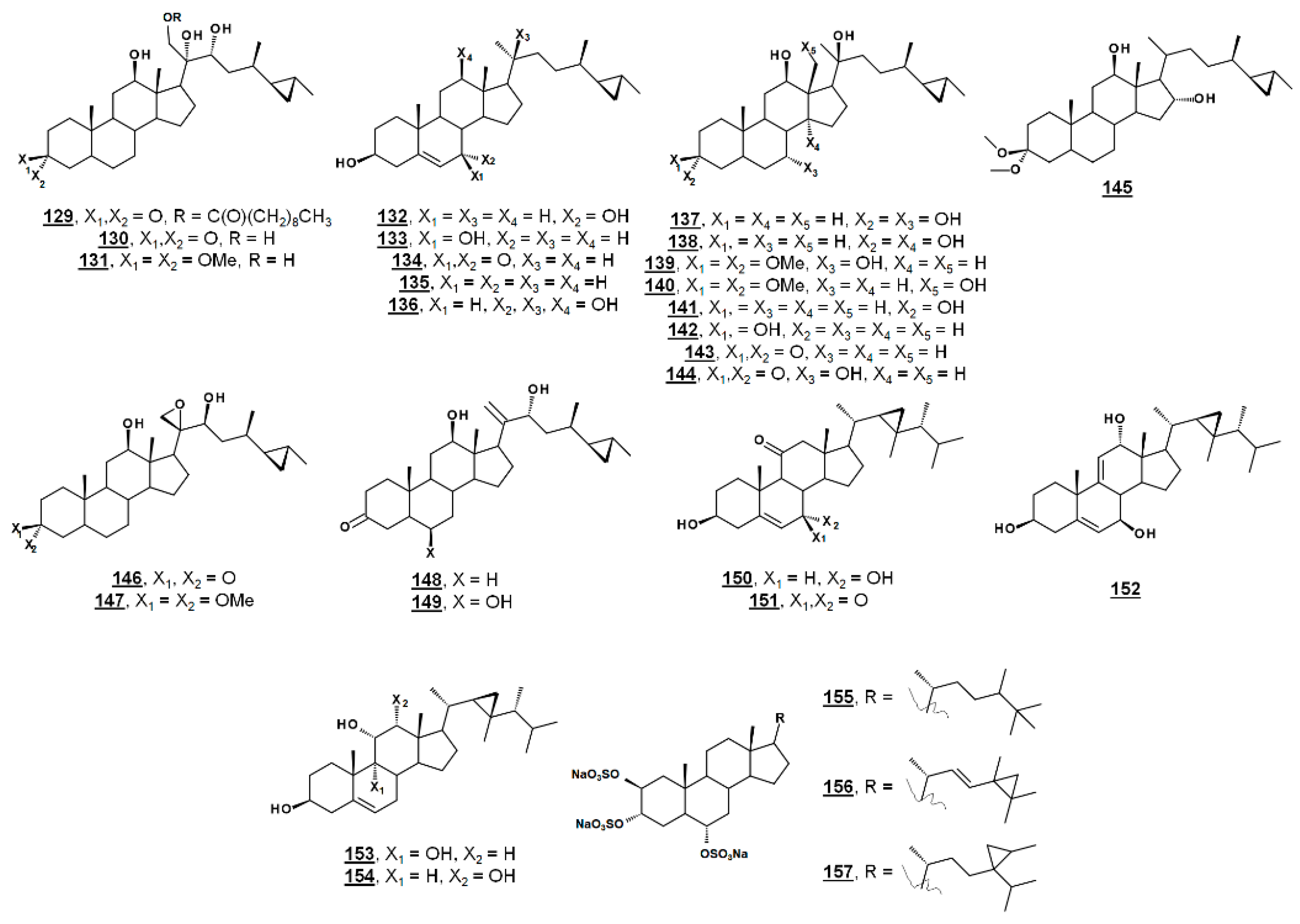
From the marine sponge Xestospongia testudinaria, oxyphytosterols 129 and 130 (Figure 15), with a side chain cyclized at C26–C27 were recently reported to posess anti-adhesion properties against bacteria Pseudoalteromonas spp. and Polaribacter sp. New compounds 129 and 133, as well as known compounds xestokerol A 130, 7α-hydroxypetrosterol 132, and aragusterol B 143 (Figure 15), had antifouling EC50s ranging from 10 to 171 µM. New compound 133 and petrosterol 135 had an EC50 > 200 µM [98]. Some of these compounds, other known analogues, and seven new analogues have also been extracted from the marine sponge Petrosia (Strongylophora) sp. Compounds 130, 131, 134–141, and 143–147 (Figure 15) displayed micromolar inhibition across various human cancer cell lines tested, with the ketal 139 showing weaker activity [99]. Representatives from this class of steroids from Xestospongia spp. tested against human cancer cell line K562 yielded IC50s for aragusterol J 149 of IC50 34.31 µM and for aragusterol A 146 of 24.19 µM. Compounds 141–143 and 148 had IC50s >10 µM [100].
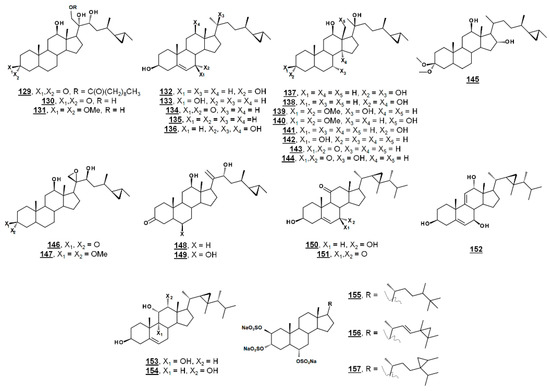
Figure 15.
Sterols bearing a 3-membered ring.
Several oxygenated gorgostenols 150–154 (Figure 15), isolated from the soft coral Klyxum flaccidum demonstrated selective biological activity. Compounds 150–152 and 154 were newly described and named klyflaccisteroids C-E [101] and klyflaccisteroid H [102], respectively. These compounds demonstrated variable inhibition across human cancer cell lines, as well as inhibition of superoxide anion generation and elastase release [101,102]. New analogues of the known trisulfate compound halistanol sulfate 155 bearing cyclopropyl rings on their side chains have been isolated from the marine sponge Halichondria sp. Halistanol sulfates I 156 and J 157 had IC50 values for sirtuins 1–3 of 45.9, 18.9, and 32.6 µM and 67.9, 21.1, and 37.5 µM, respectively, compared to IC50s of the parent structure 105 of 49.1, 19.2, and 21.8 µM [103].
4.4. Other Bioactive Steroids
Several sponge sterols, such as solomonsterol A 158 and B 159, theonellasterol 160, conicasterol 161 (Figure 16), and their analogues, can serve as ligands for human nuclear receptors; many of these compounds and their activities have been reviewed [104]. Ganoderic acid A 163 (Figure 16) and related compounds isolated from the higher fungus Ganoderma sp. possess broad therapeutic properties, including those of anti-tumor and anti-inflammation [105]. Additional recently reported bioactivities of sterols from microorganisms and algae are presented in Table 2 and Figure 16.
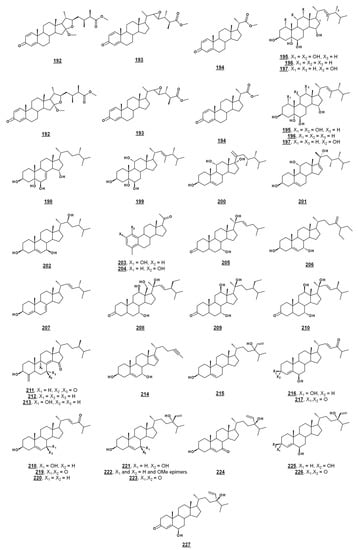
Figure 16.
Bioactive sterols and steroids. Activities are given in Table 2.

Table 2.
Recently reported biological activities from microbial steroids.
5. Conclusions
Metabolomics of sterols in microorganisms have provided insight into the biology of microorganisms. Steroidal chemotaxonomy can be used to elucidate phylogenic relationships and steroidal biomarkers can be used to monitor microbial growth and biomass production. Sterolomics additionally plays an influential role in drug discovery, through validation of drug targets, by confirmation of small molecule mechanisms, and by biological testing of microbial metabolites. The sterolome of microbiota can inform chemical biology, evolutionary traits, ecology, and pharmacology.
Author Contributions
B.A.H. wrote the paper.
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflict of interest.
Abbreviations
| [2H3]AdoMet | methyl-trideuterated S-adenosylmethionine |
| [2H3]Met | methyl-trideuterated methionine |
| 8(7)-SI | sterol C8(7)-isomerase |
| 14-SDM | sterol C14-demethylase |
| 14-SR | sterol C14-sterol reductase |
| 24-SMT | sterol C24-methyltransferase |
| Aβ | amyloid-β |
| AchE | α-acetylcholinesterase |
| BSF | bloodstream form |
| EBIs | ergosterol biosynthesis inhibitors |
| FF-MAS | follicular fluid meiosis-activating sterol |
| HEK | human epithelial kidney |
| HMGR | 3-hydroxy-3-methylglutaryl CoA reductase |
| MASs | meiosis-activating sterols |
| T-MAS | testicular meiosis-activating sterol |
| OSC | oxidosqualene cyclase |
| p38MAPK | p38 mitogen-activated protein kinase |
| PCF | procyclic form |
| PPARγ | peroxisome proliferator-activated receptor γ |
| PTP1B | protein tyrosine phosphatase 1B |
| ROCK | Rho-associated coiled-coil containing kinase |
| SqE | squalene epoxidase; TMS, trimethylsilyl(ated) |
| ZOI | zone of inhibition |
Appendix A
Table A1 gives the systematic names of all steroids discussed in the text.

Table A1.
Trivial and systematic names of sterols depicted in figures and discussed in text.
Table A1.
