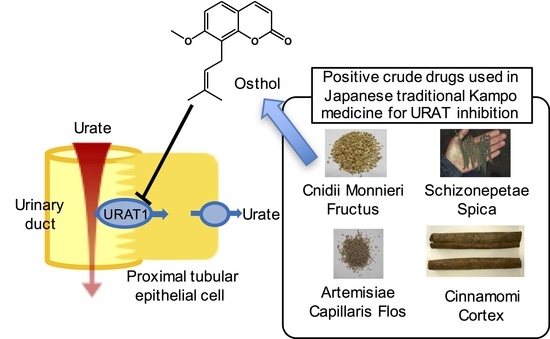
Effects of Osthol Isolated from Cnidium monnieri Fruit on Urate Transporter 1
Abstract
1. Introduction
2. Results
2.1. Screening of 107 Crude Drug Extracts
2.2. Effect of Cnidii Monnieris Fructus Extract on URAT1, and Its Activity-Guided Fractionation
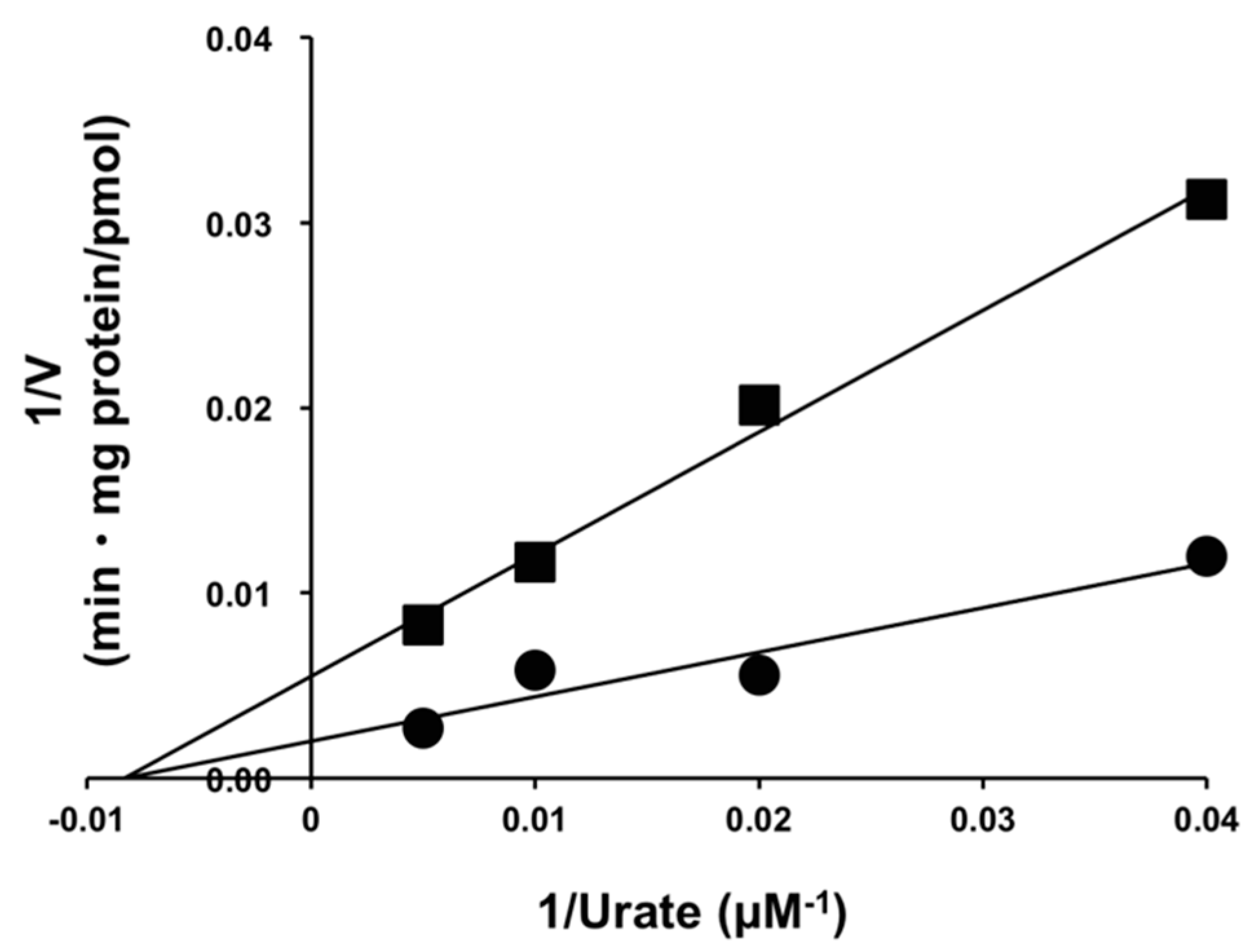
2.3. Inhibitory Effect of Osthol on URAT1 and Its Kinetics
2.4. Comparison of Inhibitory Effects on URAT1 among Coumarins
3. Discussion
4. Materials and Methods
4.1. Crude Drugs
4.2. Inhibitory Effect of the Samples on URAT1
4.3. Sample Cytotoxicity
4.4. Preparation and Fractionation of Cnidii Monnieris Fructus Extract
4.5. Coumarins
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yamanaka, H. Guideline for the Management of Hyperuricemia and Gout; Guideline Revision Committee: Tokyo, Japan, 2010. [Google Scholar]
- Redon, P.; Maloberti, A.; Facchetti, R.; Redon, J.; Lurbe, E.; Bombelli, M.; Mancia, G.; Grassi, G. Gender-related differences in serum uric acid in treated hypertensive patients from central and east European countries: Findings from the blood pressure control rate and cardiovascular risk profile study. J. Hypertens. 2018. [Google Scholar] [CrossRef] [PubMed]
- Maloberti, A.; Maggioni, S.; Occhi, L.; Triglione, N.; Panzeri, F.; Nava, S.; Signorini, S.; Falbo, R.; Casati, M.; Grassi, G.; et al. Sex-related relationships between uric acid and target organ damage in hypertension. J. Clin. Hypertens. 2018, 20, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, M. Hyperuricemia, cardiovascular disease, and hypertension. Pulse 2016, 3, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Shi, Y.; Zhuang, S.; Liu, N. Recent advances on uric acid transporters. Oncotarget 2017, 8, 100852–100862. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, A.; Kimura, H.; Chairoungdua, A.; Shigeta, Y.; Jutabha, P.; Cha, S.H.; Hosoyamada, M.; Takeda, M.; Sekine, T.; Igarashi, T.; et al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature 2002, 417, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Anzai, N.; Ichida, K.; Jutabha, P.; Kimura, T.; Babu, E.; Jin, C.J.; Srivastava, S.; Kitamura, K.; Hisatome, I.; Endou, H.; et al. Plasma urate level is directly regulated by a voltage-driven urate efflux transporter URATv1 (SLC2A9) in humans. J. Biol. Chem. 2008, 283, 26834–26838. [Google Scholar] [CrossRef] [PubMed]
