Development of Functional Phthalocyanine-Based Associate towards an Effective Fluorimetric Detection of Hg(II)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Molecular Structure of AlS4Pc and Alcian Blue 8GX
2.2. Fluorescence Properties of AlS4Pc
2.3. Spectral Behavior and the Mechanism of the Reaction System
2.4. Optimization of Experimental Conditions
2.4.1. pH and the Selection of Buffer Medium
2.4.2. Selection of Temperature
2.4.3. Selection of Reaction Time
2.4.4. Investigations on the Usage of Alcian Blue 8GX
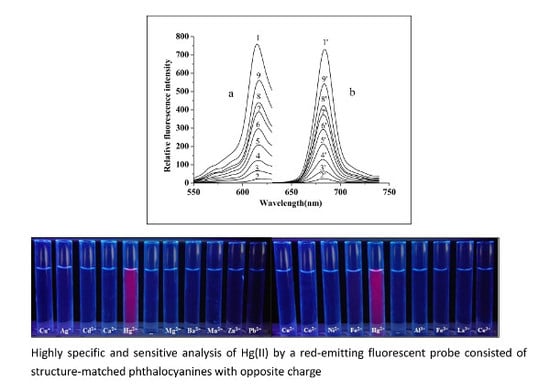
2.5. Effects of Coexisting Substances
2.6. Standard Curve
2.7. Analysis of Practical Samples
3. Materials and Methods
3.1. Materials
3.2.Experimental Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Harris, H.H.; Pickering, I.J.; George, G.N. The chemical form of mercury in fish. Science 2003, 301, 1203. [Google Scholar] [CrossRef] [PubMed]
- Renzoni, A.; Zino, F.; Franchi, E. Mercury levels along the food chain and risk for exposed populations. Environ. Res. 1998, 77, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Nolan, E.M.; Lippard, S.J. Tools and tactics for the optical detection of mercuric ion. Chem. Rev. 2008, 108, 3443–3480. [Google Scholar] [CrossRef] [PubMed]
- Krishna, M.V.B.; Ranjit, M.; Karunasagar, D.; Arunachalam, J. A rapid ultrasound-assisted thiourea extraction method for the determination of inorganic and methyl mercury in biological and environmental samples by CVAAS. Talanta 2005, 67, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Yardim, M.F.; Budinova, T.; Ekinci, E.; Petrov, N.; Razvigorova, M.; Minkova, V. Removal of mercury (II) from aqueous solution by activated carbon obtained from furfural. Chemosphere 2003, 52, 835–841. [Google Scholar] [CrossRef]
- Segade, S.R.; Tyson, J.F. Determination of methylmercury and inorganic mercury in water sample by slurry sampling cold vapor atomic absorption spectrometry in a flow injection system after preconcentration on silica C18 modified. Talanta 2007, 71, 1696–1702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Adeloju, S.B. A novel sequential injection-cold vapour atomic absorption spectrometric system for rapid and reliable determination of mercury. Talanta 2008, 74, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; Wang, Q.; Zhao, Q. Determination of mercury in sodium hydroxide as food additives by hydride generation-atomic fluorescence spectrometry. Chin. J. Health Lab. Technol. 2008, 18, 1324–1332. [Google Scholar]
- Yu, F.; Yu, T.L. Determination of ultra-trace lead and mercury in groundwater by hydride generation—Atomic Fluorescence Spectrometry with sulfhydryl cotton. Spectrosc. Spectr. Anal. 2000, 20, 898–900. [Google Scholar]
- Boaventura, G.R.; Barbosa, A.C.; East, G.A. Multivessel system for cold-vapor mercury generation determination of mercury in hair and fish. Ecol. Biol. Trace Elem. Res. 1997, 60, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Seibert, E.L.; Dressler, V.L.; Pozebon, D.; Curtius, A.J. Determination of Hg in seawater by inductively coupled plasma mass spectrometry after on-line pre-concentration. Spectrochim. Acta Part B 2001, 56, 1963–1971. [Google Scholar] [CrossRef]
- Iwashita, A.; Nakajima, T.; Takanashi, H.; Ohki, A.; Fujita, Y.; Yamashita, T. Determination of trace elements in coal fly ash by joint-use of ICP-AES and atomic absorption spectrometry. Talanta 2007, 71, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Hardy, S. Development of a capillary electrophoretic method for the separation and determination of trace inorganic and organomercury species utilizing the formation of highly absorbing water soluble dithizone sulphonate complexes. J. Chromatogr. A 1997, 765, 345–352. [Google Scholar] [CrossRef]