Trivial and systematic names of sterols depicted in figures and discussed in text.
| No.1 | Trivial Name, If Applicable (Secondary Trivial Name, If Applicable) | Systematic Name 2 (Systematic Name Relative to 5α-Cholestane) | PubChem CID |
|---|---|---|---|
| 1 | cholesterol | cholest-5-en-3β-ol | 5997 |
| 2 | ergosterol | ergosta-5,7,22E-trien-3β-ol (24β-methylcholesta-5,7,22E-trien-3β-ol) | 444679 |
| 3 | sitosterol | stigmast-5-en-3β-ol (24α-methylcholest-5-en-3β-ol) | 222284 |
| 4 | FF-MAS | 4α,4β-dimethylcholesta-8,14,24-trien-3β-ol | 443212 |
| 5 | T-MAS | 4α,4β-dimethylcholesta-8,24-dien-3β-ol | 50990081 |
| 6 | 25-hydroxycholesterol | cholest-5-en-3β,25-diol | 65094 |
| 8 | ergosterol peroxide | 5α,8α-epidioxyergosta-6,22E-dien-3β-ol (24β-methyl-5α,8α-epidioxycholesta-6,22E-dien-3β-ol) | 5351516 |
| 9 | squalamine | 3β-[3-(4-aminobutyl)amino]propyl-7α-hydroxycholestan-24β-hydrosulfate | 72495 |
| 12 | lanosterol | lanosta-8,24-dien-3β-ol (4α,4β,14α-trimethylcholesta-8,24-dien-3β-ol) | 246983 |
| 13 | 31-norlanosterol | 4α,14α-dimethylcholesta-8,24-dien-3β-ol | 15101557 |
| 14 | zymosterone | cholesta-8,24-dien-3-one | 22298942 |
| 15 | zymosterol | cholesta-8,24-dien-3β-ol | 92746 |
| 16 | fecosterol | ergosta-8,24(28)-dien-3β-ol (24-methylideneholest-8-en-3β-ol) | 440371 |
| 17 | episterol | ergosta-7,24(28)-dien-3β-ol (24-methylideneholest-7-en-3β-ol) | 5283662 |
| 18 | ergosta-5,7,22E,24(28)-tetraen-3β-ol (24-methylideneholesta-5,7,22E-trien-3β-ol) | 11090531 | |
| 25 | 25-azalanosterol | 25-azalanost-8(9)-en-3β-ol 4α,4β,14α-trimethyl-25-azacholest-8-en-3β-ol | 66746490 |
| 26 | 24(R,S),25-epiminolanosterol | 24(R,S),25-epiminolanost8(9)-en-3β-ol (4α,4β,14α-trimethyl-24,25-azanetriylcholest-8-en-3β-ol) | 163740 |
| 27 | cycloartenol | cycloart-24(25)-en-3β-ol (4α,4β,14α-trimethyl-9β,19-cyclocholest-24-en-3β-ol) | 92110 |
| 28 | cyclolaudenol | 24β-methylcycloart-25(27)-en-3β-ol (4α,4β,14α,24β-tetramethyl-9β,19-cyclocholest-25(27)-en-3β-ol) | 101729 |
| 29 | 24-methylenecycloartanol | 24-methylidenecycloartan-3β-ol (4α,4β,14α-trimethyl-24-methylidene-9β,19-cyclocholestan-3β-ol) | 94204 |
| 30 | ergosta-8,25(27)-dien-3β-ol (24β-methylcholest-8,25(27)-dien-3β-ol) | 102515129 | |
| 31 | obtusifoliol | 4α,14α-dimethylergosta-8,24(28)-dien-3β-ol (4α,14α-dimethyl-24-methylideneholest-8-en-3β-ol) | 65252 |
| 32 | 24(28)-methylenelophenol | 4α-methylergosta-7,24(28)-dien-3β-ol (4α-methyl-24-methylideneholest-7-en-3β-ol) | 5283640 |
| 33 | chlamysterol | 4α,14α-dimethylporiferasta-8,25(27)-dien-3β-ol (24β-ethyl-4α,14α-dimethyl-cholesta-8,25(27)-dien-3β-ol) | 90657605 |
| 34 | 24(28)Z-ethylidene lophenol (citrostadienol) | 4α-methylstigmasta-7,24(28)Z-dien-3β-ol (4α-methyl-24Z-ethylideneholest-7-en-3β-ol) | 9548595 |
| 35 | 7-dehydroporiferasterol | poriferasta-5,7,22E-trien-3β-ol (24β-ethylcholesta-5,7,22E-trien-3β-ol) | 20843308 |
| 36 | campesterol | campest-5-en-3β-ol (24α-methylcholest-5-en-3β-ol) | 173183 |
| 37 | clionosterol (22-dihydroporiferasterol) | poriferast-5-en-3β-ol (24β-ethylcholest-5-en-3β-ol) | 457801 |
| 38 | 22-dihydrobrassicasterol | ergost-5-en-3β-ol (24β-methylcholesta-5-en-3β-ol) | 312822 |
| 39 | stigmasterol | stigmasta-5,22E-dien-3β-ol (24α-ethylcholesta-5,22E-dien-3β-ol) | 5280794 |
| 40 | poriferasterol | poriferasta-5,22E-dien-3β-ol (24β-ethylcholesta-5,22E-dien-3β-ol) | 5281330 |
| 41 | crinosterol (epibrassiasterol) | campesta-5,22E-dien-3β-ol (24α-methylcholesta-5,22E-dien-3β-ol) | 5283660 |
| 42 | brassicasterol | ergosta-5,22E-3β-ol (24β-methylcholesta-5,22E-dien-3β-ol) | 5281327 |
| 43 | fucosterol | stigmasta-5,24(28)E-dien-3β-ol (24E-ethylideneholest-7-en-3β-ol) | 5281328 |
| 44 | isofucosterol | stigmasta-5,24(28)Z-dien-3β-ol (24Z-ethylideneholest-5-en-3β-ol) | 5281326 |
| 45 | 24(28)-methylenecholesterol (chalinasterol) | ergosta-5,24(28)-dien-3β-ol (24-methylideneholest-5-en-3β-ol) | 92113 |
| 46 | 24-ethyldesmosterol | stigmasta-5,24(25)-dien-3β-ol (24-ethylcholesta-5,24(25)-dien-3β-ol) | 22848721 |
| 47 | 24-methyldesmosterol | ergosta-5,24(25)-dien-3β-ol (24-methylcholesta-5,24(25)-dien-3β-ol) | 193567 |
| 48 | desmosterol | cholesta-5,24-dien-3β-ol | 439577 |
| 49 | schottenol | stigmast-7-en-3β-ol (24α-ethylcholest-7-en-3β-ol) | 441837 |
| 50 | 22-dihydrochondrillasterol | poriferast-7-en-3β-ol (24β-ethylcholest-7-en-3β-ol) | 5283639 |
| 51 | epifungisterol (22-dihydrostellasterol) | campest-7-en-3β-ol (24α-methylcholest-7-en-3β-ol) | 90889779 |
| 52 | fungisterol | ergost-7-en-3β-ol (24β-methylcholest-7-en-3β-ol) | 5283646 |
| 53 | stigmasta-7,22E-dien-3β-ol (24α-ethylcholesta-7,22E-dien-3β-ol) | 125122456 | |
| 54 | chondrillasterol | poriferasta-7,22E-dien-3β-ol (24β-ethylcholesta-7,22E-dien-3β-ol) | 5283663 |
| 55 | stellasterol | campesta-7,22E-dien-3β-ol (24α-methylcholest-7,22E-dien-3β-ol) | 5283669 |
| 56 | 5-dihydroergosterol | ergosta-7,22E-dien-3β-ol (24β-methylcholest-7,22E-dien-3β-ol) | 13889661 |
| 57 | 24-dehydrolathosterol | cholesta-7,24-dien-3β-ol | 5459827 |
| 58 | 22-dihydroergosterol | ergosta-5,7-dien-3β-ol (24β-methylcholest-5,7-dien-3β-ol) | 5326970 |
| 59 | stigmast-8-en-3β-ol (24α-ethylcholest-8-en-3β-ol) | 23424905 | |
| 60 | poriferast-8-en-3β-ol (24β-ethylcholest-8-en-3β-ol) | 101826503 | |
| 61 | campest-8-en-3β-ol (24α-methylcholest-8-en-3β-ol) | - | |
| 62 | ergost-8-en-3β-ol (24β-methylcholest-8-en-3β-ol) | 60077053 | |
| 63 | cholest-8-enol (24-dihydrozymosterol) | cholest-8-en-3β-ol | 101770 |
| 64 | ergosta-7,25(27)-dien-3β-ol (24β-methylcholesta-7,25(27)-dien-3β-ol) | 60077052 | |
| 65 | poriferasta-7,25(27)-dien-3β-ol (24β-ethylcholesta-7,25(27)-dien-3β-ol) | 5283655 | |
| 66 | lichesterol | ergosta-5,8,22E-trien-3β-ol (24β-methylcholesta-5,8,22E-trien-3β-ol) | 5281329 |
| 67 | clerosterol | poriferasta-5,25(27)-dien-3β-ol (24β-ethylcholesta-5,25(27)-dien-3β-ol) | 5283638 |
| 68 | epiclerosterol | stigmasta-5,25(27)-dien-3β-ol (24α-ethylcholesta-5,25(27)-dien-3β-ol) | 185472 |
| 69 | 5-dehydrostellasterol (epiergosterol) | campesta-5,7,22E-trien-3β-ol (24α-methylcholesta-5,7,22E-trien-3β-ol) | 124427258 |
| 70 | occelasterol | 27-norcholesta-5,22E-dien-3β-ol | 15481847 |
| 71 | lathosterol | cholest-7-en-3β-ol | 65728 |
| 72 | 7-dehydrocholesterol (provitamin D3) | cholesta-5,7-dien-3β-ol | 439423 |
| 73 | fucosteryl epoxide | 24,28-epoxyergost-5-en-3β-ol 24-(3-methyloxiran-2-yl)-cholest-5-en-3β-ol | 3082427 |
| 74 | dinosterol | 4α,23-dimethylergost-22E-en-3β-ol (4α,23,24β-trimethylcholest-22E-en-3β-ol) | 44263330 |
| 75 | 24Z-propylidenecholesterol | 6443745 | |
| 76 | dihydrodinosterol | (23R)-4α,23-dimethylergostan-3β-ol ((23R)-4α,23,24β-trimethylcholestan-3β-ol) | 133309 |
| 77 | amphisterol | 4α-methylergosta-8(14),24(28)-dien-3β-ol (4α-methyl-24-methylidenechoest-8(14)-en-3β-ol) | 60077061 |
| 78 | 4α-methylergosterol | 4α-methylergosta-5,7,22E-trien-3β-ol (4α,24β-dimethylcholesta-5,7,22E-trien-3β-ol) | - |
| 79 | 4α-methylgorgosterol | 4α-methylgorgost-5-en-3β-ol ((22R,23R)-4α,23,24β-trimethyl-22,23-methanocholest-5-en-3β-ol) | - |
| 80 | gorgosterol | gorgost-5-en-3β-ol ((22R,23R)-23,24β-dimethyl-22,23-methanocholest-5-en-3β-ol) | 52931413 |
| 81 | cholestanol | (5α) cholestan-3β-ol | 6710664 |
| 82 | cholesta-5,7,24-trienol 7-dehydrodesmosterol | cholesta-5,7,24-trien-3β-ol | 440558 |
| 83 | ergosta-5,7,24(28)-trien-3β-ol (24β-methylcholesta-5,7,24(28)-trien-3β-ol) | 10894570 | |
| 84 | ergosta-5,7,24(25)-trien-3β-ol (24β-methylcholesta-5,7,24(25)-trien-3β-ol) | 58104987 | |
| 85 | ergosta-5,7,25(27)-trien-3β-ol (24β-methylcholesta-5,7,25(27)-trien-3β-ol) | 101600336 | |
| 86 | 24,24-dimethylcholesta-5,7,25(27)-trien-3β-ol | - | |
| 87 | protothecasterol | ergosta-5,7,22E,25(27)-tetraen-3β-ol (24β-methylcholesta-5,7,22E,25(27)-tetraen-3β-ol) | 101600338 |
| 88 | 26-fluorolanosterol | 26-fluorolanosta-8,24-dien-3β-ol (26-fluoro-4α,4β,14α-trimethylcholesta-8,24-dien-3β-ol) | - |
