Quinazolin-4(3H)-ones and 5,6-Dihydropyrimidin-4(3H)-ones from β-Aminoamides and Orthoesters
Abstract
:1. Introduction
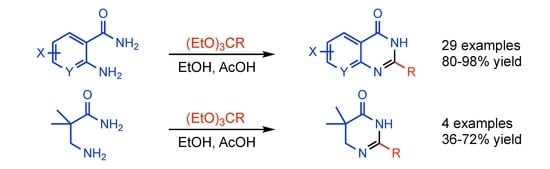
2. Results and Discussion
3. Experimental Section
3.1. General Methods
3.2. General Procedures to Prepare Quinazolin-4(3H)-ones
3.2.1. Method 1
3.2.2. Method 2
3.2.3. Method 3: General Procedure to Prepare 5,6-dihydropyrimidin-4(3H)-ones
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Michael, J.P. Quinoline, quinazoline and acridone alkaloids. Nat. Prod. Rep. 2005, 22, 627–646. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.-B.; Geng, H.-Q.; Wang, W.; Qi, X.; Ying, J.; Wu, X.-F. Palladium-catalyzed four-component carbonylative synthesis of 2,3-disubstituted quinazolin-4(3H)-ones: Convenient methaqualone preparation. J. Catal. 2018, 365, 10–13. [Google Scholar] [CrossRef]
- Inoue, I.; Oine, T.; Yamada, Y.; Tani, J.; Ishida, R.; Ochiai, T. 2-Fluoromethyl-3-(o-tolyl)- 6-amino-4(3H)-quinazolinone. German Patent DE 2,449,113 A1, 24 April 1975. [Google Scholar]
- Ochiai, T.; Ishida, R. Pharmacological studies on 6-amino-2-fluoromethyl-3-(o-tolyl)- 4(3H)-quinazolinone (afloqualone), a new centrally acting muscle relaxant. (II). Effects on the spinal reflex potential and the rigidity. Jpn. J. Pharmacol. 1982, 32, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Bartroli, J.; Turmo, E.; Agueró, M.; Boncompte, E.; Vericat, M.L.; Conte, L.; Ramis, J.; Merlos, M.; García-Rafanell, J.; Forn, J. New azole antifungals. 3. Synthesis and antifungal activity of 3-substituted-4(3H)-quinazolinones. J. Med. Chem. 1998, 41, 1869–1882. [Google Scholar] [CrossRef] [PubMed]
- Mabkhot, Y.N.; Al-Har, M.S.; Barakat, A.; Aldawsari, F.D.; Aldelbahi, A.; Ul-Haq, Z. Synthesis, anti-microbial and molecular docking studies of quinazolin-4(3H)-ones derivatives. Molecules 2014, 19, 8725–8739. [Google Scholar] [CrossRef] [PubMed]
- Bunce, R.A.; Nammalwar, B. New conditions for the synthesis of (±)-2-monosubstituted and (±)-2,2-disubstituted 2,3-dihydro-4(1H)-quinazolinones from 2-nitro- and 2-aminobenzamide. J. Heterocycl. Chem. 2011, 48, 991–997. [Google Scholar] [CrossRef]
- Fortenberry, C.; Nammalwar, B.; Bunce, R.A. Ammonium chloride-catalyzed synthesis of benzo-fused heterocycles from o-substituted anilines and orthoesters. Org Prep. Proced. Int. 2013, 45, 57–65. [Google Scholar] [CrossRef]
- Khajavi, M.S.; Rad-Moghadam, K.; Hazarkhani, H. A facile synthesis of 6-substituted benzimidazo[1,2-c]quinazolines under microwave irradiation. Synth. Commun. 1999, 29, 2617–2624. [Google Scholar] [CrossRef]
- Helali, A.Y.H.; Sarg, M.T.M.; Koraa, M.M.S.; El-Zoghbi, M.S.F. Utility of 2-methyl-quinazolin-4(3H)-one in the synthesis of heterocyclic compounds with anticancer activity. Open J. Med. Chem. 2014, 4, 12–37. [Google Scholar] [CrossRef]
- Von Niementowski, S. Synthesen von Chinazolinverbindungen. J. Prakt. Chem. 1895, 51, 564–572. [Google Scholar] [CrossRef]
