Improved Resolution of 4-Chloromandelic Acid and the Effect of Chlorine Interactions Using (R)-(+)-Benzyl-1-Phenylethylamine as a Resolving Agent
Abstract
:1. Introduction
2. Results and Discussion
2.1. Solvent Screening and Resolution Condition Determination
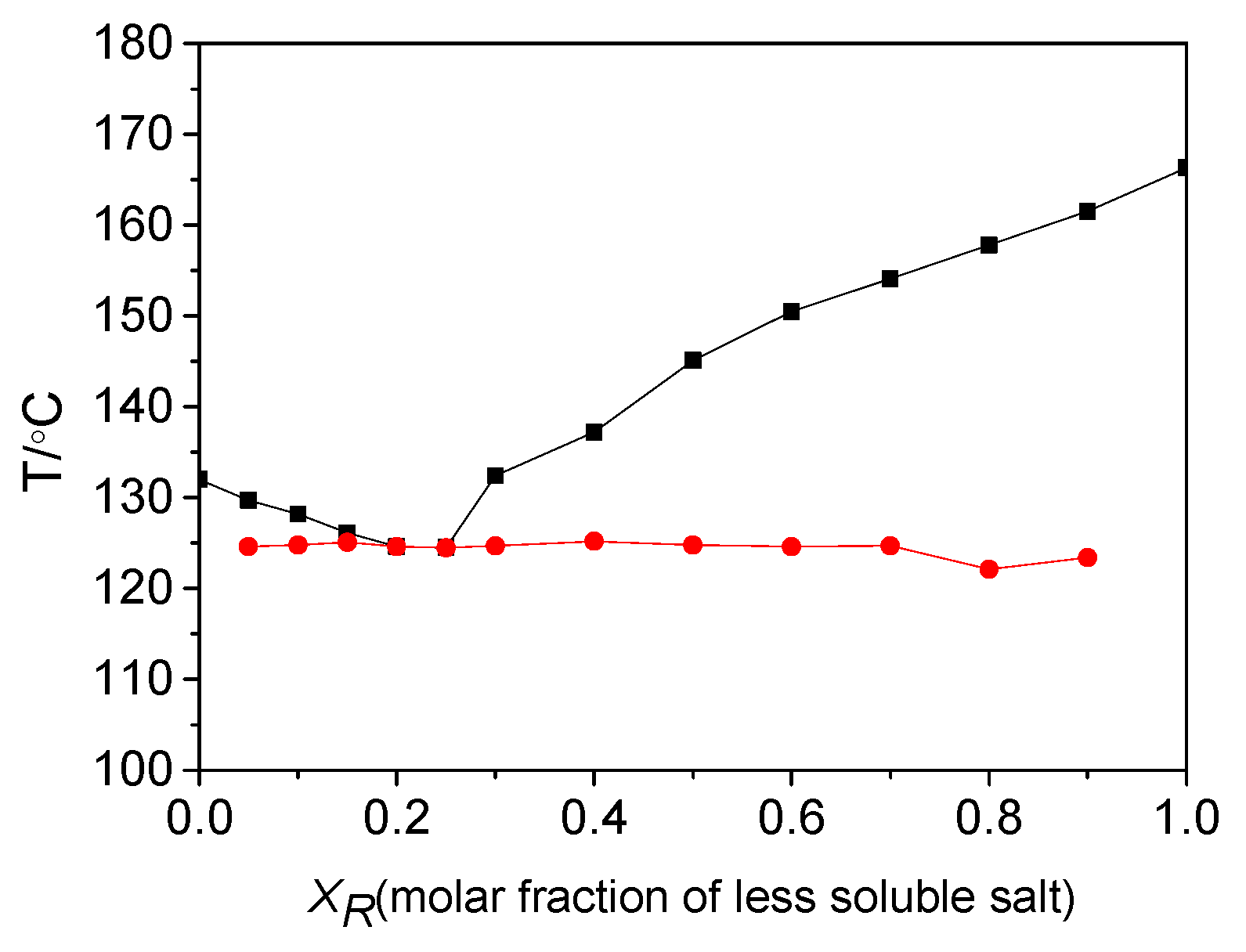
2.2. Thermodynamic Properties of Diastereomeric Salts
2.3. Crystal Structure of Diastereomeric Salts
2.3.1. Hydrogen-Bonding Network
2.3.2. CH/π and π/π Interaction
2.3.3. Chlorine…Chlorine/π Interactions
2.3.4. Stacking Mode
3. Materials and Methods
3.1. Materials
3.2. Analytical Methods
3.3. Preparation of the Less Soluble Salt (R)-(−)-4-ClMA·(R)-(+)-BPA
3.4. Preparation of the More Soluble Salt (R)-(−)-4-ClMA·(S)-(−)-BPA
3.5. Solubility Determination
3.6. Determination of Binary Phase Diagram of Diastereomeric Salts
3.7. Resolution Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A
References
- FDA’s Policy Statement for the Development of New Stereoisomeric Drugs; Wiley-Liss, Inc.: Hoboken, NJ, USA, 21 September 2004; pp. 338–340. [CrossRef]
- Pellissier, H. Recent advances in enantioselective vanadium-catalyzed transformations. Corordination Chem. Rev. 2015, 284, 93–110. [Google Scholar] [CrossRef]
- Tang, K.W.; Yi, J.M.; Liu, Y.B. Enantioselective separation of R,S-phenylsuccinic acid by biphasic recognition chiral extraction. Chem. Eng. Sci. 2009, 64, 4081–4088. [Google Scholar] [CrossRef]
- Xie, R.; Chu, L.Y.; Deng, J.G. Membranes and membrane process for chiral resolution. Chem. Soc. Rev. 2008, 37, 1243–1263. [Google Scholar] [CrossRef] [PubMed]
- Bocian, S.; Skoczylas, M.; Buszewski, B. Amino acids, peptides, and proteins as chemically bonded stationary phases—A review. J. Sep. Sci. 2016, 39, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, Q.; Zhu, Du. Enzymatic synthesis of chiral 2-hydroxy carboxylic acids. Process Biochem. 2015, 50, 759–770. [Google Scholar] [CrossRef]
- Faigl, F.; Fogassy, E.; Nógrádi, M.; Pálovics, E.; Schindler, J. Strategies in optical resolution: A practical guide. Tetrahedron Asymmetry 2008, 19, 519–536. [Google Scholar] [CrossRef]
- Kozma, D. Resolution by Formation and Fractional Crystallization of Diastereomeric Salts. In CRC Handbook of Optical Resolutions via Diastereomeric Salt Formation, 1st ed.; CRC Press: Boca Raton, FL, USA, 2001; pp. 9–50. [Google Scholar]
- Faigl, F.; Schindler, J.; Fogassy, E. Advantages of structural similarities of reactants in optical resolution. ChemInform 2007, 38. [Google Scholar] [CrossRef]
- Szeleczky, Z.; Semsey, S.; Bagi, P.; Fódi, B.; Faigl, F.; Pálovics, E.; Fogassy, E. An aspect of selecting resolving agents: The role of differences in molecule length in diastereomeric salt resolutions. Sep. Sci. Technol. 2016, 51, 727–732. [Google Scholar] [CrossRef]