| 89 | 26-fluorocholesta-5,7,24-trien-3β-ol | - | |
| 90 | 26-fluoro-4α,4β-dimethylcholesta-8,24-dien-3β-ol | - | |
| 91 | 26-fluoro-4α-methylcholesta-8,24-dien-3β-ol | - | |
| 92 | 26-fluorocholesta-8,24-dien-3β-ol | - | |
| 93 | 26-fluoroergosta-8,25(27)-dien-3β-ol 26-fluoro-24β-methylcholesta-8,25(27)-dien-3β-ol | - | |
| 94 | amebasterol-1 | 19(10→6)-abeo-ergosta-5,7,9,22E-tetraen-3β-ol (24β-methyl-19(10→6)-abeo-cholesta-5,7,9,22E-tetraen-3β-ol) | 11596359 |
| 95 | amebasterol-2 | 19(10→6)-abeo-poriferasta-5,7,9,22E-tetraen-3β-ol (24β-ethyl-19(10→6)-abeo-cholesta-5,7,9,22E-tetraen-3β-ol) | - |
| 96 | amebasterol-3 | 19(10→6)-abeo-ergosta-5,7,9-trien-3β-ol (24β-methyl-19(10→6)-abeo-ergosta-5,7,9-trien-3β-ol) | - |
| 97 | amebasterol-4 | 19(10→6)-abeo-poriferasta-5,7,9,22E,25-pentaen-3β-ol (24β-ethyl-19(10→6)-abeo-cholesta-5,7,9,22E,25-pentaen-3β-ol) | - |
| 98 | amebasterol-5 | 19(10→6)-abeo-poriferasta-5,7,9,25(27)-tetraen-3β-ol (24β-ethyl-19(10→6)-abeo-cholesta-5,7,9,25(27)-tetraen-3β-ol) | - |
| 99 | amebasterol-6 | 19(10→6)-abeo-poriferasta-5,7,9-trien-3β-ol (24β-ethyl-19(10→6)-abeo-poriferasta-5,7,9-trien-3β-ol) | - |
| 102 | eburicol | 24-methylidenelanost-8-en-3β-ol 4α,4β,14α-trimethyl-24-methylidenecholest-8-en-3β-ol | 9803310 |
| 103 | 14-methylergosta-8,24(28)-dien-3β,6α-diol 14,24β-dimethyl-24-methylidenecholest-8-en--3β,6α-diol | 148910 | |
| 105 | FR171456 | 24-methylidene-3β,8α,11α-trihydroxy-1,6-dioxocycloartan-30-oic acid (4α,14α-dimethyl-24-methylidene-9β,19-cyclo-3β,8α,11α-trihydroxy-1,6-dioxocholestan-4β-carboxylic acid) | - |
| 107 | michosterol A | (20S,23R)-23-methyl-20-hydroperoxy-25-acetoxyergost-16-en-3β,5β,6α-triol ((20S,23R)-23,24β-dimethyl-20-hydroperoxy-25-acetoxycholest-16-en-3β,5β,6α-triol) | - |
| 108 | michosterol B | (17E,23R)-23-methyl-16-hydroperoxy-25-acetoxyergost-17-en-3β,5β,6α-triol ((17E,23R)-23,24β-di-methyl-16-hydroperoxy-25-acetoxycholest-17-en-3β,5β,6α-triol) | - |
| 109 | nigerasterol A | 5α,9α-epidioxyergosta-6,8(14),22E-trien-3β,15α-diol (24β-methyl-5α,9α-epidioxycholesta-6,8(14),22E-trien-3β,15α-diol) | - |
| 110 | nigerasterol B | 5α,9α-epidioxyergosta-6,8(14),22E-trien-3β,15β-diol (24β-methyl-5α,9α-epidioxycholesta-6,8(14),22E-trien-3β,15β-diol) | - |
| 111 | 24-ethenyl-24-hydroperoxycholest-5-en-3β-ol | 10411225 | |
| 112 | 29-hydroperoxyisofucosterol | (24Z)-29-hydroperoxystigmasta-5,24(28)-dien-3β-ol 24Z-(2-hydroperoxyethylidnene)cholest-5-en-3β-ol | 46224335 |
| 113 | michosterol C | 6α-acetoxyergostan-3β,5β,25-triol (24β-methyl-6α-acetoxycholestan-3β,5β,25-triol) | - |
| 114 | anicequol (NGA0187) | 16β-acetoxy-3β,7β,11β-trihydroxyergost-22E-en-6-one (24β-methyl-16β-acetoxy-3β,7β,11β-trihydroxycholest-22E-en-6-one) | 10413810 |
| 115 | penicisteroid A | 24-methyl-16β-acetoxycholest-22E-en-3β,6β,7β,11β-tetrol | - |
| 116 | penicisteroid C | 24-methyl-16β-acetoxycholesta-5,22E-dien-3β,6β-diol | - |
| 117 | 11α-acetoxycholest-24-en-3β,5α,6β-triol | - | |
| 118 | 11α-acetoxyergosta-22E,25-dien-3β,5α,6β-triol 24β-methyl-11α-acetoxycholesta-22E,25-dien-3β,5α,6β-triol | - | |
| 119 | 11α-acetoxygorgostan-3β,5α,6β-triol ((22R,23R)-23,24β-dimethyl-22,23-methano-11α-acetoxycholestan-3β,5α,6β-triol) | 54769262 | |
| 120 | halicrasterol D | 11α-acetoxyergost-22E-en-3β,5α,6β-triol 24β-methyl-11α-acetoxycholest-22E-en-3β,5α,6β-triol | - |
| 121 | 11α,19-diacetoxycholest-7-en-2α,3β,5α,6β,9α-pentol | - | |
| 122 | 6β-acetoxyergost-24(28)-en-3β,5α-diol 24-methylidene-6β-acetoxycholestan -3β,5α-diol | 101687891 | |
| 123 | methyl 25-acetoxy-3β-hydroxycholest-5-en-19-carboxylate | - | |
| 124 | 11α-acetoxygorgostan-3β,5α,6β,12α-tetrol ((22R,23R)-23,24β-dimethyl-22,23-methano-11α-acetoxycholest-5-en-3β,5α,6β,12α-tetrol) | 56962930 | |
| 125 | 12α-acetoxygorgostan-3β,5α,6β,11α-tetrol ((22R,23R)-23,24β-dimethyl-22,23-methano-12α-acetoxycholestan-3β,5α,6β,11α-tetrol) | - | |
| 126 | 11α-acetoxygorgostan-3β,5α,6β,15α-tetrol ((22R,23R)-23,24β-dimethyl-22,23-methano-12α-acetoxycholestan-3β,5α,6β,15α-tetrol) | - | |
| 127 | 7β-acetoxyergosta-5,24(28)-dien-3β,19-diol 24-methylidene-7β-acetoxycholest-5-en-3β,19-diol | 477494 | |
| 128 | halymeniaol | 3β,15α,16β-triacetoxy-12β-hydroxycholest-5-en-7-one | - |
| 129 | 21-O-octadecanoyl-xestokerol A | (20S,21R)-21-octadecanoyl-11β,20,22-trihydroxypetrostan-3-one ((20S,21R,25R,26R)-24α,26-dimethyl-26,27-cyclo-21-octadecanoyl-11β,20,22-trihydroxycholestan-3-one) | 71747680 |
| 130 | xestokerol A | (20S,21R)-11β,20,21,22-tetrahydroxypetrostan-3-one ((20S,21R,25R,26R)-24α,26-dimethyl-26,27-cyclo-11β,20,21,22-tetrahydroxycholestan-3-one) | 44584465 |
| 131 | xestokerol A dimethyl ketal | (20S,21R)-3,3-dimethoxypetrostan-11β,20,21,22-tetrol ((20S,21R,25R,26R)-24α,26-dimethyl-26,27-cyclo-3,3-dimethoxycholestan--11β,20,21,22-tetrol) | - |
| 132 | 7α-hydroxypetrosterol | petrost-5-en-3β,7α-diol ((25R,26R)-24α,26-dimethyl-26,27-cyclocholest-5-en-3β,7α-diol) | 101209535 |
| 133 | 7β-hydroxypetrosterol | petrost-5-en-3β,7β-diol ((25R,26R)-24α,26-dimethyl-26,27-cyclocholest-5-en-3β,7β-diol) | 71747681 |
| 134 | 7-ketopetrosterol | 3β-hydroxypetrost-5-en-7-one ((25R,26R)-24α,26-dimethyl-26,27-cyclo-3β-hydroxycholest-5-en-7-one) | 101209534 |
| 135 | petrosterol | petrost-5-en-3β-ol ((25R,26R)-24α,26-dimethyl-26,27-cyclocholest-5-en-3β-ol) | 194249 |
| 136 | 11β-hydroxypetrosterol | petrost-5-en-3β,11βα-diol ((25R,26R)-24α,26-dimethyl-26,27-cyclocholest-5-en-3β,11β-diol) | - |
| 137 | (20S)-petrostan-3α,7α,12β,20-tetrol ((20S,25R,26R)-24α,26-dimethyl-26,27-cyclocholestan-3α,7α,12β,20-tetrol) | - | |
| 138 | (20S)-petrostan-3α,12β,14α,20-tetrol ((20S,25R,26R)-24α,26-dimethyl-26,27-cyclocholestan-3α,12β,14α,20-tetrol) | - | |
| 139 | (20S)-3,3-dimethoxypetrostan-7α,12β,20-triol ((20S,25R,26R)-24α,26-dimethyl-26,27-cyclo-3,3-dimethoxycholestan-7α,12β,20-triol) | - | |
| 140 | (20S)-3,3-dimethoxypetrostan-7α,12β,19,20-tetrol ((20S,25R,26R)-24α,26-dimethyl-26,27-cyclo-3,3-dimethoxycholestan-7α,12β,19,20-tetrol) | - | |
| 141 | (20S)-petrostan-3α,12β,20-triol ((20S,25R,26R)-24α,26-dimethyl-26,27-cyclocholestan-3α,12β,20-triol) | - | |
| 142 | (20S)-petrostan-3β,12β,20-triol ((20S,25R,26R)-24α,26-dimethyl-26,27-cyclocholestan-3β,12β,20-triol) | - | |
| 143 | aragusterol B | (20S)-12β,20-dihydroxypetrostan-3-one ((20S,25R,26R)-24α,26-dimethyl-26,27-cyclo-12β,20-dihydroxycholestan-3-one) | 44566420 |
| 144 | (20S)-7α,12β,20-trihydroxypetrostan-3-one ((20S,25R,26R)-24α,26-dimethyl-26,27-cyclo-7α,12β,20-trihydroxycholestan-3-one) | - | |
| 145 | 3,3-dimethoxypetrostan-12β,16α-diol ((25R,26R)-24α,26-dimethyl-26,27-cyclo-3,3-dimethoxycholestan-12β,16α-diol) | - | |
| 146 | aragusterol A | (20R,22S)-20,21-epoxy-12β,22-dihydroxypetrostan-3-one ((20R,22S,25R,26R)-24α,26-dimethyl-20,21-epoxy-26,27-cyclo-12β,22-dihydroxycholestan-3-one) | 9933873 |
| 147 | (20R,22S)-20,21-epoxy-3,3-dimethoxypetrostan-12β,22-diol ((20R,22S,25R,26R)-24α,26-dimethyl-20,21-epoxy-26,27-cyclo-3,3-dimethoxycholestan-12β,22-diol) | 10696885 | |
| 148 | (22R)-12β,22-dihydroxypetrost-20(21)-en-3-one ((22R,25R,26R)-24α,26-dimethyl-26,27-cyclo-12β,22-dihydroxycholest-20(21)-en--3-one) | - | |
| 149 | aragusterol J | (22R)-7β,12β,22-trihydroxypetrost-20(21)-en-3-one ((22R,25R,26R)-24α,26-dimethyl-26,27-cyclo-7β,12β,22-trihydroxycholest-20(21)-en--3-one) | - |
| 150 | klyflaccisteroid C | 3β,7α-dihydroxygorgost-5-en-11-one ((22R,23R)-23,24β-dimethyl-22,23-methano-3β,7α-dihyroxycholest-5-en-11-one) | - |
| 151 | klyflaccisteroid D | 3β-hydroxygorgost-5-en-7,11-dione ((22R,23R)-23,24β-dimethyl-22,23-methano-3β,7α-dihyroxycholest-5-en-7,11-dione) | - |
| 152 | klyflaccisteroid E | gorgosta-5,9(11)-dien-3β,7β,12α-triol ((22R,23R)-23,24β-dimethyl-22,23-methanocholesta-5,9(11)-dien-3β,7β,12α-triol) | - |
| 153 | gorgost-5-en-3β,9α,11α-triol ((22R,23R)-23,24β-dimethyl-22,23-methanocholest-5,-dien-3β,9α,11α-triol) | 10742556 | |