- Meyer, J.F.; Wagner, E.C. The Niementowski reaction. The use of methyl anthranilate or isatoic anhydride with substituted amides or amidines in the formation of 3-substituted-4-keto-3,4-dihydroquinazolines. The course of the reaction. J. Org. Chem. 1943, 8, 239–252. [Google Scholar] [CrossRef]
- Li, F.; Feng, Y.; Meng, Q.; Li, W.; Li, A.; Wang, Q.; Tao, Z. An efficient construction of quinazolin-4(3H)-ones under microwave irradiation. Arkivoc 2007, 2007, 40–50. [Google Scholar]
- Baghbanzadeh, M.; Molnar, M.; Damm, M.; Reidlinger, C.; Dabiri, M.; Kappe, C.O. Parallel microwave synthesis of 2-styrylquinazolin-4(3H)-ones in a high throughput platform using HPLC/GC vials as reaction vessels. J. Comb. Chem. 2009, 11, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-L.; Liu, X.-X.; Zhang, T.; Zhang, J.-M.; Zhou, J.-H. New Facile and solvent-free method for the one-pot synthesis of quinazolin-4(3H)-ones catalyzed by SbCl3 under microwave irradiation. J. Chem. Soc. Pak. 2016, 38, 1196–1202. [Google Scholar]
- Endicott, M.M.; Wick, E.; Mercury, M.L.; Sherrill, M.L. Quinazoline derivatives. I. The synthesis of 4-(4’-diethylamino-1’-methylbutylamino)-quinazoline (SN 11,534) and the corresponding 2-phenylquinazoline (SN 11,535). J. Am. Chem. Soc. 1946, 68, 1299–1301. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Jadhavar, P.S.; Nautiyal, M.; Sharma, H.; Meena, P.K.; Adane, L.; Pancholia, S.; Chakraborti, A.K. Convenient synthesis of 2,3-disubstituted quinazolin-4(3H)-ones and 2-styryl-3-substituted quinazolin-4(3H)-ones: Applications towards the synthesis of drugs. RSC Adv. 2015, 5, 30819–30825. [Google Scholar] [CrossRef]
- Sharma, R.; Abdullaha, M.; Bharate, S.B. Metal-free ionic liquid-mediated synthesis of benzimidazoles and quinazolin-4(3H)-ones from benzylamines. Asian J. Org. Chem. 2017, 6, 1370–1374. [Google Scholar] [CrossRef]
- Li, Z.; Dong, J.; Chen, X.; Li, Q.; Zhou, Y.; Yin, S.-F. Metal- and oxidant-free synthesis of quinazolinones from -ketoesters with o-aminobenzamides via phosphorous acid-catalyzed cyclocondensation and selective C–C bond cleavage. J. Org. Chem. 2015, 80, 9392–9400. [Google Scholar] [CrossRef] [PubMed]
- Almeida, S.; Marti, R.; Vanoli, E.; Abele, S.; Tortoioli, S. One-pot synthesis of trifluoromethylated quinazolin-4(3H)-ones with trifluoroacetic acid as a CF3 source. J. Org. Chem. 2018, 83, 514–5113. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.A.; Uma-Maheswari, C.; Ghantasala, S.; Jyothi, C.; Reddy, K.R. Synthesis of 3H-quinazolin-4-ones and 4H-3,1-benzoxazin-4-ones via benzylic oxidation and oxidative dehydrogenation using potassium iodide-tert-butyl hydroperoxide. Adv. Synth. Catal. 2011, 353, 401–410. [Google Scholar] [CrossRef]
- Sun, J.; Tao, T.; Xu, D.; Cao, H.; Kong, Q.; Wang, X.; Liu, Y.; Zhao, J.; Wang, Y.; Pan, Y. Metal-free oxidative cyclization of 2-aminobenzamides, 2-aminobenzenesulfonamide or 2-(aminomethyl)-anilines with primary alcohols for the synthesis of quinazolines and their analogues. Tetrahedron Lett. 2018, 59, 2099–2102. [Google Scholar] [CrossRef]