- Kodama, K.; Kobayashi, Y.; Saigo, K. Role of the relative molecular length of the component in ternary inclusion crystals in the chiral recognition and assembly of superamolecular helical architectures. Cryst. Growth Des. 2007, 7, 935–939. [Google Scholar] [CrossRef]
- Saigo, K.; Sakai, K. Toward efficient optical resolution by diastereomeric salt formation. J. Syn. Org. Chem. Jpn. 2011, 69, 499–505. [Google Scholar] [CrossRef]
- Kinbara, K. Design of resolving agents based on crystal engineering. Synlett 2005, 5, 732–743. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kokubo, Y.; Aisaka, T.; Saigo, K. Hydrogen-bonding sheets in crystals for chirality recognition: Synthesis and application of (2S,3S)-2,3-dihydroxy- and (2S,3S)-2,3-dibenzyloxy-1,4-bis(hydroxyamino)butanes. Tetrahedron Asymmetry 2008, 19, 2536–2541. [Google Scholar] [CrossRef]
- Suezawa, H.; Ishihara, S.; Umezawa, Y.; Tsunoyama, S.; Nishio, M. The aromatic CH/pi hydrogen bond as an important factor in determining the relative stability of diastereomeric salts relevant to enantiomeric resolution—A crystallographic database study. Eur. J. Org. Chem. 2004, 23, 4816–4822. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Saigo, K. The role of CH/π interaction in the stabilization of less-soluble diastereomeric salt crystals. Chem. Rec. 2007, 7, 47–56. [Google Scholar] [CrossRef]
- Sakai, K.; Sakurai, R.; Hirayama, N. Molecular mechanisms of dielectrically controlled resolution (DCR). Top. Curr. Chem. 2007, 233–271. [Google Scholar] [CrossRef]
- Sakai, K.; Sakurai, R.; Hirayama, N. Chiral discrimination controlled by the solvent dielectric constant. Tetrahedron Asymmetry 2004, 15, 1073–1076. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Maeda, J.; Ando, T.; Saigo, K. Halogen-bonding interaction stabilizing cluster-type diastereomeric salt crystals. Cryst. Growth Des. 2010, 10, 685–690. [Google Scholar] [CrossRef]
- Farina, A.; Meille, S.V.; Messina, M.T.; Metrango, P.; Resnati, G.; Vecchio, G. Resolution of racemic 1,2-dibromohexafluoropropane through halogen-bonded supramolecular helices. Angrew. Chem. Int. Ed. 1999, 38, 2433–2434. [Google Scholar] [CrossRef]
- Yin, L.; Shan, W.; Jia, X.; Li, X.; Chan, A.S.C. Ru-catalyzed enantioselective preparation of methyl (R)-O-chloromandelate and its application in the synthesis of (S)-clopidogrel. J. Organomet. Chem. 2009, 694, 2092–2095. [Google Scholar] [CrossRef]
- Bousquet, A.; Musolino, A. Hydroxyacetic Ester Derivatives, Preparation Method and Use as Synthesis Intermediates. U.S. Patent 6,573,381, 3 June 2003. [Google Scholar]