| 154 | klyfaccisteroid H | gorgost-5-en-3β,11α,12α-triol ((22R,23R)-23,24β-dimethyl-22,23-methanocholesta-5,9(11)-dien-3β,11α,12α-triol) | - |
| 155 | halistanol sulfate | 24,25-dimethylcholestane-2β,3α,6α-trisulfate | 73361 |
| 156 | halistanol sulfate I | 24-methyl-24,25-methanocholestane-2β,3α,6α-trisulfate | - |
| 157 | halistanol sulfate J | 24,24-(methylethano)cholestane-2β,3α,6α-trisulfate | - |
| 158 | solomonsterol A | trisodium cholane-2β,3α,24-trisulfate (trisodium 25,26,27-trinorcholestane-2β,3α,24-trisulfate) | 50925451 |
| 159 | solomonsterol B | trisodium 24-norcholane-2β,3α,23-trisulfate (trisodium 24,25,26,27-tetranorcholestane-2β,3α,24-trisulfate) | 53318073 |
| 160 | theonellasterol | 4-methylidineporiferast-8(14)-en-3β-ol (24β-ethyl-4-methylidinecholest-8(14)-en-3β-ol) | 52931395 |
| 161 | conicasterol | 4-methylidinecampest-8(14)-en-3β-ol (24α-methyl-4-methylidinecholest-8(14)-en-3β-ol) | 21670674 |
| 162 | ganoderic acid A | 7β,15α-dihydroxy-3,11,23-trioxolanost-8-en-26-oic acid 4α,4β,14α-trimethyl-7β,15α-dihydroxy-3,11,23-trioxocholest-8-en-26-oic acid) | 471002 |
| 163 | ergosta-7,9(11),22E-trien-3β-ol 24β-methylcholesta-7,9(11),22E-trien-3β-ol | 12308954 | |
| 164 | ergosta-4,7,22E-trien-3-one 24β-methylcholesta-4,7,22E-trien-3-one | 11003773 | |
| 165 | ergosta-4,6,8(14),22E-tetraen-3-one 24β-methylcholesta-4,6,8(14),22E-tetraen-3-one | 6441416 | |
| 166 | 14α-hydroxyergosta-4,7,9(11),22E-tetraen-3,6-dione 24β-methyl-14α-hydroxycholesta-4,7,9(11),22E-tetraen-3,6-dione | 10251684 | |
| 167 | 9α,14α-dihydroxyergosta-4,7,22E-trien-3,6-dione 24β-methyl-9α,14α-dihydroxycholesta-4,7,22E-trien-3,6-dione | - | |
| 168 | ergosta-4,6,8(14),22E,24(28)-pentaen-3-one 24-methylidenecholesta-4,6,8(14),22E-tetraen-3-one | - | |
| 169 | nodulisporiviridin E | 18-nor-1α,3β-dihydroxy-4,5,6-[2,3,4]furanoandrosta-5,8,11,13(14)-tetraen-7,17-dione 18,20,21,22,23,24,25,26,27-nonanor-1α,3β-dihydroxy-4,5,6-[2,3,4]furanoandrosta-5,8,11,13(14)-tetraen-7,17-dione | 122179368 |
| 170 | nodulisporiviridin F | 3β,11β-dihydroxy-4,5,6-[2,3,4]furanoandrosta-5,8-dien-7,17-dione 20,21,22,23,24,25,26,27-octanor-3β,11β-dihydroxy-4,5,6-[2,3,4]furanocholesta-5,8-dien-7,17-dione | 122179369 |
| 171 | nodulisporiviridin G | 11β-hydroxy-4,5,6-[2,3,4]furanoandrosta-5,8-dien-3,7,17-trione 20,21,22,23,24,25,26,27-octanor-11β-hydroxy-4,5,6-[2,3,4]furanocholesta-5,8-dien-3,7,17-trione | 122179370 |
| 172 | nodulisporiviridin H | 3β,12β-dihydroxy-4,5,6-[2,3,4]furanoandrosta-5,8-dien-7,17-dione 20,21,22,23,24,25,26,27-octanor-3β,12β-dihydroxy-4,5,6-[2,3,4]furanocholesta-5,8-dien-7,17-dione | 122179371 |
| 173 | 16-O-desmethylasporyergosterol-β-d-mannoside | β-d-mannosyloxyergosta-6,8(14),17(20)E, 22E-tetraen-3β-ol 24β-methyl-β-d-mannosyloxycholesta-6,8(14),17(20)E,22E-tetraen-3β-ol | - |
| 174 | (24S)-24,28-epoxyergost-5-en-3β,4α-diol (24S)-24-oxyranylcholest-5-en-3β,4α-diol | 44575614 | |
| 175 | ergosta-5,24(28)-dien-3β,7α-diol (24-methylidenecholest-5-en-3β,7α-diol) | 10949727 | |
| 176 | ergosta-5,24(28)-dien-3β,7β-diol (24-methylidenecholest-5-en-3β,7β-diol) | 11373355 | |
| 177 | ergost-5-en-3β,7β-diol (24β-methylcholest-5-en-3β,7β-diol) | 11475561 | |
| 178 | ergost-24(28)-en-3β,5α,6β-triol (24-methylidenecholestan-3β,5α,6β-triol) | 21775108 | |
| 179 | ergostan-3β,5α,6β-triol (24β-methylcholestan-3β,5α,6β-triol) | 44558918 | |
| 180 | 3β,5α,6β,11α-tetrahydroxyergostan-1-one (24β-methyl-3β,5α,6β,11α-tetrahydroxycholestan-1-one) | - | |
| 181 | ergostan-1α,3β,5α,6β,11α-pentol (24β-methylcholestan-1α,3β,5α,6β,11α-pentol) | - | |
| 182 | sarcoaldesterol B | ergostan-3β,5α,6β,11α-tetrol (24β-methylcholestan-3β,5α,6β,11α-tetrol) | 10718409 |
| 183 | ergostan-1β,3β,5α,6β-tetrol (24β-methylcholestan-1β,3β,5α,6β-tetrol) | - | |
| 184 | pregnedioside A | 4α-O-β-d-arabinopyranosyloxypregn-20-en-3β-ol22,23,24,25,26,27-hexanor-4α-O-β-d-arabinopyranosyloxycholest-20-en-3β-ol | 21673267 |
| 185 | gorgostan-1α,3β,5α,6β,11α-pentol ((22R,23R)-23,24β-dimethyl-22,23-methanocholestan-1α,3β,5α,6β,11α-pentol) | 23426029 | |
| 186 | sarcoaldesterol A | gorgostan-3β,5α,6β,11α-tetrol ((22R,23R)-23,24β-dimethyl-22,23-methanocholestan-3β,5α,6β,11α-tetrol) | 10790775 |
| 187 | (20R,23R)-23-methylergost-16-en-3β,20-diol (20R,23R)-23,24β-methylcholest-16-en-3β,20-diol | - | |
| 188 | ximaosteroid E | (16S)-16,22-epoxycholesta-1,22E-dien-3-one | - |
| 189 | ximaosteroid F | (20R,22R)-20,22-dihydroxycholesta-1,4-dien-3-one | - |
| 190 | (20S)-20-hydroxycholest-1-en-3,16-dione | 53997071 | |
| 191 | sinubrasone A | methyl (22R)-22-O-β-d-xylopyranosyloxy-3-oxoergosta-1,4-diene-26-carboxylate methyl (22R)-24β-methyl-22-O-β-d-xylopyranosyloxy-3-oxocholesta-1,4-diene-26-carboxylate | - |
| 192 | sinubrasone B | methyl (16S,22R)-16-methoxy-16,22-epoxy-3-oxoergosta-1,4-diene-26-carboxylate methyl (16S,22R)-24β-methyl-16-methoxy-16,22-epoxy-3-oxocholesta-1,4-diene-26-carboxylate | - |
| 193 | sinubrasone C | methyl (22R,23R)-22,23-epoxy-3-oxoergosta-1,4-diene-26-carboxylate methyl (22R,23R)-24β-methyl-22,23-epoxy-3-oxocholesta-1,4-diene-26-carboxylate | - |
| 194 | sinubrasone D | methyl (20S)-20-methyl-3-oxopregna-1,4-diene-21-carboxylate methyl 23,24,25,26,27-pentanor-3-oxocholesta-1,4-diene-21-carboxylate | 15929041 |
| 195 | ergostan-1α,3β,5α,6β,11α,15α-hexol (24β-methylcholestan-1α,3β,5α,6β,11α,15α-hexol) | - | |
| 196 | ergostan-3β,5α,6β,15α-tetrol (24β-methylcholestan-3β,5α,6β,15α-tetrol) | - | |
| 197 | ergostan-3β,5α,6β,11α,15α-pentol (24β-methylcholestan-3β,5α,6β,11α,15α-pentol) | - | |
| 198 | ergost-7-en-3β,5α,6β,15α-tetrol (24β-methylcholest-7-en-3β,5α,6β,15α-tetrol) | - | |
| 199 | 23-methylergost-22E-en-3β,5α,6β,11α-tetrol (23,24β-dimethylcholest-22E-en-3β,5α,6β,11α-tetrol) | - | |
| 200 | klyflaccisteroid A | (17S,23R)-23-methylergosta-5,20(21)-dien-3β,17α-diol (17S,23R)-23,24β-dimethylcholesta-5,20(21)-dien-3β,17α-diol | - |
| 201 | klyfaccisteroid J | (20R,23R)-23-methylergosta-5,16-dien-3β,11α,20-triol (20R,23R)-23,24β-dimethylcholesta-5,16-dien-3β,11α,20-triol | - |
| 202 | klyflaccisteroid M | (22S)-ergost-5-en-3β,7β,22-triol ((22S)-24β-methylcholest-5-en-3β,7β,22-triol) | - |
| 203 | subergorgol U | 19(10→4)-abeo-2-hydroxypregna-2,4,1(10)-trien-20-one (22,23,24,25,26,27-hexnor-19(10→4)-abeo-2-hydroxycholesta-2,4,1(10)-trien-20-one) | 132918691 |
| 204 | 19(10→4)-abeo-1-hydroxypregna-2,4,1(10)-trien-20-one (22,23,24,25,26,27-hexnor-19(10→4)-abeo-2-hydroxycholesta-2,4,1(10)-trien-20-one) | 54484024 | |
| 205 | (20S)-7α,12β,20-trihydroxycholest-22E-en-3-one | - | |
| 206 | langcosterol A | 26,27-dimethylergosta-5,24(28)-dien-3β,7α-diol (26,27-dimethyl-24-methylidenecholest-5-en-3β,7α-diol) | 23426186 |
| 207 | ergosta-4,7,22E,25-tetraen-3-one (24β-methylcholesta-4,7,22E,25-tetraen-3-one) | 132280531 | |
| 208 | 7α,12β,18-trihydoxystigmast-22E-en-3-one (24α-methyl-7α,12β,18-trihydoxycholest-22E-en-3-one) | - | |
| 209 | (20S)-24-ethyl-7α,12β,20-trihydroxycholestan-3-one | - | |
| 210 | (20S)-24-methyl-7α,12β,20-trihydroxycholest-22E-en-3-one | - | |
| 211 | 7,15-dioxoconicasterol | 4-methylidene-3β-hydroxycampest-8(14)-en-7,15-dione (24α-methyl-4-methylidene-3β-hydroxycholest-8(14)-en-7,15-dione) | - |
| 212 | 15-oxoconicasterol | 4-methylidene-3β-hydroxycampest-8(14)-en-15-one (24α-methyl-4-methylidene-3β-hydroxycholest-8(14)-en-15-one) | - |
| 213 | 4-methylidene-3β,9α-dihydroxycampest-8(14)-en-15-one (24α-methyl-4-methylidene-3β,9α-dihydroxycholest-8(14)-en-15-one) | - | |
| 214 | gelliusterol E | 24-methylchola-5,16-dien-23-yn-3β,7α-diol (26,27-dinorcholesta-5,16-dien-23-yn-3β,7α-diol) | - |
| 215 | saringosterol | 24-ethenylcholest-5-en-3β,24-diol | 14161394 |
| 216 | dictyosterol A | 3β,6β-dihydroxycholesta-4,22E-dien-24-one | - |
| 217 | dictyosterol B | 6β-hydroxycholesta-4,22E-dien-3,24-dione | - |
| 218 | dictyosterol C | 3β,7α-dihydroxycholesta-5,22E-dien-24-one | - |