- Ge, W.; Zhu, X.; Wei, Y. Iodine-catalyzed oxidative system for the cyclization of primary alcohols with o-aminobenzamides to quinazolinones using DMSO as the oxidant in dimethyl carbonate. RSC Adv. 2013, 3, 10817–10822. [Google Scholar] [CrossRef]
- Mohammed, S.; Vishwakarma, R.A.; Bharate, S.B. Iodine catalyzed oxidative synthesis of quinazolin-4(3H)-ones and pyrazolo[4.3-d]pyrimidin-7(6H)-ones via amination of sp3 C–H bind. J. Org. Chem. 2015, 80, 6915–6921. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, M.; Zhang, C.; Zhang, Z.; Lu, J.; Wang, F. The cascade synthesis of quinazolinones and quinazolines using α-MnO2 catalyst and tert-butyl hydroperoxide (TBHP) as an oxidant. Chem. Commun. 2015, 51, 9205–9207. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Zhou, Y.-R.; Shen, Q.; Li, J.-X. Iron-catalyzed oxidative synthesis of N-heterocycles from primary alcohols. RSC Adv. 2014, 4, 6486–6489. [Google Scholar] [CrossRef]
- Parua, S.; Das, S.; Sikari, R.; Sinha, S.; Paul, N.D. One-pot cascade synthesis of quinazolin-4(3H)-ones via nickel-catalyzed dehydrogenative coupling of o-aminobenzamides with alcohols. J. Org. Chem. 2017, 82, 7165–7175. [Google Scholar] [CrossRef] [PubMed]
- Sharif, M.; Opalach, J.; Langer, P.; Beller, M.; Wu, X.-F. Oxidative synthesis of quinazolinones and benzothiadiazine 1,1-dioxides from 2-aminobenzamide and 2-aminobenzenesufonamide with benzyl alcohols and aldehydes. RSC Adv. 2014, 4, 8–17. [Google Scholar] [CrossRef]
- Zhang, W.; Meng, C.; Liu, Y.; Tang, Y.; Li, F. Auto-tandem catalysis with ruthenium: From o-aminobenzamides and allylic alcohols to quinazolinones via redox isomerization/acceptorless dehydrogenation. Adv. Synth. Catal. 2018, 360, 1–10. [Google Scholar] [CrossRef]
- Zhou, J.; Fang, J. One-pot synthesis of quinazolinones via iridium-catalyzed hydrogen transfers. J. Org. Chem. 2011, 76, 7730–7736. [Google Scholar] [CrossRef] [PubMed]
- Hakim Siddiki, S.M.A.; Kon, K.; Touchy, A.S.; Shimizu, K. Direct synthesis of quinazolinones by acceptorless dehydrogenative coupling of o-aminobenzamide and alcohols by heterogeneous Pt catalysts. Catal. Sci. Technol. 2014, 4, 1716–1719. [Google Scholar] [CrossRef]
- Couture, A.; Cornet, H.; Grandclaudon, P. An expeditious synthesis of 2-aryl- and 2-alkylquinazolin-4(3H)-ones. Synthesis 1991, 1009–1010. [Google Scholar] [CrossRef]
- Liu, X.; Fu, H.; Jiang, Y.; Zhao, Y. A simple efficient approach to quinazolinones under mild copper-catalyzed conditions. Angew. Chem. Int. Ed. 2009, 48, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Liu, H.; Jiang, Y.; Fu, H. Copper-catalyzed domino synthesis of quinazolinones via Ullmann-type coupling and aerobic oxidative C–H amidation. Org. Lett. 2011, 13, 1274–1277. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chen, M.; Yang, L.; Xiong, Z.; Wang, Y.; Li, F.; Chen, D. Copper-catalyzed consecutive reaction to construct quinazolin-4(3H)-ones and pyrido[2,3-d]pyrimidin-4(3H)-ones. Tetrahedron 2016, 72, 868–874. [Google Scholar] [CrossRef]