- Eraly, S.A.; Vallon, V.; Rieg, T.; Gangoiti, J.A.; Wikoff, W.R.; Siuzdak, G.; Barshop, B.A.; Nigam, S.K. Multiple organic anion transporters contribute to net renal excretion of uric acid. Physiol. Genom. 2008, 33, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Jutabha, P.; Anzai, N.; Kitamura, K.; Taniguchi, A.; Kaneko, S.; Yan, K.; Yamada, H.; Shimada, H.; Kimura, T.; Katada, T.; et al. Human sodium phosphate transporter 4 (hNPT4/SLC17A3) as a common renal secretory pathway for drugs and urate. J. Biol. Chem. 2010, 285, 35123–35132. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, H.; Takada, T.; Ichida, K.; Nakamura, T.; Nakayama, A.; Ikebuchi, Y.; Ito, K.; Kusanagi, Y.; Chiba, T.; Tadokoro, S.; et al. Common defects of ABCG2, a high-capacity urate exporter, cause gout: A function-based genetic analysis in a Japanese population. Sci. Transl. Med. 2009, 1. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Takeda, M.; Enomoto, A.; Fujimura, M.; Miyazaki, H.; Anzai, N.; Endou, H. Interactions of urate transporter URAT1 in human kidney with uricosuric drugs. Nephrology 2011, 16, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Stamp, L.K.; Haslett, J.; Frampton, C.; White, D.; Gardner, D.; Stebbings, S.; Taylor, G.; Grainger, R.; Kumar, R.; Kumar, S.; et al. The safety and efficacy of benzbromarone in gout in Aotearoa New Zealand. Intern. Med. J. 2016, 46, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Wu, J.; Liu, W.; Xie, Y.; Liu, Y.; Zhang, S.; Xu, W.; Tang, L.; Wang, J.; Zhao, G. Systematic structure-activity relationship (SAR) exploration of diarylmethane backbone and discovery of a highly potent novel uric acid transporter 1 (URAT1) inhibitor. Molecules 2018, 23, 252. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Wakayama, T.; Mamada, H.; Shirasaka, Y.; Nakanishi, T.; Tamai, I. Identification and functional characterization of uric acid transporter Urat1 (Slc22a12) in rats. Biochim. Biophys. Acta 2011, 1808, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Anzai, N.; Miyazaki, H.; Noshiro, R.; Khamdang, S.; Chairoungdua, A.; Shin, H.J.; Enomoto, A.; Sakamoto, S.; Hirata, T.; Tomita, K.; et al. The multivalent PDZ domain-containing protein PDZK1 regulates transport activity of renal urate-anion exchanger URAT1 via its C terminus. J. Biol. Chem. 2004, 279, 45942–45950. [Google Scholar] [CrossRef] [PubMed]
- Bensky, D.; Clavey, S.; Stöger, E. Chinese Herbal Medicine—Materia Medica, 3rd ed.; Eastland Press: Seattle, WA, USA, 2004. [Google Scholar]
- Japan Kampo Medicines Manufacturers’ Association. Handbook on OTC Medicinal Product in Kampo; Jiho: Tokyo, Japan, 2013. [Google Scholar]