| 219 | 3β-hydroxycholesta-5,22E-dien-7,24-dione | - | |
| 220 | 3β-hydroxycholesta-5,22E-dien-24-one | - | |
| 221 | dictyopterisin C | (24R)-stigmasta-4,28(29)-dien-3β,7β,24-triol (24R)-24-ethenylcholest-4-en-3β,7β,24-triol | - |
| 222 | (24R)-7-methoxystigmasta-4,28(29)-dien-3β,24-diol (24R)-7-methoxy-24-ethenylcholest-4-en-3β,24-diol | - | |
| 223 | dictyopterisin F | (24R)-3β,24-dihydroxystigmasta-4,28(29)-dien-7-one (24R)-24-ethenyl-3β,24-dihydroxycholest-4-en-7-one | - |
| 224 | dictyopterisin G | (24S)-3β,24-dihydroxyporiferasta-4,28(29)-dien-7-one (24S)-24-ethenyl-3β,24-dihydroxycholest-4-en-7-one | - |
| 225 | dictyopterisin H | (24R)-stigmasta-4,28(29)-dien-3β,6β,24-triol (24R)-24-ethenylcholest-4-en-3β,6β,24-triol | - |
| 226 | dictyopterisin I | (24R)-6β,24-dihydroxystigmasta-4,28(29)-dien-3-one (24R)-24-ethenyl-6β,24-dihydroxycholest-4-en-3-one | - |
| 227 | dictyopterisin J | (24S)-6β,24-dihydroxyporiferasta-4,28(29)-dien-3-one (24S)-24-ethenyl-6β,24-dihydroxycholest-4-en-3-one | - |
1 Compound number. 2 Systematic names use carbon numbering and side chain α/β designations of the Nes system presented in Figure 1 and Ref. [1].
References
- Nes, W.D. Biosynthesis of cholesterol and other sterols. Chem. Rev. 2011, 111, 6423–6451. [Google Scholar] [CrossRef] [PubMed]
- Volkman, J.K. Sterols and other triterpenoids: Source specificity and evolution of biosynthetic pathways. Org. Geochem. 2005, 36, 139–159. [Google Scholar] [CrossRef]
- Cheng, L.L.; Wang, G.Z.; Zhang, H.Y. Sterol biosynthesis and prokaryotes-to-eukaryotes evolution. Biochem. Biophys. Res. Commun. 2007, 363, 885–888. [Google Scholar] [CrossRef] [PubMed]
- Desmond, E.; Gribaldo, S. Phylogenomics of sterol synthesis: Insights into the origin, evolution, and diversity of a key eukaryotic feature. Genome Biol. Evol. 2009, 1, 364–381. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.H.; Yin, X.; Welander, P.V. Sterol synthesis in diverse bacteria. Front. Microbiol. 2016, 7, 990. [Google Scholar] [CrossRef] [PubMed]
- Blackstone, N.W. An evolutionary framework for understanding the origin of eukaryotes. Biology 2016, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I. Cholesterol in health and disease. J. Clin. Investig. 2002, 110, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Santori, F.R.; Huang, P.; van de Pavert, S.A.; Douglass, E.F., Jr.; Leaver, D.J.; Haubrich, B.A.; Keber, R.; Lorbek, G.; Konijn, T.; Rosales, B.N.; et al. Identification of natural RORgamma ligands that regulate the development of lymphoid cells. Cell. Metab. 2015, 21, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Poirot, M.; Silvente-Poirot, S. The tumor-suppressor cholesterol metabolite, dendrogenin A, is a new class of LXR modulator activating lethal autophagy in cancers. Biochem. Pharmacol. 2018, 153, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Zerbinati, C.; Caponecchia, L.; Puca, R.; Ciacciarelli, M.; Salacone, P.; Sebastianelli, A.; Pastore, A.; Palleschi, G.; Petrozza, V.; Porta, N.; et al. Mass spectrometry profiling of oxysterols in human sperm identifies 25-hydroxycholesterol as a marker of sperm function. Redox Biol. 2017, 11, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, W.J.; Abdel-Khalik, J.; Hearn, T.; Yutuc, E.; Morgan, A.H.; Wang, Y. Current trends in oxysterol research. Biochem. Soc. Trans. 2016, 44, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Testa, G.; Rossin, D.; Poli, G.; Biasi, F.; Leonarduzzi, G. Implication of oxysterols in chronic inflammatory human diseases. Biochimie 2018. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, W.J.; Wang, Y. Sterolomics: State of the art, developments, limitations and challenges. Biochim. Biophys. Acta. 2017, 1862, 771–773. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, W.J.; Wang, Y. An update on oxysterol biochemistry: New discoveries in lipidomics. Biochem. Biophys. Res. Commun. 2018. [Google Scholar] [CrossRef] [PubMed]
- Moreau, R.A.; Nystrom, L.; Whitaker, B.D.; Winkler-Moser, J.K.; Baer, D.J.; Gebauer, S.K.; Hicks, K.B. Phytosterols and their derivatives: Structural diversity, distribution, metabolism, analysis, and health-promoting uses. Prog. Lipid. Res. 2018, 70, 35–61. [Google Scholar] [CrossRef] [PubMed]
- Nes, W.R.; Krevitz, K.; Joseph, J.; Nes, W.D. The phylogenetic distribution of sterols in tracheophytes. Lipids 1977, 12, 511–527. [Google Scholar] [CrossRef]
- Weete, J.D. Structure and function of sterols in fungi. In Advances in Lipid Research; Paoletti, R., Kritchevsky, D., Eds.; Elsevier: New York, NY, USA, 1989; Volume 23, pp. 115–167. [Google Scholar]
- Weete, J.D.; Gandhi, S.R. Sterols of the phylum zygomycota: Phylogenetic implications. Lipids 1997, 32, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Furlong, S.T.; Samia, J.A.; Rose, R.M.; Fishman, J.A. Phytosterols are present in Pneumocystis carinii. Antimicrob. Agents Chemother. 1994, 38, 2534–2540. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nes, W.D.; Norton, R.A.; Crumley, F.G.; Madigan, S.J.; Katz, E.R. Sterol phylogenesis and algal evolution. Proc. Natl. Acad. Sci. USA 1990, 87, 7565–7569. [Google Scholar] [CrossRef] [PubMed]
- Halevy, S.; Avivi, L.; Katan, H. Sterols of soil amoebas and Ochromonas danica: Phylogenetic approach. J. Protozool. 1966, 13, 480–483. [Google Scholar] [CrossRef] [PubMed]
- Raederstorff, D.; Rohmer, M. Sterol biosynthesis de nova via cycloartenol by the soil amoeba Acanthamoeba polyphaga. Biochem. J. 1985, 231, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Burden, R.S.; Cooke, D.T.; Carter, G.A. Inhibitors of sterol biosynthesis and growth in plants and fungi. Phytochemistry 1989, 28, 1791–1804. [Google Scholar] [CrossRef]
- Odds, F.C.; Brown, A.J.; Gow, N.A. Antifungal agents: Mechanisms of action. Trends Microbiol. 2003, 11, 272–279. [Google Scholar] [CrossRef]
- Leaver, D.J. Synthesis and Biological Activity of Sterol 14alpha-Demethylase and Sterol C24-Methyltransferase Inhibitors. Molecules 2018, 23, 1753. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Nes, W.D. Steroidal triterpenes: Design of substrate-based inhibitors of ergosterol and sitosterol synthesis. Molecules 2009, 14, 4690–4706. [Google Scholar] [CrossRef] [PubMed]
- Godtfredsen, W.O.; Jahnsen, S.; Lorck, H.; Roholt, K.; Tybring, L. Fusidic acid: A new antibiotic. Nature 1962, 193, 987. [Google Scholar] [CrossRef] [PubMed]
- Verbist, L. The antimicrobial activity of fusidic acid. J. Antimicrob. Chemother. 1990, 25 (Suppl. B), 1–5. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Godecke, T.; Gunn, J.; Duan, J.A.; Che, C.T. Protostane and fusidane triterpenes: A mini-review. Molecules 2013, 18, 4054–4080. [Google Scholar] [CrossRef] [PubMed]
- Bu, M.; Yang, B.B.; Hu, L. Natural bioactive sterol 5α, 8α-endoperoxides as drug lead compounds. Med. Chem. 2014, 4, 709–716. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Bioactive fungal endoperoxides. Med. Mycol. 2015, 1, 5. [Google Scholar] [CrossRef]
- Liu, D.; Li, X.M.; Li, C.S.; Wang, B.G. Nigerasterols A and B, antiproliferative sterols from the mangrove-derived endophytic fungus Aspergillus niger MA-132. Helvetica. Chimica. Acta 2013, 96, 1055–1061. [Google Scholar] [CrossRef]
- Zhou, X.; Sun, J.; Ma, W.; Fang, W.; Chen, Z.; Yang, B.; Liu, Y. Bioactivities of six sterols isolated from marine invertebrates. Pharm. Biol. 2014, 52, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Kitchawalit, S.; Kanokmedhakul, K.; Kanokmedhakul, S.; Soytong, K. A new benzyl ester and ergosterol derivatives from the fungus Gymnoascus reessii. Nat. Prod. Res. 2014, 28, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Brunel, J.M.; Salmi, C.; Loncle, C.; Vidal, N.; Letourneux, Y. Squalamine: A polyvalent drug of the future? Curr. Cancer Drug Targets 2005, 5, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Warrilow, A.G.S.; Thomas, C.D.; Ramos, E.; Parker, J.E.; Price, C.L.; Vanderloop, B.H.; Fisher, P.M.; Loftis, M.D.; Kelly, D.E.; et al. Functional importance for developmental regulation of sterol biosynthesis in Acanthamoeba castellanii. Biochim. Biophys. Acta 2018, 1863, 1164–1178. [Google Scholar] [CrossRef] [PubMed]