- Thakur, M.S.; Nayal, O.S.; Bhatt, V.; Sharma, S.; Kumar, N. Rapid and efficient cascade synthesis of 4-amino-4(3H)-quinazolinones over an in situ-generated heterogeneous CuCO3-K2CO3 nanocomposite. Asian J. Org. Chem. 2016, 5, 750–754. [Google Scholar] [CrossRef]
- Soheilizad, M.; Soroosh, S.; Pashazadeh, R. Solvent-free copper-catalyzed three-component synthesis of 2-substituted quinazolin-4(3H)-ones. Monatsh. Chem. 2017, 148, 739–743. [Google Scholar] [CrossRef]
- Abe, T.; Kida, K.; Yamada, K. A copper-catalyzed Ritter-type cascade via iminoketene for the synthesis of quinazolin-4(3H)-ones and diazocines. Chem. Commun. 2017, 53, 4362–4365. [Google Scholar] [CrossRef] [PubMed]
- Nisha; Sharma, M.C.; Kumar, R.; Kumar, Y. Regioselective copper(I) catalyzed Ullmann amination of halopyridyl carboxylates using sodium azide: A route for aminopyridyl carboxylates and their transformation to pyrido[2,3-d]pyrimidin-4(1H)-ones. Chem. Sel. 2018, 3, 4822–4826. [Google Scholar] [CrossRef]
- Yu, X.; Gao, L.; Jia, L.; Yamamoto, Y.; Bao, M. Synthesis of quinazolin-4(3H)-ones via the reaction of 2-halobenzamides with nitriles. J. Org. Chem. 2018, 83, 10352–10358. [Google Scholar] [CrossRef] [PubMed]
- Hikawa, H.; Ino, Y.; Suzuki, H.; Yokoyama, Y. Pd-catalyzed benzylic C–H amidation with benzyl alcohols in water: A strategy to construct quinazolinones. J. Org. Chem. 2012, 77, 7046–7051. [Google Scholar] [CrossRef] [PubMed]
- Hikawa, H.; Matsuda, N.; Suzuki, H.; Yokoyama, Y.; Azumaya, I. N-Benzylation/benzylic C–H amidation cascade by the (η3-benzyl)palladium system in aqueous media: An effective pathway for the direct construction of 3-phenyl-3,4-dihydro-(2H)-1,2,4-benzothiadiazine 1,1-dioxides. Adv. Synth. Catal. 2013, 355, 2308–2320. [Google Scholar] [CrossRef]
- Jiang, X.; Tang, T.; Wang, J.-M.; Chen, Z.; Zhu, Y.-M.; Ji, S.-J. Palladium-catalyzed one-pot synthesis of quinazolinones via tert-butyl isocyanide insertion. J. Org. Chem. 2014, 79, 5082–5087. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Liu, K.; Tao, S.-W.; Zhang, F.-L.; Zhu, Y.M.; Yang, S.-L. Palladium-catalyzed oxidative three-component coupling of anthranilamides with isocyanides and arylboronic acids: Access to 2,3-disubstituted quinazolinones. J. Org. Chem. 2018, 83, 9201–9209. [Google Scholar] [CrossRef] [PubMed]
- Hammond, G.S. Steric Effects in Organic Chemistry; Newman, M.S., Ed.; Wiley: New York, NY, USA, 1956; p. 425. [Google Scholar]
- Anslyn, E.V.; Dougherty, D.A. Modern Physical Organic Chemistry; University Science Books: Sausalito, CA, USA, 2006; p. 497. [Google Scholar]
- Zhao, G.; Souers, A.J.; Voorbach, M.; Falls, H.D.; Droz, B.; Brodjian, S.; Lai, Y.Y.; Iyengar, R.R.; Gao, J.; Judd, A.S.; Wagaw, S.H.; et al. Validation of diacyl glycerolacyltransferase I as a novel target for the treatment of obesity and dyslipidemia using a potent and selective small molecule inhibitor. J. Med. Chem. 2008, 51, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Kakuta, K.; Zheng, X.; Oda, H.; Harada, S.; Sugimoto, Y.; Sasaki, K.; Tai, A. Cyclooxygenase-1-selective inhibitors are attractive candidates for analgesics that do not cause gastric damage. Design and in vitro/in vivo evaluation of a benzamide-type cyclooxygenase-1-selective inhibitor. J. Med. Chem. 2008, 51, 2400–2411. [Google Scholar] [CrossRef] [PubMed]