- Young, T.K.; Yun, J.B.; Yoon, B.S. Determination of Hg2+ ions using a modified glassy carbon electrode with 2,2′:6′:2″-Terpyridine. Bull. Korean Chem. Soc. 2002, 23, 346–350. [Google Scholar]
- Liu, Z.H.; Huan, S.Y.; Jiang, J.H.; Shen, G.L.; Yu, R.Q. Molecularly imprinted TiO2 thin film using stable ground state complex as template as applied to selective electrochemical determination of mercury. Talanta 2006, 68, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- Tabak, M.; Borisevitch, I.E. Interaction of dipyridamole with micelles of lysophosphatidylcholine and with bovine serum-albumin-fluorescence studies. Biochim. Biophys. Acta 1992, 1116, 241–249. [Google Scholar] [CrossRef]
- Husain, N.; Agbaria, R.A.; Warner, I.M. Spectroscopic analysis of the binding of doxorubicin to human alpha-1-acid glycoprotein. J. Phys. Chem. 1993, 97, 10857–10861. [Google Scholar] [CrossRef]
- Karoui, R.; Bosset, J.O.; Mazerolles, G.; Kulmyrzaev, A.; Dufour, E. Monitoring the geographic origin of both experimental French Jura hard cheeses and Swiss Gruyere and L’Etivaz PDO cheeses using mid-infrared and fluorescence spectroscopies: A preliminary investigation. Int. Dairy J. 2005, 15, 275–286. [Google Scholar] [CrossRef]
- Wen, Z.Q. Raman spectroscopy of protein pharmaceuticals. J. Pharm. Sci. 2007, 96, 2861–2878. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wu, P.; Xu, K.L.; Lv, Y.; Hou, X.D. Highly sensitive and interference-free determination of bismuth in environmental samples by electrothermal vaporization atomic fluorescence spectrometry after hydride trapping on iridium-coated tungsten coil. Spectrochim. Acta Part B 2008, 63, 704–709. [Google Scholar] [CrossRef]
- De Silva, A.P.; Gunaratne, H.N.; Gunnlaugsson, T.; Huxley, A.J.M.; McCoy, C.P.; Rademacher, J.T.; Rice, T.E. Signaling recognition events with fluorescent sensors and switches. Chem. Rev. 1997, 97, 1515–1566. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.N.; Ren, W.X.; Kim, J.S.; Yoon, J. Fluorescence and colorimetric sensors for detection of lead, cadium, and mercury ion. Chem. Soc. Rev. 2012, 41, 3210–3244. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Guo, Z.; Zeng, G.; Tang, L. Fluorescent and colorimetric sensors for environmental mercury detection. Analyst 2015, 140, 5400–5443. [Google Scholar] [CrossRef] [PubMed]
- Vedamalai, M.; Wu, S.P. A BODIPY-based reactive probe for Hg(II) ions and its application to living cell imaging. Org. Biomol. Chem. 2012, 10, 5410–5416. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, P.; Thirumalivasan, N.; Wu, S.P. A Rhodamine-based chemosensor with diphenylselenium for highly selective fluorescence turn-on detection of Hg2+ in vitro and in vivo. RSC Adv. 2017, 7, 21733–21739. [Google Scholar] [CrossRef]
- Dong, M.; Wang, Y.W.; Peng, Y. Highly selective ratiometric fluorescent sensing for Hg2+ and Au3+ respectively in aqueous media. Org. Lett. 2010, 12, 5310–5313. [Google Scholar] [CrossRef] [PubMed]
- Ambroz, M.; Beeby, A.; MacRobert, A.J.; Simpson, M.S.C.; Svensen, R.K.; Phillips, D. Preparative, analytical and fluorescence spectroscopic studies of sulphonated aluminum phthalocyanine photosensitizers. J. Photochem. Photobiol. B 1991, 9, 87–95. [Google Scholar] [CrossRef]
