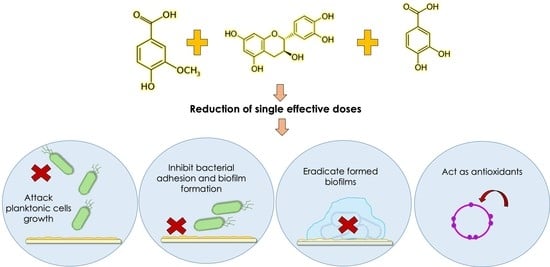
Comparison of Single and Combined Use of Catechin, Protocatechuic, and Vanillic Acids as Antioxidant and Antibacterial Agents against Uropathogenic Escherichia Coli at Planktonic and Biofilm Levels
Abstract
1. Introduction
2. Results
2.1. Antibacterial Activity of Individual and Combined Phenolic Compounds against Planktonic UPEC
2.2. Minimum Inhibitory Concentrations of Individual and Combined Phenolic Compounds against Uropathogenic E. coli Adhesion on Silicone Urinary Catheters
2.3. Effect of Individual and Combined Phenolic Compounds to Eradicate Pre-Formed Uropathogenic E. coli Biofilms
2.4. Antioxidant Activity of Individual and Combined Phenolic Compounds
3. Discussion
4. Materials and Methods
4.1. Antibacterial Effect of Individual Phenolic Compounds Against Planktonic Bacteria
4.2. Antibacterial Effect of Combined Phenolic Compounds against Planktonic Bacteria
4.3. The Minimum Inhibitory Concentrations of Phenolic Compounds against Biofilm Formation of Uropathogenic E. coli
4.4. The Minimum Inhibitory Concentrations of Phenolic Compounds to Eradicate Pre-Formed Biofilms of Uropathogenic E. coli
4.5. Crystal Violet Assay to Evaluate the Inhibition and Eradication of Uropathogenic E. coli Biofilm by Exposure to Individual and Combined Phenolic Compounds
4.6. Antioxidant Capacity of Individual and Combined Phenolic Compounds Using the DPPH•, TEAC, and FRAP Assays
4.6.1. DPPH• Radical Scavenging Activity
4.6.2. ABTS Radical Scavenging Activity
4.6.3. FRAP Assay
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Trautner, B.W.; Darouiche, R.O. Role of biofilm in catheter-associated urinary tract infection. Am. J. Infect. Control 2004, 32, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.A.; Mensa, J. Infección urinaria asociada a catéteres urinarios en la comunidad. Enferm. Infecc. Microbiol. Clin. 2005, 23, 57–66. [Google Scholar] [CrossRef] [PubMed]
- De Lira Torres, M.; Flores, A.; Fragoso, L.; Oliva, B.; López, E.; Márquez, M. Infecciones del tracto urinario asociado a catéter vesical. Áreas de cirugía y medicina interna de dos hospitales del sector público. Enf. Inf. Microbiol. 2012, 33, 13–18. [Google Scholar]
- Nicolle, L.E. Catheter associated urinary tract infections. Antimicrob. Resist. Infect. Control 2014, 3, 23. [Google Scholar] [CrossRef] [PubMed]
- Gordiushina, I.V.; Savchenko, R.P.; Sukhanov, D.S.; Petrov, A.; Romantsov, M.G. Antioxidant and membranoprotector treatment of chronic pyelonephritis. Eksperimental′naia i klinicheskaia farmakologiia 2011, 74, 27–30. [Google Scholar] [PubMed]
- Ribeiro, S.M.; Felicio, M.R.; Boas, E.V.; Goncalves, S.; Costa, F.F.; Samy, R.P.; Santos, N.C.; Franco, O.L. New frontiers for anti-biofilm drug development. Pharmacol. Ther. 2016, 160, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Jaimes, E.; Casanova-Román, G.; Galindo-Fraga, A.; Gutiérrez-Escoto, P.; Landa-Juárez, S.; Moreno-Espinosa, S.; Rodríguez-Covarrubias, F.; Simón-Pereira, L.; Valdez-Vázquez, R. Diagnóstico y tratamiento de las infecciones en vías urinarias: Un enfoque multidisciplinario para casos no complicados. Bol. Med. Hosp. Infant. Mex. 2013, 70, 3–10. [Google Scholar]
- Ito, A.; Taniuchi, A.; May, T.; Kawata, K.; Okabe, S. Increased antibiotic resistance of Escherichia coli in mature biofilms. Appl. Environ. Microbiol. 2009, 75, 4093–4100. [Google Scholar] [CrossRef] [PubMed]
- Amalaradjou, M.A.R.; Venkitanarayanan, K. Natural Approaches for Controlling Urinary Tract Infections; InTech: Hong Kong, China, 2011; pp. 228–244. [Google Scholar]