- Malla, B.; Chang, B.Y.; Kim, S.B.; Park, H.; Lee, M.K.; Kim, S.Y. Potential of the Cnidium monnieri fruits as an immune enhancer in Escherichia coli infection model. J. Pharm. Pharmacol. 2016, 68, 1430–1439. [Google Scholar] [CrossRef] [PubMed]
- Kwak, H.G.; Lim, H.B. Inhibitory effects of Cnidium monnieri fruit extract on pulmonary inflammation in mice induced by cigarette smoke condensate and lipopolysaccharide. Chin. J. Nat. Med. 2014, 12, 641–647. [Google Scholar] [CrossRef]
- Matsuda, H.; Ido, Y.; Hirata, A.; Ino, Y.; Naruto, S.; Amamiya, T.; Kubo, M. Antipruritic effect of Cnidii Monnieri Fructus (fruits of Cnidium monnieri CUSSON). Biol. Pharm. Bull. 2002, 25, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Tomohiro, N.; Ido, Y.; Kubo, M. Anti-allergic effects of cnidii monnieri fructus (dried fruits of Cnidium monnieri) and its major component, osthol. Biol. Pharm. Bull. 2002, 25, 809–812. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Qin, L.; He, W.; Van Puyvelde, L.; Maes, D.; Adams, A.; Zheng, H.; De Kimpe, N. Coumarins from Cnidium monnieri and their antiosteoporotic activity. Planta Med. 2007, 73, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China, 1st ed.; The Stationery Office: Beijing, China, 2017. [Google Scholar]
- Yu, Z.; Fong, W.P.; Cheng, C.H. Morin (3,5,7,2′,4′-pentahydroxyflavone) exhibits potent inhibitory actions on urate transport by the human urate anion transporter (hURAT1) expressed in human embryonic kidney cells. Drug Metab. Dispos. 2007, 35, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.W.; Juang, L.J.; Wang, B.S.; Wang, M.Y.; Tai, H.M.; Hung, W.J.; Chen, Y.J.; Huang, M.H. Antioxidant and antityrosinase activity of mulberry (Morus alba L.) twigs and root bark. Food Chem. Toxicol. 2011, 49, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Li, J.M.; Zhang, X.; Wang, X.; Xie, Y.C.; Kong, L.D. Protective effects of cortex fraxini coumarines against oxonate-induced hyperuricemia and renal dysfunction in mice. Eur. J. Pharmacol. 2011, 666, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Shokoohinia, Y.; Jafari, F.; Mohammadi, Z.; Bazvandi, L.; Hosseinzadeh, L.; Chow, N.; Bhattacharyya, P.; Farzaei, M.H.; Farooqi, A.A.; Nabavi, S.M.; et al. Potential anticancer properties of osthol: A comprehensive mechanistic review. Nutrients 2018, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Y.; Shi, F.; Wang, L.; Li, L.; Yang, D. Osthole attenuates right ventricular remodeling via decreased myocardial apoptosis and inflammation in monocrotaline-induced rats. Eur. J. Pharmacol. 