Tetra-glucopyranosyl Diterpene ent-Kaur-16-en-19-oic Acid and ent-13(S)-Hydroxyatisenoic Acid Derivatives from a Commercial Extract of Stevia rebaudiana (Bertoni) Bertoni
Abstract
:1. Introduction
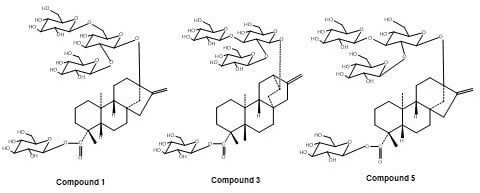
2. Results and Discussion
Structure Elucidation
3. Materials and Methods
3.1. Chemicals
3.2. General Experimental Procedures
3.3. Plant Material
3.4. Isolation Procedure
3.5. Alkaline Hydrolysis of Compounds 1 and 3
3.6. Physicochemical Parameters of Compounds
3.7. RP-C18 HPLC Analysis
3.8. Determination of the Sugar Unit Absolute Configuration
3.9. X-ray Crystallography of Iso-Stevioside
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Whiting, D.R.; Guariguata, L.; Weil, C.; Shaw, J. IDF diabetes atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res. Clin. Pract. 2011, 94, 311–321. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Ferrannini, E. Insulin resistance: A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care 1991, 14, 173–194. [Google Scholar] [CrossRef] [PubMed]
- Midmore, D.; Rank, A. A Report for the Rural Industries Research and Development Corporation, RIRDC Project No UCQ-16A; Rural Industries Research and Development Corporation: ACT, Barton, Australia, 2002. [Google Scholar]
- Ohtani, K.; Yamasaki, K. Methods to improve the taste of the sweet principles of Stevia rebaudiana. In Stevia: The Genus Stevia; Kinghorn, A.D., Ed.; Taylor & Francis: London, UK, 2002; pp. 138–159. [Google Scholar]
- Philippaert, K.; Pironet, A.; Mesuere, M.; Sones, W.; Vermeiren, L.; Kerselaers, S.; Pinto, S.; Segal, A.; Antoine, N.; Gysemans, C. Steviol glycosides enhance pancreatic beta-cell function and taste sensation by potentiation of TRPM5 channel activity. Nat. Commun. 2017, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- McChesney, J.; Rodenburg, D. Chromatography Methods. U.S. patent 8,801,924B2, 3 January 2014. [Google Scholar]
- Perera, W.H.; Avula, B.; Khan, I.A.; McChesney, J.D. Assignment of sugar arrangement in branched steviol glycosides using electrospray ionization quadrupole time-of-flight tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2017, 31, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Perera, W.H.; Docampo, M.L.; Wiggers, F.T.; Hufford, C.D.; Fronczek, F.R.; Avula, B.; Khan, I.A.; McChesney, J.D. Endocyclic double bond isomers and by-products from rebaudioside A and stevioside formed under acid conditions. Phytochem Lett. 2018, 25, 163–170. [Google Scholar] [CrossRef]