- Vysakh, A.; Raji, N.R.; Suma, D.; Jayesh, K.; Jyothis, M.; Latha, M.S. Role of antioxidant defence, renal toxicity markers and inflammatory cascade in disease progression of acute pyelonephritis in experimental rat model. Microb. Pathog. 2017, 109, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Monagas, M.; Urpi-Sarda, M.; Sánchez-Patán, F.; Llorach, R.; Garrido, I.; Gómez-Cordovés, C.; Andres-Lacueva, C.; Bartolomé, B. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct. 2010, 1, 233–253. [Google Scholar] [CrossRef] [PubMed]
- Iswaldi, I.; Arráez-Román, D.; Gómez-Caravaca, A.M.; del Mar Contreras, M.; Uberos, J.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Identification of polyphenols and their metabolites in human urine after cranberry-syrup consumption. Food Chem. Toxicol. 2013, 55, 484–492. [Google Scholar] [CrossRef] [PubMed]
- McKay, D.L.; Chen, C.-Y.O.; Zampariello, C.A.; Blumberg, J.B. Flavonoids and phenolic acids from cranberry juice are bioavailable and bioactive in healthy older adults. Food Chem. 2015, 168, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, P.; Sakharkar, M.K.; Lim, C.S.; Tang, T.H.; Sakharkar, K.R. Activity and interactions of antibiotic and phytochemical combinations against Pseudomonas aeruginosa in vitro. Int. J. Biol. Sci. 2010, 6, 556–568. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.J.; Ferreira, I.C.; Froufe, H.J.; Abreu, R.; Martins, A.; Pintado, M. Antimicrobial activity of phenolic compounds identified in wild mushrooms, SAR analysis and docking studies. J. Appl. Microbiol. 2013, 115, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Shimatani, K.; Ozawa, T.; Shigemune, N.; Tsugukuni, T.; Tomiyama, D.; Kurahachi, M.; Nonaka, A.; Miyamoto, T. A study of the antibacterial mechanism of catechins: Isolation and identification of Escherichia coli cell surface proteins that interact with epigallocatechin gallate. Food Control 2013, 33, 433–439. [Google Scholar] [CrossRef]
- Van Vuuren, S.; Viljoen, A. Plant-based antimicrobial studies–methods and approaches to study the interaction between natural products. Planta Med. 2011, 77, 1168–1182. [Google Scholar] [CrossRef] [PubMed]
- Simoes, M.; Bennett, R.N.; Rosa, E.A. Understanding antimicrobial activities of phytochemicals against multidrug resistant bacteria and biofilms. Nat. Prod. Rep. 2009, 26, 746–757. [Google Scholar] [CrossRef] [PubMed]
- Negi, P.S. Plant extracts for the control of bacterial growth: Efficacy, stability and safety issues for food application. Int. J. Food Microbiol. 2012, 156, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Miyague, L.; Macedo, R.E.; Meca, G.; Holley, R.A.; Luciano, F.B. Combination of phenolic acids and essential oils against Listeria monocytogenes. LWT-Food Sci. Technol. 2015, 64, 333–336. [Google Scholar] [CrossRef]
- Taylor, P.W.; Hamilton-Miller, J.M.; Stapleton, P.D. Antimicrobial properties of green tea catechins. Food Sci. Technol. Bull. 2005, 2, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Wojnicz, D.; Kucharska, A.Z.; Sokół-Łętowska, A.; Kicia, M.; Tichaczek-Goska, D. Medicinal plants extracts affect virulence factors expression and biofilm formation by the uropathogenic Escherichia coli. Urol. Res. 2012, 40, 683–697. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Saavedra, M.J.; Simões, M. The activity of ferulic and gallic acids in biofilm prevention and control of pathogenic bacteria. Biofouling 2012, 28, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Song, B.; Neto, C.; Camesano, T.A. Atomic force microscopy-guided fractionation reveals the influence of cranberry phytochemicals on adhesion of Escherichia coli. Food Funct. 2016, 7, 2655–2666. [Google Scholar] [CrossRef] [PubMed]