2018, 818, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Li, Y.; Qian, Z.; Zhu, L.; Yang, D. Osthole attenuates pulmonary arterial hypertension in monocrotalinetreated rats. Mol. Med. Rep. 2017, 16, 2823–2829. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.C.; Wang, F.; Xie, M.L.; Cheng, Z.Q.; Qin, Q.; Chen, L.; Chen, R. Osthole inhibits the expressions of collagen I and III through Smad signaling pathway after treatment with TGF-β1 in mouse cardiac fibroblasts. Int. J. Cardiol. 2017, 228, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fu, Y.; Wei, Z.; He, X.; Shi, M.; Kou, J.; Zhou, E.; Liu, W.; Yang, Z.; Guo, C. Anti-asthmatic activity of osthole in an ovalbumin-induced asthma murine model. Respir. Phys. Neurobiol. 2017, 239, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Cai, Y.; Zhang, X.X.; Chen, H.; Lin, Y.D.; Li, H. Osthole pretreatment alleviates TNBS-induced colitis in mice via both cAMP/PKA-dependent and independent pathways. Acta Pharmacol. Sinica 2017, 38, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Ying, J.; Luo, C.; Jin, X.; Zhang, S.; Xu, T.; Zhang, L.; Mi, M.; Chen, D.; Tong, P.; et al. Osthole promotes bone fracture healing through activation of BMP signaling in chondrocytes. Int. J. Biol Sci. 2017, 13, 996–1007. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.R.; Leung, W.N.; Li, G.; Kong, S.K.; Lu, X.; Wong, Y.M.; Chan, C.W. Osthole enhances osteogenesis in osteoblasts by elevating transcription factor osterix via cAMP/CREB signaling in vitro and in vivo. Nutrients 2017, 9, 588. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Dong, Z. Osthole protects against inflammation in a rat model of chronic kidney failure via suppression of nuclear factor-κB, transforming growth factor-β1 and activation of phosphoinositide 3-kinase/protein kinase B/nuclear factor (erythroid-derived 2)-like 2 signaling. Mol. Med. Rep. 2017, 16, 4915–4921. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Li, P.; Qi, D.; Wang, L.; Qu, H.L.; Zhang, Y.J.; Wang, X.K.; Fan, H.Y. Osthole protects sepsis-induced acute kidney injury via down-regulating NF-κB signal pathway. Oncotarget 2017, 8, 4796–4813. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Wang, J.Y.; Qian, Z.Q.; Li, Y.L.; Li, W.N.; Gao, Y.; Yang, D.L. Osthole inhibits intimal hyperplasia by regulating the NF-κB and TGF-β1/Smad2 signalling pathways in the rat carotid artery after balloon injury. Eur. J. Pharmacol. 2017, 811, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Miner, J.N.; Tan, P.K.; Hyndman, D.; Liu, S.; Iverson, C.; Nanavati, P.; Hagerty, D.T.; Manhard, K.; Shen, Z.; Girardet, J.L.; et al. Lesinurad, a novel, oral compound for gout, acts to decrease serum uric acid through inhibition of urate transporters in the kidney. Arthritis Res. Ther. 2016, 18, 214. [Google Scholar] [CrossRef] [PubMed]