- Adams, A.D.; Jones, A.B.; Berger, J.P. Preparation of 2-Aryloxy-2-Arylalkanoic Acids for Diabetes and Lipid Disorders. WO Patent 2,002,064,094, 2 May 2002. [Google Scholar]
- Bereczki, L.; Marthi, K.; Pokol, G. Structure-resolvability relationship of several diastereomeric salt pairs of 1-phenylethylamine—A cambridge structural database study. Period. Polytech. Chem. Eng. 2009, 53, 71. [Google Scholar] [CrossRef]
- Bandala, Y.; Juaristi, E. Recent advances in the application of alpha-phenylethylamine (alpha-PEA) in the preparation of enantiopure compounds. Aldrichimica Acta 2010, 43, 65–78. [Google Scholar]
- He, Q.; Peng, Y.F.; Rohani, S. Diastereomeric resolution of p-chloromandelic acid with (R)-phenylethylamine. Chirality 2010, 22, 16–23. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Gomma, H.; Rohani, S.; Zhu, J.; Jennings, M. Chiral discrimination in diastereometric salts of chlorine substituted mandelic acid and phenylethyamine. Chirality 2010, 22, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.F.; He, Q.; Rohani, S.; Jenkins, H. Resolution of 2-chloromandelic acid with (R)-(+)-N-benzyl-1-phenylethylamine: Chiral discrimination mechanism. Chirality 2012, 24, 349–355. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Rohani, S.; Zhu, J.; Gomaa, H. Crystallization of racemic compound and conglomerate of (RS)-2-chloromandelic acid. Cryst. Growth Des. 2010, 10, 5136–5145. [Google Scholar] [CrossRef]
- Peng, Y.F.; Cai, S.H. Research on Extraction for (S)-(−)-α-Methylbenzylamine. J. ECUST 1999, 25, 329–332. [Google Scholar] [CrossRef]
- Wen, L.F.; Zhang, N.; Li, H.C.; Huang, Q.; Wu, X.; Hao, X.; Wu, M.; Ban, C.; Zhao, J. Ternary liquid-liquid equilibrium for mixtures of water + (±)α-phenylethylamine + n-hexane at T = 298.2, 318.2, and 333.2 K. J. Chem. Eng. Data 2017, 62, 1819–1824. [Google Scholar] [CrossRef]
- Suzuki, M.; Sakai, K. Optical active N-benzyl-1-phenylethylamine. J. Synth. Org. Chem. 2000, 58, 147–148. [Google Scholar] [CrossRef]
- Yoshioka, R.; Hiramatsu, H.; Okamura, K.; Tsujioka, I.; Yamada, S. Crystal structure-solubility relationships in optical resolution by diastereomeric salt formation of dl-phenylglycine with (1S)-(+)-camphor-10-sulfonic acid. J. Chem. Soc. Perkin Trans. 2000, 10, 2121–2128. [Google Scholar] [CrossRef]
- Leusen, F.J.J.; Noordik, J.H.; Karfunkel, H.R. Racemate resolution via crystallization of diastereomeric salts: Thermodynamic considerations and molecular mechanics calculations. Tetrahedron 1993, 49, 5377–5396. [Google Scholar] [CrossRef]
- Bolchi, C.; Pallavicini, M.; Fumagalli, L. Highly efficient resolutions of 1, 4-benzodioxane-2-carboxylic acid with para substituted 1-phenylethylamines. Tetrahedron Asymmetry 2005, 16, 1639–1643. [Google Scholar] [CrossRef]