- Tanaka, T.; Nakashima, T.; Ueda, T.; Tomii, K.; Kouno, I. Facile Discrimination of Aldose Enantiomers by Reversed-Phase HPLC. Chem. Pharm. Bull. 2007, 55, 899–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perera, W.H.; Ghiviriga, I.; Rodenburg, D.L.; Carvalho, R.; Alves, K.; McChesney, J.D. Development of a high-performance liquid chromatography procedure to identify known and detect novel C-13 oligosaccharide moieties in diterpene glycosides from Stevia rebaudiana (Bertoni) Bertoni (Asteraceae): Structure elucidation of rebaudiosides V and W. J. Sep. Sci. 2017, 40, 3771–3781. [Google Scholar] [CrossRef] [PubMed]
- Kohda, H.; Kasai, R.; Yamasaki, K.; Murakami, K.; Tanaka, O. New sweet diterpene glucosides from Stevia rebaudiana. Phytochemistry 1976, 15, 981–983. [Google Scholar] [CrossRef]
- Ortega, A.; Morales, F.; Salmon, M. Kaurenic acid derivatives from Stevia eupatoria. Phytochemistry 1985, 24, 1850–1852. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Rodenburg, D.L.; Alves, K.; Fronczek, F.R.; McChesney, J.D.; Wu, C.; Nettles, B.J.; Venkataraman, S.K.; Jaksch, F.J. Minor diterpene glycosides from the leaves of Stevia rebaudiana. J. Nat. Prod. 2014, 77, 1231–1235. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedula, V.; Prakash, I.J. Additional minor diterpene glycosides from Stevia rebaudiana. Nat. Prod. Commun. 2011, 6, 1059–1062. [Google Scholar] [PubMed]
- Perera, W.H.; Ramsaroop, T.; Carvalho, R.; Rodenburg, D.L.; McChesney, J.D. A silica gel orthogonal high-performance liquid chromatography method for the analyses of steviol glycosides: Novel tetra-glucopyranosyl steviol. Nat. Prod. Res. 2018, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Starratt, A.N.; Kirby, C.W.; Pocs, R.; Brandle, J.E. Rebaudioside F, a diterpene glycoside from Stevia rebaudiana. Phytochemistry 2002, 59, 367–370. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Rodenburg, D.L.; Alves, K.; Perera, W.H.; Fronczek, F.R.; Bowling, J.; McChesney, J.D. Rebaudiosides R and S, minor diterpene glycosides from the leaves of Stevia rebaudiana. J. Nat. Prod. 2016, 79, 1468–1472. [Google Scholar] [CrossRef] [PubMed]
- Ohta, M.; Sasa, S.; Inoue, A.; Tamai, T.; Fujita, I.; Morita, K.; Matsuura, F. Characterization of Novel Steviol Glycosides from Leaves of Stevia rebaudiana Morita. J. Appl. Glycosci. 2010, 57, 199–209. [Google Scholar] [CrossRef]