- Thomson, S.; Rice, C.A.; Zhang, T.; Edrada-Ebel, R.; Henriquez, F.L.; Roberts, C.W. Characterisation of sterol biosynthesis and validation of 14alpha-demethylase as a drug target in Acanthamoeba. Sci. Rep. 2017, 7, 8247. [Google Scholar] [CrossRef] [PubMed]
- Korn, E.D.; Von Brand, T.; Tobie, E.J. The sterols of Trypanosoma cruzi and Crithidia fasciculata. Comp. Biochem. Physiol. 1969, 30, 601–610. [Google Scholar] [CrossRef]
- Goad, L.J.; Holz, G.G., Jr.; Beach, D.H. Sterols of Leishmania species. Implications for biosynthesis. Mol. Biochem. Parasitol. 1984, 10, 161–170. [Google Scholar] [CrossRef]
- Nakamura, C.V.; Waldow, L.; Pelegrinello, S.R.; Ueda-Nakamura, T.; Filho, B.A.; Filho, B.P. Fatty acid and sterol composition of three phytomonas species. Mem. Inst. Oswaldo Cruz. 1999, 94, 519–525. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Haubrich, B.A.; Singha, U.K.; Miller, M.B.; Nes, C.R.; Anyatonwu, H.; Lecordier, L.; Patkar, P.; Leaver, D.J.; Villalta, F.; Vanhollebeke, B.; et al. Discovery of an ergosterol-signaling factor that regulates Trypanosoma brucei growth. J. Lipid Res. 2015, 56, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Nes, C.R.; Singha, U.K.; Liu, J.; Ganapathy, K.; Villalta, F.; Waterman, M.R.; Lepesheva, G.I.; Chaudhuri, M.; Nes, W.D. Novel sterol metabolic network of Trypanosoma brucei procyclic and bloodstream forms. Biochem. J. 2012, 443, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Cross, G.A.; Nes, W.D. Cholesterol import fails to prevent catalyst-based inhibition of ergosterol synthesis and cell proliferation of Trypanosoma brucei. J. Lipid. Res. 2007, 48, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Urbina, J.A. Ergosterol biosynthesis and drug development for Chagas disease. Mem. Inst. Oswaldo Cruz 2009, 104 (Suppl. 1), 311–318. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.B.; Haubrich, B.A.; Wang, Q.; Snell, W.J.; Nes, W.D. Evolutionarily conserved Delta(25(27))-olefin ergosterol biosynthesis pathway in the alga Chlamydomonas reinhardtii. J. Lipid. Res. 2012, 53, 1636–1645. [Google Scholar] [CrossRef] [PubMed]
- Gealt, M.A.; Adler, J.H.; Nes, W.R. The sterols and fatty acids from purified flagella of Chlamydomonas reinhardtii. Lipids 1981, 16, 133–136. [Google Scholar] [CrossRef]
- Patterson, G.W. The distribution of sterols in algae. Lipids 1971, 6, 120–127. [Google Scholar] [CrossRef]
- Volkman, J.K. Sterols in microalgae. In The Physiology of Microalgae; Borowitzka, M., Beardall, J., Raven, J., Eds.; Springer: Cham, Switzerland, 2016; Volume 6, pp. 485–505. [Google Scholar]
- Patkar, P.; Haubrich, B.A.; Qi, M.; Nguyen, T.T.; Thomas, C.D.; Nes, W.D. C-24-methylation of 26-fluorocycloartenols by recombinant sterol C-24-methyltransferase from soybean: Evidence for channel switching and its phylogenetic implications. Biochem. J. 2013, 456, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Haubrich, B.A.; Collins, E.K.; Howard, A.L.; Wang, Q.; Snell, W.J.; Miller, M.B.; Thomas, C.D.; Pleasant, S.K.; Nes, W.D. Characterization, mutagenesis and mechanistic analysis of an ancient algal sterol C24-methyltransferase: Implications for understanding sterol evolution in the green lineage. Phytochemistry 2015, 113, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Nes, W.D.; Marshall, J.A.; Jia, Z.; Jaradat, T.T.; Song, Z.; Jayasimha, P. Active site mapping and substrate channeling in the sterol methyltransferase pathway. J. Biol. Chem. 2002, 277, 42549–42556. [Google Scholar] [CrossRef] [PubMed]
- Dahmen, A.S.; Houle, H.M.; Leblond, J.D. Free sterols of the historically important green algal genera, Acetabularia and Acicularia (Polyphysaceae): A modern interpretation. Algol. Stud. 2017, 2017, 59–70. [Google Scholar] [CrossRef]
- Martin-Creuzburg, D.; Merkel, P. Sterols of freshwater microalgae: Potential implications for zooplankton nutrition. J. Plankton Res. 2016, 38, 865–877. [Google Scholar] [CrossRef]
- Giner, J.L.; Zhao, H.; Tomas, C. Sterols and fatty acids of three harmful algae previously assigned as Chattonella. Phytochemistry 2008, 69, 2167–2171. [Google Scholar] [CrossRef] [PubMed]
- Giner, J.L.; Zhao, H.; Dixon, M.S.; Wikfors, G.H. Bioconversion of (13)C-labeled microalgal phytosterols to cholesterol by the Northern Bay scallop, Argopecten irradians irradians. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2016, 192, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gergs, R.; Steinberger, N.; Beck, B.; Basen, T.; Yohannes, E.; Schulz, R.; Martin-Creuzburg, D. Compound-specific delta(13)C analyses reveal sterol metabolic constraints in an aquatic invertebrate. Rapid. Commun. Mass Spectrom. 2015, 29, 1789–1794. [Google Scholar] [CrossRef] [PubMed]
- Martin-Creuzburg, D.; von Elert, E. Ecological significance of sterols in aquatic food webs. In Lipids in Aquatic Ecosystems; Kainz, M., Brett, M., Arts, M., Eds.; Springer: New York, NY, USA, 2009. [Google Scholar]
- Behmer, S.T.; Nes, W.D. Insect sterol nutrition and physiology: A global overview. Adv. Insect. Physiol. 2003, 31, 72. [Google Scholar] [CrossRef]
- Taipale, S.J.; Hiltunen, M.; Vuorio, K.; Peltomaa, E. Suitability of Phytosterols Alongside Fatty Acids as Chemotaxonomic Biomarkers for Phytoplankton. Front. Plant. Sci. 2016, 7, 212. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.X.; Yu, R.C.; Chen, Z.F.; Peng, Q.C.; Yan, T.; Zhou, M.J. Analysis of sterols in selected bloom-forming algae in China. Harmful. Algae. 2017, 66, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Giner, J.L.; Ceballos, H.; Tang, Y.Z.; Gobler, C.J. Sterols and Fatty Acids of the Harmful Dinoflagellate Cochlodinium polykrikoides. Chem. Biodivers. 2016, 13, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Houle, H.M.; Lopez, C.B.; Leblond, J.D. Sterols of the Toxic Marine Dinoflagellate, Pyrodinium bahamense. J. Eukaryot. Microbiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Gori, A.; Tolosa, I.; Orejas, C.; Rueda, L.; Viladrich, N.; Grinyo, J.; Flogel, S.; Grover, R.; Ferrier-Pages, C. Biochemical composition of the cold-water coral Dendrophyllia cornigera under contrasting productivity regimes: Insights from lipid biomarkers and compound-specific isotopes. Deep Sea Res. Part 1 Oceanogr. Res. Pap. 2018. [Google Scholar] [CrossRef]
- Crandell, J.B.; Teece, M.A.; Estes, B.A.; Manfrino, C.; Ciesla, J.H. Nutrient acquisition strategies in mesophotic hard corals using compound specific stable isotope analysis of sterols. J. Exp. Mar. Bio. Ecol. 2016, 474, 133–141. [Google Scholar] [CrossRef]
- Zhou, W.; Lepesheva, G.I.; Waterman, M.R.; Nes, W.D. Mechanistic analysis of a multiple product sterol methyltransferase implicated in ergosterol biosynthesis in Trypanosoma brucei. J. Biol. Chem. 2006, 281, 6290–6296. [Google Scholar] [CrossRef] [PubMed]
- Millerioux, Y.; Mazet, M.; Bouyssou, G.; Allmann, S.; Kiema, T.R.; Bertiaux, E.; Fouillen, L.; Thapa, C.; Biran, M.; Plazolles, N.; et al. De novo biosynthesis of sterols and fatty acids in the Trypanosoma brucei procyclic form: Carbon source preferences and metabolic flux redistributions. PLoS Pathog. 2018, 14, e1007116. [Google Scholar] [CrossRef] [PubMed]
- Leaver, D.J.; Patkar, P.; Singha, U.K.; Miller, M.B.; Haubrich, B.A.; Chaudhuri, M.; Nes, W.D. Fluorinated Sterols Are Suicide Inhibitors of Ergosterol Biosynthesis and Growth in Trypanosoma brucei. Chem. Biol. 2015, 22, 1374–1383. [Google Scholar] [CrossRef] [PubMed]
- Dauchy, F.A.; Bonhivers, M.; Landrein, N.; Dacheux, D.; Courtois, P.; Lauruol, F.; Daulouede, S.; Vincendeau, P.; Robinson, D.R. Trypanosoma brucei CYP51: Essentiality and Targeting Therapy in an Experimental Model. PLoS Negl. Trop Dis. 2016, 10, e0005125. [Google Scholar] [CrossRef] [PubMed]