- Silvestro, G.D.; Palmisano, G.; Pellegata, R. Phase diagram of (R)-and (S)-4-hydroxy-2-pyrrolldone mixtures: A new calculation of a conglomerate forming system. J. Pharm. Sci. 1993, 82, 758–760. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, H.; Seidel-Morgenstern, S. Binary and ternary phase diagrams of two enantiomers in solvent systems. Thermochim. Acta 2002, 382, 129–142. [Google Scholar] [CrossRef]
- Dunitz, J.D.; Gavezzotti, A. How molecules stick together in organic crystals: The weak intermolecular interaction. Chem. Soc. Rev. 2009, 38, 2622–2633. [Google Scholar] [CrossRef] [PubMed]
- Nishio, M. The CH/π hydrogen bond in chemistry. Conformation, supramolecules, optical resolution and interactions involving carbohydrates. Phys. Chem. Chem. Phys. 2011, 13, 13873–13900. [Google Scholar] [CrossRef] [PubMed]
- Gilday, L.C.; Robinson, S.W.; Barends, T.A.; Langton, M.J.; Mullaney, B.R.; Beer, P.D. Halogen bonding in supramolecular chemistry. Chem. Rev. 2015, 115, 7118–7195. [Google Scholar] [CrossRef] [PubMed]
- Desiraju, G.R.; Ho, P.S.; Kloo, L.; Legon, A.C.; Marquardt, R.; Metrangolo, P.; Polotzer, P.; Resnati, G.; Rissanen, K. Definition of the halogen bond. Pure Appl. Chem. 2013, 85, 1711–1713. [Google Scholar] [CrossRef]
- Ribeiro, N.; Kobayashi, Y.; Maeda, J.; Saigo, K. Enantiopure cyclic O-substitrted phenylphosphonothioic acid-synthesis and chirality recognition ability. Chirality 2011, 23, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, A.; Ono, H.; Mikata, Y. Characteristic conformations and molecular packings in crystal structures of diastereomeric esters prepared from (S)-2-methoxy-2-(1-naphthy) propanoic acid. Tetrahedron Asymmetry 2008, 19, 2693–2698. [Google Scholar] [CrossRef]
- Donatella, A.; Adrian, S.P.; Carlos, R.D.; Jose, M.L. Halogen…Halogen interaction in the self-assembly of one-dimensional 2,2′-bipyrimidine-based CuIIReIV systems. CrystEngComm 2018, 20, 4575–4581. [Google Scholar] [CrossRef]
- Nagels, N.; Hauchecorne, D.; Herrebout, W.A. Ecploring the C-X…π halogen bonding motif: An infared and raman study of the complexes of CF3X(X = Cl, Br, and I) with the aromatic model compounds benzene and toluene. Molecules 2013, 18, 6829–6851. [Google Scholar] [CrossRef] [PubMed]
- Hauchecorne, D.; Nagels, N.; van der Veken, B.J.; Herrebout, W.A. C-X…π halogen and C-H…π hydron bonding: Interactions of CF3X(X=Cl, Br, I or H) with ethane and propene. Phys. Chem. Chem. Phys. 2012, 14, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Chang, Y.K.; Zin, H.M.; Chen, C.H. Solid liquid equilibria for 4-methoxyphenol with catechol, ethylenediamine, or piperazine. J. Chem. Eng. Data 1997, 42, 349–352. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds (R)-(−)-4-ClMA·(R)-(+)-BPA and (S)-(+)-4-ClMA·(R)-(+)-BPA are not available from the authors. |