- Serra, D.O.; Mika, F.; Richter, A.M.; Hengge, R. The green tea polyphenol EGCG inhibits E. coli biofilm formation by impairing amyloid curli fibre assembly and downregulating the biofilm regulator CsgD via the σE-dependent sRNA RybB. Mol. Microbiol. 2016, 101, 136–151. [Google Scholar] [PubMed]
- Vikram, A.; Jayaprakasha, G.; Jesudhasan, P.; Pillai, S.; Patil, B. Suppression of bacterial cell–cell signalling, biofilm formation and type III secretion system by citrus flavonoids. J. Appl. Microbiol. 2010, 109, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Regmi, S.C.; Kim, J.-A.; Cho, M.H.; Yun, H.; Lee, C.-S.; Lee, J. Apple flavonoid phloretin inhibits Escherichia coli O157: H7 biofilm formation and ameliorates colon inflammation in rats. Infect. Immun. 2011, 79, 4819–4827. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Davila, M.J. Role of old antibiotics in the era of antibiotic resistance. Highlighted nitrofurantoin for the treatment of lower urinary tract infections. Antibiotics 2014, 3, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Konar, J.; Ghosh, R.; Chatterjee, S.S.; Majumdar, A.K.; Pathak, M.; Bhattacharya, S. Nitrofurantoin: The time-tested choice in uncomplicated urinary tract infection. J. Evol. Med. Dent. Sci. 2016, 5, 1872–1875. [Google Scholar] [CrossRef] [PubMed]
- Skroza, D.; Mekinic, I.G.; Svilovic, S.; Simar, V.; Katalinic, V. Investigation of the potential synergistic effect of resveratrol with other phenolic compounds: A case of binary phenolic mixtures. J. Food Compos. Anal. 2015, 38, 13–18. [Google Scholar] [CrossRef]
- Palafox-Carlos, H.; Gil-Chavez, J.; Sotelo-Mundo, R.R.; Namiesnik, J.; Gorinstein, S.; Gonzalez-Aguilar, G.A. Antioxidant Interactions between Major Phenolic Compounds Found in ‘Ataulfo’ Mango Pulp: Chlorogenic, Gallic, Protocatechuic and Vanillic Acids. Molecules 2012, 17, 12657–12664. [Google Scholar] [CrossRef] [PubMed]
- Choe, E.; Min, D.B. Mechanisms of Antioxidants in the Oxidation of Foods. Compr. Rev. Food Sci. Food Saf. 2009, 8, 345–358. [Google Scholar] [CrossRef]
- Kurutas, E.B.; Gumusalan, Y.; Cetinkaya, A.; Dogan, E. Evaluation of method performance for oxidative stress biomarkers in urine and biological variations in urine of patients with type 2 diabetes mellitus and diabetic nephropathy. Biol. Proced Online 2015, 17, 3. [Google Scholar] [CrossRef] [PubMed]
- Malinovskaya, V.; Delenian, N.; Gadgialieva, M.; Zakharova, I.; Korovina, N.; Makashova, V.; Polesko, I. Therapy of Children with Pyelonephritis by Recombinant Interferon α-2b with Antioxidants. In Proceedings of the International Cytokine Society Annual Meeting, Trinity Coll, Dublin, Ireland, 20–24 September 2003; Medimond Publishing Co: Monterey, CA, USA, 2003. [Google Scholar]
- Celik, S.; Gorur, S.; Aslantas, O.; Erdogan, S.; Ocak, S.; Hakverdi, S. Caffeic acid phenethyl ester suppresses oxidative stress in Escherichia coli-induced pyelonephritis in rats. Mol. Cell. Biochem. 2007, 297, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Asma, B.; Vicky, L.; Stephanie, D.; Yves, D.; Amy, H.; Sylvie, D. Standardised high dose versus low dose cranberry Proanthocyanidin extracts for the prevention of recurrent urinary tract infection in healthy women [PACCANN]: A double blind randomised controlled trial protocol. BMC Urol. 2018, 18, 29. [Google Scholar] [CrossRef] [PubMed]