- Ceunen, S.; Geuns, J.M. Steviol glycosides: Chemical diversity, metabolism, and function. J. Nat. Prod. 2013, 76, 1201–1228. [Google Scholar] [CrossRef] [PubMed]
- Perera, W.H.; Ghiviriga, I.; Rodenburg, D.L.; Alves, K.; Bowling, J.J.; Avula, B.; Khan, I.A.; McChesney, J.D. Rebaudiosides T and U, minor C-19 xylopyranosyl and arabinopyranosyl steviol glycoside derivatives from Stevia rebaudiana (Bertoni) Bertoni. Phytochemistry 2017, 135, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Rodenburg, D.L.; Alves, K.; Perera, W.H.; Ramsaroop, T.; Carvalho, R.; McChesney, J.D. Development of HPLC analytical techniques for diterpene glycosides from Stevia rebaudiana (Bertoni) Bertoni: Strategies to scale-up. J. Braz. Chem. Soc. 2016, 27, 1406–1412. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Flack, H.D. Crystal Structure and Synthesis of 3β-(p-Iodobenzoyloxy)-16α,17α-Epoxypregn-4-En-6,20-Dione. Acta Crystallogr. 1983, A39, 876–881. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1–7 are available from the authors. |
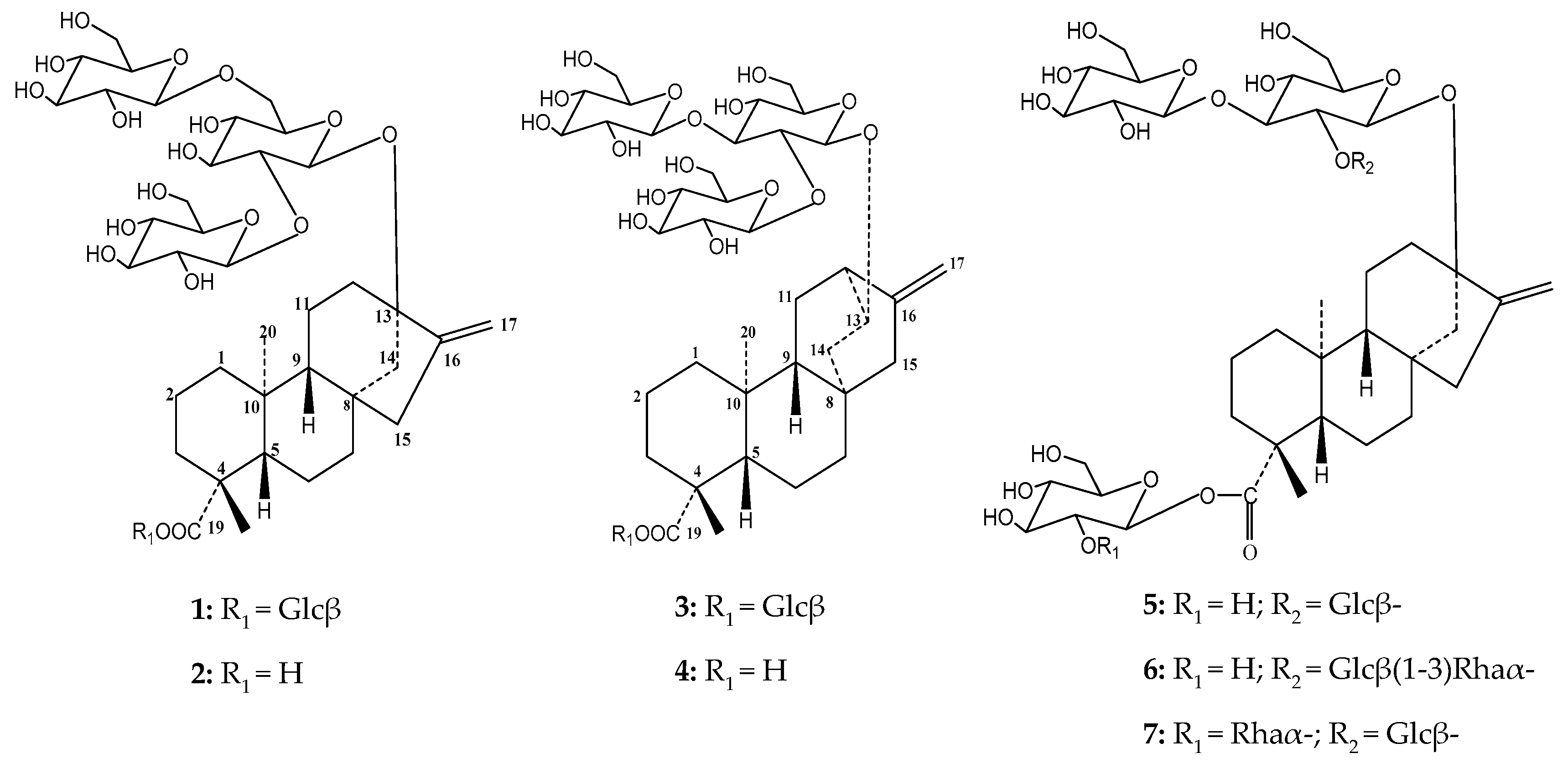



| Moiety | Position | 1 a | 2 b | 3 b | 4 b |
|---|---|---|---|---|---|
| δ (ppm) | δ (ppm) | δ (ppm) c | δ (ppm) c | ||
| Aglycone | 1 | 0.73, 1.73 | 0.95, 2.13 | 0.92, 1.59 | 0.85, 1.56 |
| 2 | 2.20, 1.43 | 1.96, 1.37 | 1.92, 1.40 | 2.01, 1.30 | |
| 3 | 2.28, 1.79 | 1.97, 1.54 | 2.20, 1.09 | 2.14, 0.88 | |
| 5 | 1.03 | 1.01 | 1.12 | 0.90 | |
| 6 | 1.91, 2.50 | 1.87, 1.87 | 1.78, 2.05 | 1.80, 1.98 | |
| 7 | 1.28, 1.28 | 1.52; 1.40 | 1.57, 1.16 | 1.53, 1.13 | |
| 9 | 0.87 | 0.96 | 1.09 | 1.04 | |
| 11 | 1.68, 1.68 | 1.79,1.63 | 1.63, 1.45 | 1.60, 1.44 | |
| 12 | 1.02, 2.35 | 0.83, 1.86 | 2.51 | 2.49 | |
| 13 | - | - | 4.04 | 4.05 | |
| 14 | 1.97, 2.74 | 1.51, 2.27 | 1.15, 2.51 | 1.14, 2.51 | |
| 15 | 2.06, 2.06 | 2.05, 2.10 | 1.89, 2.11 | 1.86, 2.08 | |
| 17 | 5.10, 5.73 | 4.85, 5.24 | 4.70, 4.84 | 4.68,4.82 | |
| 18 | 1.23 | 1.14 | 1.24 | 1.11 | |
| 20 | 1.31 | 1.03 | 0.89 | 0.95 | |
| Glcβ-C19 | 1′ | 6.12 | - | 5.43 | - |
| 2′ | 4.17 | - | 3.39 | - | |
| 3′ | 3.98 | - | 3.43 | - | |
| 4′ | 4.33 | - | 3.39 | - | |
| 5′ | 4.22 | - | 3.39 | - | |
| 6′ | 4.43, 4.57 | - | 3.85, 3.71 | - | |
| Glc-C13 | 1′′ | 5.19 | 4.59 | 4.56 | 4.55 |
| 2′′ | 4.23 | 3.60 | 3.65 | 3.64 | |
| 3′′ | 4.27 | 3.54 | 3.71 | 3.71 | |
| 4′′ | 4.45 | 3.35 | 3.38 | 3.42 | |
| 5′′ | 4.08 | 3.17 | 3.34 | 3.30 | |
| 6′′ | 4.51, 4.78 | 3.78, 4.10 | 3.91, 3.68 | 3.85, 3.70 | |