- Lepesheva, G.I.; Ott, R.D.; Hargrove, T.Y.; Kleshchenko, Y.Y.; Schuster, I.; Nes, W.D.; Hill, G.C.; Villalta, F.; Waterman, M.R. Sterol 14alpha-demethylase as a potential target for antitrypanosomal therapy: Enzyme inhibition and parasite cell growth. Chem. Biol. 2007, 14, 1283–1293. [Google Scholar] [CrossRef] [PubMed]
- Lorente, S.O.; Rodrigues, J.C.; Jimenez Jimenez, C.; Joyce-Menekse, M.; Rodrigues, C.; Croft, S.L.; Yardley, V.; de Luca-Fradley, K.; Ruiz-Perez, L.M.; Urbina, J.; et al. Novel azasterols as potential agents for treatment of leishmaniasis and trypanosomiasis. Antimicrob. Agents Chemother. 2004, 48, 2937–2950. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.B.; Patkar, P.; Singha, U.K.; Chaudhuri, M.; David Nes, W. 24-Methylenecyclopropane steroidal inhibitors: A Trojan horse in ergosterol biosynthesis that prevents growth of Trypanosoma brucei. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2017, 1862, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Kidane, M.E.; Vanderloop, B.H.; Zhou, W.; Thomas, C.D.; Ramos, E.; Singha, U.; Chaudhuri, M.; Nes, W.D. Sterol methyltransferase a target for anti-amoeba therapy: Towards transition state analog and suicide substrate drug design. J. Lipid. Res. 2017, 58, 2310–2323. [Google Scholar] [CrossRef] [PubMed]
- Lamb, D.C.; Warrilow, A.G.; Rolley, N.J.; Parker, J.E.; Nes, W.D.; Smith, S.N.; Kelly, D.E.; Kelly, S.L. Azole Antifungal Agents to Treat the Human Pathogens Acanthamoeba castellanii and Acanthamoeba polyphaga through Inhibition of Sterol 14alpha-Demethylase (CYP51). Antimicrob. Agents Chemother. 2015, 59, 4707–4713. [Google Scholar] [CrossRef] [PubMed]
- Nakaminami, H.; Tanuma, K.; Enomoto, K.; Yoshimura, Y.; Onuki, T.; Nihonyanagi, S.; Hamada, Y.; Noguchi, N. Evaluation of In Vitro Antiamoebic Activity of Antimicrobial Agents Against Clinical Acanthamoeba Isolates. J. Ocul. Pharmacol. Ther. 2017, 33, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Gueudry, J.; Le Goff, L.; Compagnon, P.; Lefevre, S.; Colasse, E.; Aknine, C.; Duval, F.; Francois, A.; Razakandrainibe, R.; Ballet, J.J.; et al. Evaluation of voriconazole anti-Acanthamoeba polyphaga in vitro activity, rat cornea penetration and efficacy against experimental rat Acanthamoeba keratitis. J. Antimicrob. Chemother. 2018. [Google Scholar] [CrossRef] [PubMed]
- Taravaud, A.; Loiseau, P.M.; Pomel, S. In vitro evaluation of antimicrobial agents on Acanthamoeba sp. and evidence of a natural resilience to amphotericin B. Int. J. Parasitol. Drugs Drug Resist. 2017, 7, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, X.; Wei, P.; Su, X.; Zhao, L.; Wu, M.; Hao, C.; Liu, C.; Zhao, D.; Cheng, M. Design, synthesis and evaluation of aromatic heterocyclic derivatives as potent antifungal agents. Eur. J. Med. Chem. 2017, 137, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wei, P.; Wu, M.; Zhang, X.; Zhao, L.; Jiang, X.; Hao, C.; Su, X.; Zhao, D.; Cheng, M. Design, synthesis and evaluation of benzoheterocycle analogues as potent antifungal agents targeting CYP51. Bioorg. Med. Chem. 2018, 26, 3242–3253. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.; Neugebauer, T.; Zill, P.; Lass-Florl, C.; Bracher, F.; Binder, U. Sterol Composition of Clinically Relevant Mucorales and Changes Resulting from Posaconazole Treatment. Molecules 2018, 23, 1218. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Shi, H.; Chang, W.; Li, Y.; Zhang, M.; Qiao, Y.; Lou, H. Eudesmane sesquiterpenes from Chinese liverwort are substrates of Cdrs and display antifungal activity by targeting Erg6 and Erg11 of Candida albicans. Bioorg. Med. Chem. 2017, 25, 5764–5771. [Google Scholar] [CrossRef] [PubMed]
- Helliwell, S.B.; Karkare, S.; Bergdoll, M.; Rahier, A.; Leighton-Davis, J.R.; Fioretto, C.; Aust, T.; Filipuzzi, I.; Frederiksen, M.; Gounarides, J.; et al. FR171456 is a specific inhibitor of mammalian NSDHL and yeast Erg26p. Nat. Commun. 2015, 6, 8613. [Google Scholar] [CrossRef] [PubMed]
- Warrilow, A.G.; Parker, J.E.; Price, C.L.; Nes, W.D.; Garvey, E.P.; Hoekstra, W.J.; Schotzinger, R.J.; Kelly, D.E.; Kelly, S.L. The Investigational Drug VT-1129 Is a Highly Potent Inhibitor of Cryptococcus Species CYP51 but Only Weakly Inhibits the Human Enzyme. Antimicrob. Agents Chemother. 2016, 60, 4530–4538. [Google Scholar] [CrossRef] [PubMed]
- Alhanout, K.; Rolain, J.M.; Brunel, J.M. Squalamine as an example of a new potent antimicrobial agents class: A critical review. Curr. Med. Chem. 2010, 17, 3909–3917. [Google Scholar] [CrossRef] [PubMed]
- Savage, P.B.; Li, C.; Taotafa, U.; Ding, B.; Guan, Q. Antibacterial properties of cationic steroid antibiotics. FEMS Microbiol. Lett. 2002, 217, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Tseng, W.R.; Ahmed, A.F.; Chiang, P.L.; Tai, C.J.; Hwang, T.L.; Dai, C.F.; Sheu, J.H. Anti-Inflammatory Polyoxygenated Steroids from the Soft Coral Lobophytum michaelae. Mar. Drugs 2018, 16, 93. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.M.; Ito, T.; Win, N.N.; Vo, H.Q.; Nguyen, H.T.; Morita, H. A new sterol from the Vietnamese marine sponge Xestospongia testudinaria and its biological activities. Nat. Prod. Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, Y.; Sekine, A.; Fukazawa, H.; Uehara, Y.; Yamaguchi, K.; Endo, Y.; Okuda, T.; Furumai, T.; Oki, T. Anicequol, a novel inhibitor for anchorage-independent growth of tumor cells from Penicillium aurantiogriseum Dierckx TP-F0213. J. Antibiot. 2002, 55, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Nozawa, Y.; Sakai, N.; Matsumoto, K.; Mizoue, K. A novel neuritogenic compound, NGA0187. J. Antibiot. 2002, 55, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.R.; Noguchi, K.; Fukazawa, H.; Igarashi, Y.; Arai, H.; Uehara, Y. p38MAPK and Rho-dependent kinase are involved in anoikis induced by anicequol or 25-hydroxycholesterol in DLD-1 colon cancer cells. Biochem. Biophys. Res. Commun. 2013, 430, 1240–1245. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.M.; Liu, S.X.; Huang, C.H.; Pang, J.Y.; Lin, Y.C. Secondary metabolites of a mangrove endophytic fungus Aspergillus terreus (No. GX7-3B) from the South China Sea. Mar. Drugs 2013, 11, 2616–2624. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.S.; Li, X.M.; Li, C.S.; Proksch, P.; Wang, B.G. Penicisteroids A and B, antifungal and cytotoxic polyoxygenated steroids from the marine alga-derived endophytic fungus Penicillium chrysogenum QEN-24S. Bioorg. Med. Chem. Lett. 2011, 21, 2894–2897. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Razek, A.S.; Hamed, A.; Frese, M.; Sewald, N.; Shaaban, M. Penicisteroid C: New polyoxygenated steroid produced by co-culturing of Streptomyces piomogenus with Aspergillus niger. Steroids 2018, 138, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.B.; Xiao, H.; Fan, C.Q.; Lu, Y.N.; Zhang, G.; Yin, S. Bioactive polyhydroxylated sterols from the marine sponge Haliclona crassiloba. Steroids 2013, 78, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Ngoc, N.T.; Huong, P.T.; Thanh, N.V.; Chi, N.T.; Dang, N.H.; Cuong, N.X.; Nam, N.H.; Thung, D.C.; Kiem, P.V.; Minh, C.V. Cytotoxic Steroids from the Vietnamese Soft Coral Sinularia conferta. Chem. Pharm. Bull. 2017, 65, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tang, H.; Wang, P.; Gong, W.; Xue, M.; Zhang, H.; Liu, T.; Liu, B.; Yi, Y.; Zhang, W. Bioactive polyoxygenated steroids from the South China sea soft coral, Sarcophyton sp. Mar. Drugs 2013, 11, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.W.; Su, J.H.; Lin, C.C.; Li, Y.R.; Chao, Y.H.; Lin, S.H.; Chan, H.L. 24-Methyl-Cholesta-5,24(28)-Diene-3beta,19-diol-7beta-Monoacetate Inhibits Human Small Cell Lung Cancer Growth In Vitro and In Vivo via Apoptosis Induction. Mar. Drugs 2017, 15, 210. [Google Scholar] [CrossRef] [PubMed]