- Burt, S.A.; Reinders, R.D. Antibacterial activity of selected plant essential oils against Escherichia coli O157: H7. Lett. Appl. Microbiol. 2003, 36, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.V.; Ortega-Ramirez, L.A.; Gutierrez-Pacheco, M.M.; Bernal-Mercado, A.T.; Rodriguez-Garcia, I.; Gonzalez-Aguilar, G.A.; Ponce, A.; Moreira, M.d.R.; Roura, S.I.; Ayala-Zavala, J.F. Oregano essential oil-pectin edible films as anti-quorum sensing and food antimicrobial agents. Front. Microbiol. 2014, 5, 699. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Ramirez, L.A.; Silva-Espinoza, B.A.; Vargas-Arispuro, I.; Gonzalez-Aguilar, G.A.; Cruz-Valenzuela, M.R.; Nazzaro, F.; Ayala-Zavala, J.F. Combination of Cymbopogon citratus and Allium cepa essential oils increased antibacterial activity in leafy vegetables. J. Sci. Food Agric. 2017, 97, 2166–2173. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Rodriguez, M.R.; Hernandez-Mendoza, A.; Gonzalez-Aguilar, G.A.; Martinez-Tellez, M.A.; Martins, C.M.; Ayala-Zavala, J.F. Carvacrol as potential quorum sensing inhibitor of Pseudomonas aeruginosa and biofilm production on stainless steel surfaces. Food Control 2017, 75, 255–261. [Google Scholar] [CrossRef]
- Gutierrez-Pacheco, M.M.; Gonzalez-Aguilar, G.G.; Martinez-Tellez, M.A.; Lizardi-Mendoza, J.; Madera-Santana, T.; Bernal-Mercado, A.T.; Vazquez-Armenta, F.J.; Ayala-Zavala, J.F. Carvacrol inhibits biofilm formation and production of extracellular polymeric substances of Pectobacterium carotovorum subsp. carotovorum. Food Control 2018, 89, 210–218. [Google Scholar] [CrossRef]
- Burton, E.; Yakandawala, N.; LoVetri, K.; Madhyastha, M. A microplate spectrofluorometric assay for bacterial biofilms. J. Ind. Microbiol. Biotechnol. 2007, 34, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Melgarejo-Flores, B.G.; Ortega-Ramírez, L.A.; Silva-Espinoza, B.A.; González-Aguilar, G.A.; Miranda, M.R.A.; Ayala-Zavala, J.F. Antifungal protection and antioxidant enhancement of table grapes treated with emulsions, vapors, and coatings of cinnamon leaf oil. Postharvest Biol. Technol. 2013, 86, 321–328. [Google Scholar] [CrossRef]
- Velderrain-Rodriguez, G.R.; Torres-Moreno, H.; Villegas-Ochoa, M.A.; Ayala-Zavala, J.F.; Robles-Zepeda, R.E.; Wall-Medrano, A.; Gonzalez-Aguilar, G.A. Gallic Acid Content and an Antioxidant Mechanism Are Responsible for the Antiproliferative Activity of ‘Ataulfo’ Mango Peel on LS180 Cells. Molecules 2018, 23, 3. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from Sigma-Aldrich. |





| Compounds | MIC (mM) | MBC (mM) |
|---|---|---|
| Protocatechuic acid | 12.98 | 19.46 |
| Vanillic acid | 11.80 | 17.84 |
| Catechin | 13.78 | ND |
| Nitrofurantoin | 0.4 | 0.4 |
| Phenolic Compound (mM) | Effect | |||
|---|---|---|---|---|
| Protocatechuic Acid | Vanillic Acid | Catechin | ΣFIC | Interaction |
| 0.81 | 5.94 | 0.0 | 0.5 | Synergistic |
| 12.98 | 0.0 | 0.20 | 1.0 | Additive |
| 0.0 | 5.94 | 6.89 | 1.0 | Additive |
| 1.62 | 0.17 | 6.89 | 0.6 | Additive |
| 1.62 | 0.74 | 0.05 | 0.3 | Synergistic |
| 6.48 | 2.97 | 3.44 | 1.0 | Additive |
| Combination (mM) | ||||||
|---|---|---|---|---|---|---|
| Protocatechuic Acid | Vanillic Acid | Catechin | Adhered Cells (Log CFU/cm2) | Planktonic Cells (Log CFU/mL) | ΣFIC | Combination Effect |
| 0.0 | 0.0 | 0.0 | 5.96 ± 0.1 | 8.76 ± 0.01 | ||
| 2.75 | 1.48 | 13.78 | 4.23 ± 0.0 | 8.59 ± 0.09 | 0.62 | Additive |