| 4-ClMA/mol | BPA/mol | Solvent a | %d.e. | Yield b/% | Ec/% |
|---|---|---|---|---|---|
| 0.005 | 0.005 | Methanol | 94.5 | 55.9 | 52.8 |
| 0.005 | 0.005 | Ethanol | 94.8 | 83.1 | 78.8 |
| 0.005 | 0.005 | 95% ethanol | 96.3 | 76.3 | 73.5 |
| 0.005 | 0.005 | 50% ethanol | 55.2 | 158.5 | 87.5 |
| 0.005 | 0.005 | 2-propanol | 56.3 | 156.9 | 88.4 |
| 0.005 | 0.005 | Acetonitrile | 52.1 | 172.0 | 89.6 |
| 0.005 | 0.005 | Ethyl acetate | 52.8 | 176.4 | 93.2 |
| 0.005 | 0.005 | chloroform | No salts | ||
| Solubility a/g | Melting Point/°C | Heat of fusion/kJ·mol−1 | |
|---|---|---|---|
| Less soluble salt | 1.47 | 166.3 | 57.41 |
| More soluble salt | 4.81 | 132.0 | 52.58 |
| D-H…A | d(D-H) | d(H…A) | d(D…A) | <(DHA) |
|---|---|---|---|---|
| O3A-H3A…O1A a | 0.87(2) | 1.84(2) | 2.6878(15) | 164.9(17) |
| O3A-H3A…O2A a | 0.87(2) | 2.52 (2) | 3.1629(14) | 130.6(16) |
| N1B-H1BA…O2A a | 0.85(2) | 1.90(2) | 2.7457(16) | 176.1(16) |
| N1B-H1BB…O1A | 0.98(2) | 1.78(2) | 2.7337(15) | 163.7(18) |
| N1B-H1BB…O3A | 0.98(2) | 2.42(2) | 3.0019(14) | 117.4(15) |
| D-H…A | d(D-H) | d(H…A) | d(D…A) | <(DHA) |
|---|---|---|---|---|
| O(3)-H(3)...O(1) a | 0.90(3) | 1.81(3) | 2.707(3) | 170(3) |
| N(1)-H(1A)...O(1) b | 1.04(3) | 1.84(3) | 2.867(3) | 169(2) |
| N(1)-H(1B)...O(2) c | 0.93(3) | 1.80(3) | 2.690(3) | 158(3) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.; Feng, C.; Rohani, S.; He, Q. Improved Resolution of 4-Chloromandelic Acid and the Effect of Chlorine Interactions Using (R)-(+)-Benzyl-1-Phenylethylamine as a Resolving Agent. Molecules 2018, 23, 3354. https://doi.org/10.3390/molecules23123354
Peng Y, Feng C, Rohani S, He Q. Improved Resolution of 4-Chloromandelic Acid and the Effect of Chlorine Interactions Using (R)-(+)-Benzyl-1-Phenylethylamine as a Resolving Agent. Molecules. 2018; 23(12):3354. https://doi.org/10.3390/molecules23123354
Chicago/Turabian StylePeng, Yangfeng, Cai Feng, Sohrab Rohani, and Quan He. 2018. "Improved Resolution of 4-Chloromandelic Acid and the Effect of Chlorine Interactions Using (R)-(+)-Benzyl-1-Phenylethylamine as a Resolving Agent" Molecules 23, no. 12: 3354. https://doi.org/10.3390/molecules23123354
APA StylePeng, Y., Feng, C., Rohani, S., & He, Q. (2018). Improved Resolution of 4-Chloromandelic Acid and the Effect of Chlorine Interactions Using (R)-(+)-Benzyl-1-Phenylethylamine as a Resolving Agent. Molecules, 23(12), 3354. https://doi.org/10.3390/molecules23123354