- Meesala, S.; Gurung, P.; Karmodiya, K.; Subrayan, P.; Watve, M.G. Isolation and structure elucidation of halymeniaol, a new antimalarial sterol derivative from the red alga Halymenia floresii. J. Asian Nat. Prod. Res. 2018, 20, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, X.C.; Longeon, A.; Pham, V.C.; Urvois, F.; Bressy, C.; Trinh, T.T.; Nguyen, H.N.; Phan, V.K.; Chau, V.M.; Briand, J.F.; et al. Antifouling 26,27-cyclosterols from the Vietnamese marine sponge Xestospongia testudinaria. J. Nat. Prod. 2013, 76, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- Pailee, P.; Mahidol, C.; Ruchirawat, S.; Prachyawarakorn, V. Sterols from Thai Marine Sponge Petrosia (Strongylophora) sp. and Their Cytotoxicity. Mar. Drugs 2017, 15, 54. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Liu, D.; de Voogd, N.J.; Proksch, P.; Lin, W. New sterol derivatives from the marine sponge Xestospongia sp. Helvetica. Chim. Acta 2016, 99, 588–596. [Google Scholar] [CrossRef]
- Tsai, C.-R.; Huang, C.Y.; Chen, B.W.; Tsai, Y.Y.; Shih, S.-P.; Hwang, T.-L.; Dai, C.-F.; Wang, S.-Y.; Sheu, J.-H. New bioactive steroids from the soft coral Klyxum flaccidum. RSC Adv. 2015, 5, 12546–12554. [Google Scholar] [CrossRef]
- Tseng, W.R.; Huang, C.Y.; Tsai, Y.Y.; Lin, Y.S.; Hwang, T.L.; Su, J.H.; Sung, P.J.; Dai, C.F.; Sheu, J.H. New cytotoxic and anti-inflammatory steroids from the soft coral Klyxum flaccidum. Bioorg. Med. Chem. Lett. 2016, 26, 3253–3257. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, F.; Kudo, N.; Tomachi, Y.; Nakata, A.; Takemoto, M.; Ito, A.; Tabei, H.; Arai, D.; de Voogd, N.; Yoshida, M.; et al. Halistanol sulfates I. and J, new SIRT1-3 inhibitory steroid sulfates from a marine sponge of the genus Halichondria. J. Antibiot. 2018, 71, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Fiorucci, S.; Distrutti, E.; Bifulco, G.; D’Auria, M.V.; Zampella, A. Marine sponge steroids as nuclear receptor ligands. Trends Pharmacol. Sci. 2012, 33, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Zhang, H.; Sun, X.; Zhao, H.; Wu, L.; Zhu, D.; Yang, G.; Shao, Y.; Zhang, X.; Mao, X.; et al. A comprehensive review of the structure elucidation and biological activity of triterpenoids from Ganoderma spp. Molecules 2014, 19, 17478–17535. [Google Scholar] [CrossRef] [PubMed]
- Metwaly, A.M.; Kadry, H.A.; El-Hela, A.A.; Mohammad, A.I.; Ma, G.; Cutler, S.J.; Ross, S.A. Nigrosphaerin A a new isochromene derivative from the endophytic fungus Nigrospora sphaerica. Phytochem. Lett. 2014, 7, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, K.L.; Liu, M.; She, Z.G.; Wang, C.Y. Bioactive steroid derivatives and butyrolactone derivatives from a gorgonian-derived Aspergillus sp. fungus. Chem. Biodivers. 2015, 12, 1398–1406. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Chen, G.D.; Feng, X.L.; Yu, Y.; He, R.R.; Li, X.X.; Huang, Y.; Zhou, W.X.; Guo, L.D.; Zheng, Y.Z.; et al. Nodulisporiviridins A-H, Bioactive Viridins from Nodulisporium sp. J. Nat. Prod. 2015, 78, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Harms, H.; Kehraus, S.; Nesaei-Mosaferan, D.; Hufendieck, P.; Meijer, L.; Konig, G.M. Abeta-42 lowering agents from the marine-derived fungus Dichotomomyces cejpii. Steroids 2015, 104, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Ngoc, N.T.; Huong, P.T.; Thanh, N.V.; Cuong, N.X.; Nam, N.H.; Thung do, C.; Kiem, P.V.; Minh, C.V. Steroid Constituents from the Soft Coral Sinularia nanolobata. Chem. Pharm. Bull. 2016, 64, 1417–1419. [Google Scholar] [CrossRef] [PubMed]
- Thanh, N.V.; Ngoc, N.T.; Anh Hle, T.; Thung do, C.; Thao do, T.; Cuong, N.X.; Nam, N.H.; Kiem, P.V.; Minh, C.V. Steroid constituents from the soft coral Sinularia microspiculata. J. Asian Nat. Prod. Res. 2016, 18, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Ngoc, N.T.; Hanh, T.T.H.; Thanh, N.V.; Thao, D.T.; Cuong, N.X.; Nam, N.H.; Thung, D.C.; Kiem, P.V.; Minh, C.V. Cytotoxic Steroids from the Vietnamese Soft Coral Sinularia leptoclados. Chem. Pharm. Bull. 2017, 65, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.H.T.; Nguyen Viet, P.; Nguyen Van, T.; Tran, H.T.; Nguyen Xuan, C.; Nguyen Hoai, N.; Do Cong, T.; Phan Van, K.; Chau Van, M. Cytotoxic steroid derivatives from the Vietnamese soft coral Sinularia brassica. J. Asian Nat. Prod. Res. 2017, 19, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Li, S.W.; Chen, W.T.; Yao, L.G.; Guo, Y.W. Two new cytotoxic steroids from the Chinese soft coral Sinularia sp. Steroids 2018, 136, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Su, J.H.; Liaw, C.C.; Sung, P.J.; Chiang, P.L.; Hwang, T.L.; Dai, C.F.; Sheu, J.H. Bioactive Steroids with Methyl Ester Group in the Side Chain from a Reef Soft Coral Sinularia brassica Cultured in a Tank. Mar. Drugs 2017, 15, 280. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.Y.; Huang, C.Y.; Tseng, W.R.; Chiang, P.L.; Hwang, T.L.; Su, J.H.; Sung, P.J.; Dai, C.F.; Sheu, J.H. Klyflaccisteroids, K-M bioactive steroidal derivatives from a soft coral Klyxum flaccidum. Bioorg. Med. Chem. Lett. 2017, 27, 1220–1224. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Ren, J.; Huang, Q.; Long, H.; Jin, H.; Zhang, L.; Liu, H.; van Ofwegen, L.; Lin, W. Pregnane steroids from a gorgonian coral Subergorgia suberosa with anti-flu virus effects. Steroids 2016, 108, 99–104. [Google Scholar] [CrossRef] [PubMed]
- He, W.F.; Xue, D.Q.; Yao, L.G.; Li, J.; Liu, H.L.; Guo, Y.W. A new bioactive steroidal ketone from the South China Sea sponge Xestospongia testudinaria. J. Asian Nat. Prod. Res. 2016, 18, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Mencarelli, A.; D’Amore, C.; Renga, B.; Cipriani, S.; Carino, A.; Sepe, V.; Perissutti, E.; D’Auria, M.V.; Zampella, A.; Distrutti, E.; et al. Solomonsterol A, a marine pregnane-X-receptor agonist, attenuates inflammation and immune dysfunction in a mouse model of arthritis. Mar. Drugs 2013, 12, 36–53. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Li, Y.Y.; Tang, J.; Sun, F.; Lin, H.W. New 4-methylidene sterols from the marine sponge Theonella swinhoei. Fitoterapia 2018, 127, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Abdelmohsen, U.R.; Cheng, C.; Reimer, A.; Kozjak-Pavlovic, V.; Ibrahim, A.K.; Rudel, T.; Hentschel, U.; Edrada-Ebel, R.; Ahmed, S.A. Antichlamydial sterol from the Red Sea sponge Callyspongia aff. implexa. Planta. Med. 2015, 81, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Perumal, P.; Sowmiya, R.; Prasanna Kumar, S.; Ravikumar, S.; Deepak, P.; Balasubramani, G. Isolation, structural elucidation and antiplasmodial activity of fucosterol compound from brown seaweed, Sargassum linearifolium against malarial parasite Plasmodium falciparum. Nat. Prod. Res. 2018, 32, 1316–1319. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Cho, Y.R.; Hong, S.S.; Anh, E.K. Anti-obesity activity of saringosterol isolated from Sargassum muticum (Yendo) fensholt extract in 3t3-L1 cells. Phytother. Res. 2017, 31, 1694–1701. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.G.; Zhou, M.; Jin, Q.H.; Liu, B.; Guan, L.P. Antidepressant-like effects of saringosterol, a sterol from Sargassum fusiforme by performing in vivo behavioral tests. Med. Chem. Res. 2017, 26, 909–915. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, L.W.; Feng, M.T.; Liu, A.H.; Li, J.; Zhao, T.S.; Lai, X.P.; Wang, B.; Guo, Y.W.; Mao, S.C. Dictyoptesterols A–C, ∆22-24-oxo cholestane-type sterols with potent PTP1B inhibitory activity from the brown alga Dictyopteris undulata Holmes. Fitoterapia 2018, 130, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.T.; Wang, T.; Liu, A.H.; Li, J.; Yao, L.G.; Wang, B.; Guo, Y.W.; Mao, S.C. PTP1B inhibitory and cytotoxic C-24 epimers of Delta(28)-24-hydroxy stigmastane-type steroids from the brown alga Dictyopteris undulata Holmes. Phytochemistry 2018, 146, 25–35. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).