| 5.51 | 1.48 | 13.78 | 3.68 ± 0.17 | 8.47 ± 0.07 | 0.87 | Additive |
| 2.75 | 3.56 | 13.78 | 5.14 ± 0.04 | 8.35 ± 0.05 | 0.95 | Additive |
| 5.51 | 3.56 | 13.78 | 2.35 ± 0.05 | 3.08 ± 0.01 | 1.20 | Indifferent |
| 1.62 | 0.74 | 0.05 | ND | 8.78 ± 0.06 | 0.25 | Synergistic |
| 3.20 | 2.97 | 1.72 | 3.3 ± 0.13 | 8.71 ± 0.06 | 0.74 | Additive |
| 6.48 | 2.97 | 3.44 | 4.41 ± 0.06 | 7.03 ± 0.01 | 1.05 | Indifferent |
| 1.62 | 5.94 | 3.44 | ND | 6.37 ± 0.02 | 1.18 | Indifferent |
| 3.24 | 2.97 | - | 5.95 ± 0.07 | 8.86 ± 0.04 | 0.71 | Additive |
| 2.75 | 5.94 | - | ND | 7.02 ± 0.03 | 1.08 | Indifferent |
| 6.48 | 2.97 | - | 3.35 ± 0.1 | 8.02 ± 0.15 | 0.88 | Additive |
| - | 5.94 | 3.44 | 5.6 ± 0.1 | 8.14 ± 0.06 | 1.00 | Indifferent |
| Combination (mM) | ΣFIC | Interaction at the MBEC | ||
|---|---|---|---|---|
| Protocatechuic Acid | Vanillic Acid | Catechin | ||
| 5.51 | 3.56 | 13.78 | 0.56 | Additive |
| 3.2 | 2.97 | 1.72 | 0.30 | Synergistic |
| 1.62 | 5.94 | 3.44 | 0.43 | Synergistic |
| - | 5.94 | 13.78 | 0.38 | Synergistic |
| mmol of Trolox Equivalent/mmol of Phenolic Compound | |||
|---|---|---|---|
| Phenolic Compounds | DPPH | ABTS | FRAP |
| PA | 1.36 ± 0.008 b | 5.77 ± 0.34 b | 3.65 ± 0.03 b |
| VA | 0.15 ± 0.002 c | 3.45 ± 0.21 c | 1.96 ± 0.05 c |
| Cat | 2.78 ± 0.01 a | 11.32 ± 0.2 a | 3.86 ± 0.004 a |
| Phenolic Combination | DPPH• (µM TE) | Difference in DPPH * | ABTS (µM TE) | Difference in ABTS* | FRAP (µM TE) | Difference in FRAP * |
|---|---|---|---|---|---|---|
| MIC/MBIC a | 236.48 ± 1.41 | 141.11 | 599.0 ± 8.69 | 134.65 | 307.24 ± 3.43 | 9.48 |
| MBEC b | 413.12 ± 2.40 | 30.60 | 1342.32 ±62.6 | −213.53 | 1242.95 ±24.4 | 416.06 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernal-Mercado, A.T.; Vazquez-Armenta, F.J.; Tapia-Rodriguez, M.R.; Islas-Osuna, M.A.; Mata-Haro, V.; Gonzalez-Aguilar, G.A.; Lopez-Zavala, A.A.; Ayala-Zavala, J.F. Comparison of Single and Combined Use of Catechin, Protocatechuic, and Vanillic Acids as Antioxidant and Antibacterial Agents against Uropathogenic Escherichia Coli at Planktonic and Biofilm Levels. Molecules 2018, 23, 2813. https://doi.org/10.3390/molecules23112813
Bernal-Mercado AT, Vazquez-Armenta FJ, Tapia-Rodriguez MR, Islas-Osuna MA, Mata-Haro V, Gonzalez-Aguilar GA, Lopez-Zavala AA, Ayala-Zavala JF. Comparison of Single and Combined Use of Catechin, Protocatechuic, and Vanillic Acids as Antioxidant and Antibacterial Agents against Uropathogenic Escherichia Coli at Planktonic and Biofilm Levels. Molecules. 2018; 23(11):2813. https://doi.org/10.3390/molecules23112813
Chicago/Turabian StyleBernal-Mercado, Ariadna Thalia, Francisco Javier Vazquez-Armenta, Melvin R. Tapia-Rodriguez, Maria A. Islas-Osuna, Veronica Mata-Haro, Gustavo A. Gonzalez-Aguilar, Alonso A. Lopez-Zavala, and Jesus Fernando Ayala-Zavala. 2018. "Comparison of Single and Combined Use of Catechin, Protocatechuic, and Vanillic Acids as Antioxidant and Antibacterial Agents against Uropathogenic Escherichia Coli at Planktonic and Biofilm Levels" Molecules 23, no. 11: 2813. https://doi.org/10.3390/molecules23112813
APA StyleBernal-Mercado, A. T., Vazquez-Armenta, F. J., Tapia-Rodriguez, M. R., Islas-Osuna, M. A., Mata-Haro, V., Gonzalez-Aguilar, G. A., Lopez-Zavala, A. A., & Ayala-Zavala, J. F. (2018). Comparison of Single and Combined Use of Catechin, Protocatechuic, and Vanillic Acids as Antioxidant and Antibacterial Agents against Uropathogenic Escherichia Coli at Planktonic and Biofilm Levels. Molecules, 23(11), 2813. https://doi.org/10.3390/molecules23112813