| Glc(1–2) | 1′′′ | 5.30 | 4.62 | 4.81 | 4.81 |
| 2′′′ | 4.11 | 3.30 | 3.19 | 3.18 | |
| 3′′′ | 4.23 | 3.46 | 3.35 | 3.34 | |
| 4′′′ | 4.28 | 3.35 | 3.22 | 3.21 | |
| 5′′′ | 3.91 | 3.36 | 3.33 | 3.30 | |
| 6′′′ | 4.22, 4.57 | 3.68, 3.82 | 3.87, 3.66 | 3.85, 3.65 | |
| Glcβ(1-X) d | 1′′′′ | 5.14 | 4.61 | 4.65 | 4.65 |
| 2′′′′ | 4.06 | 3.21 | 3.28 | 3.27 | |
| 3′′′′ | 4.27 | 3.46 | 3.39 | 3.38 | |
| 4′′′′ | 4.12 | 3.35 | 3.31 | 3.33 | |
| 5′′′′ | 4.03 | 3.36 | 3.37 | 3.36 | |
| 6′′′′ | 4.22, 4.57 | 3.68, 3.82 | 3.90, 3.66 | 3.89, 3.65 |
| Moiety | Position | 1 a | 2 b | 3 b | 4 b |
|---|---|---|---|---|---|
| δ (ppm) | δ (ppm) | δ (ppm) | δ (ppm) | ||
| Aglycone | 1 | 41.2 | 40.1 | 39.5 | 40.5 |
| 2 | 19.9 | 20.9 | 18.4 | 19.3 | |
| 3 | 36.8 | 39.0 | 37.7 | 39.4 | |
| 4 | 44.5 | 45.6 | 43.7 | 44.7 | |
| 5 | 57.8 | 58.7 | 57.1 | 57.6 | |
| 6 | 22.7 | 23.7 | 19.9 | 20.6 | |
| 7 | 42.2 | 43.1 | 38.8 | 39.4 | |
| 8 | 43.3 | 43.1 | 33.9 | 33.8 | |
| 9 | 54.3 | 55.3 | 51.0 | 51.2 | |
| 10 | 40.3 | 40.9 | 38.1 | 38.0 | |
| 11 | 21.1 | 21.5 | 26.3 | 26.3 | |
| 12 | 38.9 | 42.6 | 41.0 | 41.0 | |
| 13 | 86.4 | 88.3 | 77.8 | 77.8 | |
| 14 | 45.1 | 45.8 | 37.7 | 37.7 | |
| 15 | 48.0 | 48.8 | 47.4 | 47.7 | |
| 16 | 155.0 | 154.2 | 146.7 | 147.0 | |
| 17 | 105.4 | 105.7 | 108.0 | 107.8 | |
| 18 | 28.8 | 30.1 | 27.5 | 29.1 | |
| 19 | 177.7 | 184.1 | 176.8 | 184.4 | |
| 20 | 16.0 | 17.3 | 11.9 | 12.2 | |
| Glcβ-C19 | 1′ | 96.3 | - | 94.2 | - |
| 2′ | 74.4 | - | 72.6 | - | |
| 3′ | 79.5 | - | 77.3 | - | |
| 4′ | 71.4 | - | 69.7 | - | |
| 5′ | 79.9 | - | 77.3 | - | |
| 6′ | 62.6 | - | 61.0 | - | |
| Glcβ-C13 | 1′′ | 98.3 | 97.7 | 100.5 | 100.3 |
| 2′′ | 84.4 | 81.8 | 79.0 | 78.8 | |
| 3′′ | 78.6 | 78.5 | 86.0 | 85.9 | |
| 4′′ | 71.8 | 71.8 | 68.8 | 68.6 | |
| 5′′ | 77.8 | 77.8 | 76.1 | 76.1 | |
| 6′′ | 70.3 | 70.0 | 61.4 | 61.2 | |
| Glcβ(1-2) | 1′′′ | 106.8 | 104.8 | 102.2 | 102.1 |
| 2′′′ | 75.7 | 76.3 | 74.6 | 74.5 | |
| 3′′′ | 78.8 | 77.8 | 76.5 | 76.5 | |
| 4′′′ | 72.1 | 71.4 | 70.6 | 70.6 | |
| 5′′′ | 78.3 | 77.9 | 76.6 | 76.6 | |
| 6′′′ | 63.3 | 62.8 | 61.9 | 61.8 | |
| Glcβ(1-X) | 1′′′′ | 105.9 | 104.6 | 103.1 | 103.1 |
| 2′′′′ | 77.2 | 75.3 | 73.9 | 73.9 | |
| 3′′′′ | 78.9 | 77.9 | 76.8 | 76.8 | |
| 4′′′′ | 72.6 | 71.7 | 70.1 | 70.1 | |
| 5′′′′ | 78.5 | 78.3 | 76.8 | 76.8 | |
| 6′′′′ | 63.3 | 62.8 | 61.2 | 61.1 |
| DG | Glycosylation Sites | RT (min) a | [M − H]− (m/z) | |||
|---|---|---|---|---|---|---|