- Pharmaceutical and Medical Device Regulatory Science Society of Japan. Japanese Pharmacopoeia Seventeenth Edition (JP XVII); Jiho: Tokyo, Japan, 2016. [Google Scholar]
- Pharmaceutical and Medical Device Regulatory Science Society of Japan. The Japanese Standards for Non-Pharmacopoeial Crude Drugs (Non-JP Crude Drug Standards); Yakuji Nippo Ltd. Company: Tokyo, Japan, 2015. [Google Scholar]
- Kato, Y.; Yoshida, K.; Watanabe, C.; Sai, Y.; Tsuji, A. Screening of the interaction between xenobiotic transporters and PDZ proteins. Pharmaceu. Res. 2004, 21, 1886–1894. [Google Scholar] [CrossRef]
Sample Availability: Sample of the compound osthol is available from the authors. |




| Compound | Urate Uptake (% of Control) |
|---|---|
| coumarin | 111 ± 8 |
| 7-methoxycoumarin | 137 ± 8 |
| 4-hydroxycoumarin | 87 ± 9 |
| 6-hydroxycoumarin | 83 ± 14 |
| umbelliferone | 114 ± 6 |
| daphnetin | 140 ± 10 |
| esculetin | 120 ± 5 |
| fraxetin | 136 ± 7 |
| osthol | 36 ± 10 |
| osthenol | 31 ± 4 |
| bergaptol | 83 ± 6 |
| bergamottin | 117 ± 6 |
| geraniol | 88 ± 5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tashiro, Y.; Sakai, R.; Hirose-Sugiura, T.; Kato, Y.; Matsuo, H.; Takada, T.; Suzuki, H.; Makino, T. Effects of Osthol Isolated from Cnidium monnieri Fruit on Urate Transporter 1. Molecules 2018, 23, 2837. https://doi.org/10.3390/molecules23112837
Tashiro Y, Sakai R, Hirose-Sugiura T, Kato Y, Matsuo H, Takada T, Suzuki H, Makino T. Effects of Osthol Isolated from Cnidium monnieri Fruit on Urate Transporter 1. Molecules. 2018; 23(11):2837. https://doi.org/10.3390/molecules23112837
Chicago/Turabian StyleTashiro, Yuusuke, Ryo Sakai, Tomoko Hirose-Sugiura, Yukio Kato, Hirotaka Matsuo, Tappei Takada, Hiroshi Suzuki, and Toshiaki Makino. 2018. "Effects of Osthol Isolated from Cnidium monnieri Fruit on Urate Transporter 1" Molecules 23, no. 11: 2837. https://doi.org/10.3390/molecules23112837
APA StyleTashiro, Y., Sakai, R., Hirose-Sugiura, T., Kato, Y., Matsuo, H., Takada, T., Suzuki, H., & Makino, T. (2018). Effects of Osthol Isolated from Cnidium monnieri Fruit on Urate Transporter 1. Molecules, 23(11), 2837. https://doi.org/10.3390/molecules23112837