- Yu, F.; Guo, M.L.; Deng, Y.B.; Yin, L.; Chen, L.; Huang, P.; Li, D.H. Structure-matched phthalocyanine ion pair as a red-emitting fluorescent optical probe for the analysis of sodium dodecylbenzenesulfonate with high specificity and sensitivity. Anal. Sci. 2016, 32, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, P.; Yang, H.Q.; Deng, Y.B.; Guo, M.L.; Li, D.H. Sensitive determination of chondroitin sulfate by fluorescence recovery of an anionic aluminum phthalocyanine-cationic surfactant ion-association complex used as a fluorescent probe emitting at red region. Spectrosc. Spectr. Anal. 2015, 35, 2203–2207. [Google Scholar]
- Chen, L.; Huang, P.; Yang, H.Q.; Deng, Y.B.; Li, D.H. Novel Method for Determination of Lysozyme Based on Fluorescence Recovery of a Cationic Aluminum Phthalocyanine-Mucopolysaccharides Association Complex Used as a Red Emitting Fluorogenic Substrate. Chin. J. Anal. Chem. 2014, 42, 962–967. [Google Scholar]
- Li, D.H.; Chen, X.L.; Fang, Y.; Xu, J.G. Determination of nucleic acids based on shifting the association equilibrium between tetrasulfonated aluminium phthalocyanine and Acridine Orange. Analyst 2001, 126, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Li, D.H.; Yang, H.H.; Zheng, H.; Fang, Y.; Zhu, Q.Z.; Xu, J.G. Fluorimetric determination of albumin and globulin in human serum using tetra-substituted sulphonated aluminum phthalocyanine. Anal. Chim. Acta 1999, 401, 185–189. [Google Scholar] [CrossRef]










| Metal Ions | Concentration (mol/L) | Relative Error/% | Metal Ions | Concentration (mol/L) | Relative Error/% |
|---|---|---|---|---|---|
| Ag+ | 2.0 × 10−5 | +8.71 | Fe3+ | 1.0 × 10−5 | −7.99 |
| Cu2+ | 4.0 × 10−5 | −7.42 | Al3+ | 1.5 × 10−5 | −7.53 |
| Cd2+ | 1.0 × 10−5 | −9.33 | Ce3+ | 1.0 × 10−5 | −9.25 |
| Ca2+ | 2.0 × 10−5 | −7.11 | La3+ | 1.0 × 10−5 | −8.55 |
| Ba2+ | 8.0 × 10−5 | −5.65 | F− | 1.0 × 10−5 | −7.25 |
| Mg2+ | 6.0 × 10−5 | −8.84 | Cl− | 2.0 × 10−4 | −4.64 |
| Co2+ | 1.0 × 10−5 | −9.45 | Br− | 2.0 × 10−5 | −6.75 |
| Ni2+ | 1.0 × 10−5 | −9.01 | I− | 2.0 × 10−6 | −40.37 |
| Cu2+ | 2.0 × 10−5 | −8.84 | N03− | 2.0 × 10−4 | −1.71 |
| Zn2+ | 1.0 × 10−5 | −9.31 | SO42− | 2.0 × 10−4 | −2.53 |
| Mn2+ | 1.0 × 10−5 | −9.01 | CO32− | 2.0 × 10−5 | 7.17 |
| Pb2+ | 2.0 × 10−5 | −8.52 | PO43− | 2.0 × 10−4 | 5.06 |
| Fe2+ | 1.5 × 10−5 | −5.81 | CH3COO− | 2.0 × 10−4 | 0.84 |
| Samples | Hg(II) Added (µmol/L) | Hg(II) Detected (µmol/L) | Recovery (%) | |
|---|---|---|---|---|
| Lungful Shanquan (pH 6.89) | 1 | 0 | Not detected | _ |
| 2 | 0.1 | 0.107 a ± 0.12 b | 107.0 | |
| 3 | 1.0 | 1.125 a ± 0.23 b | 112.5 | |
| Baisuishan (pH 6.86) | 1 | 0 | Not detected | _ |
| 2 | 0.1 | 0.108 a ± 0.04 b | 108.0 | |
| 3 | 1.0 | 1.201 a ± 0.21 b | 120.1 | |
| Biaozhi (pH 7.03) | 1 | 0 | Not detected | _ |
| 2 | 0.1 | 0.104 a ± 0.05 b | 104.0 | |
| 3 | 1.0 | 0.999 a ± 0.13 b | 99.9 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, G.-X.; Zhou, T.; Guo, M.-L.; Huang, P.; Deng, Y.-B.; Li, D.-H. Development of Functional Phthalocyanine-Based Associate towards an Effective Fluorimetric Detection of Hg(II). Molecules 2018, 23, 418. https://doi.org/10.3390/molecules23020418
Du G-X, Zhou T, Guo M-L, Huang P, Deng Y-B, Li D-H. Development of Functional Phthalocyanine-Based Associate towards an Effective Fluorimetric Detection of Hg(II). Molecules. 2018; 23(2):418. https://doi.org/10.3390/molecules23020418
Chicago/Turabian StyleDu, Guang-Xin, Tao Zhou, Meng-Lin Guo, Ping Huang, Ya-Bin Deng, and Dong-Hui Li. 2018. "Development of Functional Phthalocyanine-Based Associate towards an Effective Fluorimetric Detection of Hg(II)" Molecules 23, no. 2: 418. https://doi.org/10.3390/molecules23020418
APA StyleDu, G.-X., Zhou, T., Guo, M.-L., Huang, P., Deng, Y.-B., & Li, D.-H. (2018). Development of Functional Phthalocyanine-Based Associate towards an Effective Fluorimetric Detection of Hg(II). Molecules, 23(2), 418. https://doi.org/10.3390/molecules23020418