| C-12 (R2) | C-13 (R2) | C-19 (R1) | DG | Agly b | Experimental | |
| Rebaudioside Z (1) | -CH2 | Glcβ(1-2)[Glcβ(1-6)]-Glcβ1- | Glcβ1- | 3.94 | I | 965.4222 |
| Compound 3 | -CH | Glcβ(1-2)[Glcβ(1-3)]-Glcβ1- and -H | Glcβ1- | 4.35 | II | 965.4297 |
| Rebaudioside A | -CH2 | Glcβ(1-2)[Glcβ(1-3)]-Glcβ1- | Glcβ1- | 7.11 | I | 965.4178 |
| Iso-rebaudioside A | -CH2 | Glcβ(1-2)[Glcβ(1-3)]-Glcβ1- | Glcβ1- | 7.91 | III | 965.4204 |
| Rebaudioside Z1 (2) | -CH2 | Glcβ(1-2)[Glcβ(1-6)]-Glcβ1- | H | 9.32 | I | 803.3721 |
| Compound 4 | -CH | Glcβ(1-2)[Glcβ(1-3)]-Glcβ1- and -H | H | 10.5 | II | 803.3682 |
| Rebaudioside B | -CH2 | Glcβ(1-2)[Glcβ(1-6)]-Glcβ1- | H | 14.32 | I | 803.3692 |
| Iso-rebaudioside B | -CH2 | Glcβ(1-2)[Glcβ(1-3)]-Glcβ1- | H | 14.44 | III | 803.3682 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perera, W.H.; Ghiviriga, I.; Rodenburg, D.L.; Alves, K.; Wiggers, F.T.; Hufford, C.D.; Fronczek, F.R.; Ibrahim, M.A.; Muhammad, I.; Avula, B.; et al. Tetra-glucopyranosyl Diterpene ent-Kaur-16-en-19-oic Acid and ent-13(S)-Hydroxyatisenoic Acid Derivatives from a Commercial Extract of Stevia rebaudiana (Bertoni) Bertoni. Molecules 2018, 23, 3328. https://doi.org/10.3390/molecules23123328
Perera WH, Ghiviriga I, Rodenburg DL, Alves K, Wiggers FT, Hufford CD, Fronczek FR, Ibrahim MA, Muhammad I, Avula B, et al. Tetra-glucopyranosyl Diterpene ent-Kaur-16-en-19-oic Acid and ent-13(S)-Hydroxyatisenoic Acid Derivatives from a Commercial Extract of Stevia rebaudiana (Bertoni) Bertoni. Molecules. 2018; 23(12):3328. https://doi.org/10.3390/molecules23123328
Chicago/Turabian StylePerera, Wilmer H., Ion Ghiviriga, Douglas L. Rodenburg, Kamilla Alves, Frank T. Wiggers, Charles D. Hufford, Frank R. Fronczek, Mohamed A. Ibrahim, Ilias Muhammad, Bharathi Avula, and et al. 2018. "Tetra-glucopyranosyl Diterpene ent-Kaur-16-en-19-oic Acid and ent-13(S)-Hydroxyatisenoic Acid Derivatives from a Commercial Extract of Stevia rebaudiana (Bertoni) Bertoni" Molecules 23, no. 12: 3328. https://doi.org/10.3390/molecules23123328
APA StylePerera, W. H., Ghiviriga, I., Rodenburg, D. L., Alves, K., Wiggers, F. T., Hufford, C. D., Fronczek, F. R., Ibrahim, M. A., Muhammad, I., Avula, B., Khan, I. A., & McChesney, J. D. (2018). Tetra-glucopyranosyl Diterpene ent-Kaur-16-en-19-oic Acid and ent-13(S)-Hydroxyatisenoic Acid Derivatives from a Commercial Extract of Stevia rebaudiana (Bertoni) Bertoni. Molecules, 23(12), 3328. https://doi.org/10.3390/molecules23123328