- Okano, M.; Mito, J.; Maruyama, Y.; Masuda, H.; Niwa, T.; Nakagawa, S.-C.; Nakamura, Y.; Matsuura, A. Discovery and structure-activity relationships of 4-aminoquinazoline derivatives, a novel class of opioid receptor like-1 (ORL1) antagonists. Bioorg. Med. Chem. 2009, 17, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Takada, A.; Ueda, T. Reaction of triethyloxonium fluoroborate with acid amide. III. Formation of quinazoline and 4H-3,1-benzooxazin-4-one derivatives. Chem. Pharm. Bull. 1976, 24, 431–436. [Google Scholar] [CrossRef]
- Youssef, A.S.A.; Hemdan, M.M.; El-Mariah, F.A.; Hashem, H.E. Synthesis of some quinazolinone derivatives functionalized with N-3 heterocyclic side chain. J. Heterocycl. Chem. 2018, 55, 1626–1633. [Google Scholar] [CrossRef]
- Koohang, A.; Stanchina, C.L.; Coates, R.M. Regio- and stereoselective synthesis of N-H aziridines by N-N bond reduction of quinazolinyl aziridines. Tetrahedron 1999, 55, 9669–9686. [Google Scholar] [CrossRef]
- Abdel-Rahman, H.M.; Abdel-Azziz, M.; Canzoneri, J.C.; Gary, B.D. Novel quinazolin-4(3H)-one/Schiff base hybrids as antiproliferative and phosphodiesterase 4 inhibitors: Design, synthesis, and docking studies. Arch. Pharm. Chem. Life Sci. 2014, 347, 650–657. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |




| Substrate | Product | R | Method a | Yield (%) |
|---|---|---|---|---|
 |  | a: Me b: Et c: Pr d: P he: H | 1 1 1 1 1 | 92 89 93 81 87 |
 |  | a: Me b: Et c: Pr d: Ph | 2 2 2 2 | 90 91 ND b 84 |
 |  | a: Me b: Et c: Pr d: Ph | 2 2 2 1(2) | 92 92 88 33(97) |
 |  | a: Me b: Et c: Pr d: Ph | 2 2 2 1(2) | 94 93 89 7(98) |
 |  | a: Me b: Et c: Pr d: Ph | 1(2) 1(2) 1(2) 1(2) | 71(86) 70(92) 55(87) 68(80) |
 |  | a: Me b: Et c: Pr d: Ph | 2 2 2 2 | 87 83 ND b 89 |
 |  | a: Me b: Et c: Pr d: Ph | 2 2 2 2 | 95 92 ND b 95 |
 |  | a: Me b: Et c: Pr d: Ph | 1(2) 2 2 2 | 21(82) 80 ND b 95 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gavin, J.T.; Annor-Gyamfi, J.K.; Bunce, R.A. Quinazolin-4(3H)-ones and 5,6-Dihydropyrimidin-4(3H)-ones from β-Aminoamides and Orthoesters. Molecules 2018, 23, 2925. https://doi.org/10.3390/molecules23112925
Gavin JT, Annor-Gyamfi JK, Bunce RA. Quinazolin-4(3H)-ones and 5,6-Dihydropyrimidin-4(3H)-ones from β-Aminoamides and Orthoesters. Molecules. 2018; 23(11):2925. https://doi.org/10.3390/molecules23112925
Chicago/Turabian StyleGavin, Joshua T., Joel K. Annor-Gyamfi, and Richard A. Bunce. 2018. "Quinazolin-4(3H)-ones and 5,6-Dihydropyrimidin-4(3H)-ones from β-Aminoamides and Orthoesters" Molecules 23, no. 11: 2925. https://doi.org/10.3390/molecules23112925
APA StyleGavin, J. T., Annor-Gyamfi, J. K., & Bunce, R. A. (2018). Quinazolin-4(3H)-ones and 5,6-Dihydropyrimidin-4(3H)-ones from β-Aminoamides and Orthoesters. Molecules, 23(11), 2925. https://doi.org/10.3390/molecules23112925