3.1. Chemistry
All melting points were measured on a Gallenkamp melting point apparatus and are uncorrected. The IR spectra were recorded (KBr disk) on a 1650 FT-IR instrument (Perkin Elmer, Waltham, MA, USA). 1H-NMR (400 MHz) and 13C-NMR (100 MHz) spectra were recorded on a Varian spectrometer (Varian, Inc., Palo Alto, CA, USA) using DMSO-d6 or CDCl3 as solvent and TMS as an internal standard. Chemical shifts are reported in ppm. Coupling constants (J) are expressed in Hz. Mass spectra were recorded on a Varian MAT 112 spectrometer at 70 eV. Elemental analyses were performed at the Microanalytical Center, Cairo University, Egypt. The progress of the reactions was monitored by thin-layer chromatography (TLC) using aluminum sheets coated with silica gel F254 (Merck, Darmstadt, Germany), viewing under a short-wavelength UV lamp effected detection. All evaporations were carried out under reduced pressure at 40 °C.
Synthesis of 5-amino-3-(arylamino)-1H-pyrazole-4-carboxamides11a–c. Compounds of this series were prepared according to the literature procedure.
5-Amino-3-(4-methoxyphenylamino)-N-phenyl-1H-pyrazole-4-carboxamide (
11a). White crystals; m.p. 175–177 °C [
29].
5-Amino-3-(4-methoxyphenylamino)-N-(4-methylphenyl)-1H-pyrazole-4-carboxamide (
11b). White crystals; m.p. 198–200 °C [
29].
5-Amino-3-(4-methoxyphenylamino)-N-(4-chlorophenyl)-1H-pyrazole-4-carboxamide (
11c). White crystals; m.p. 190–192 °C [
29].
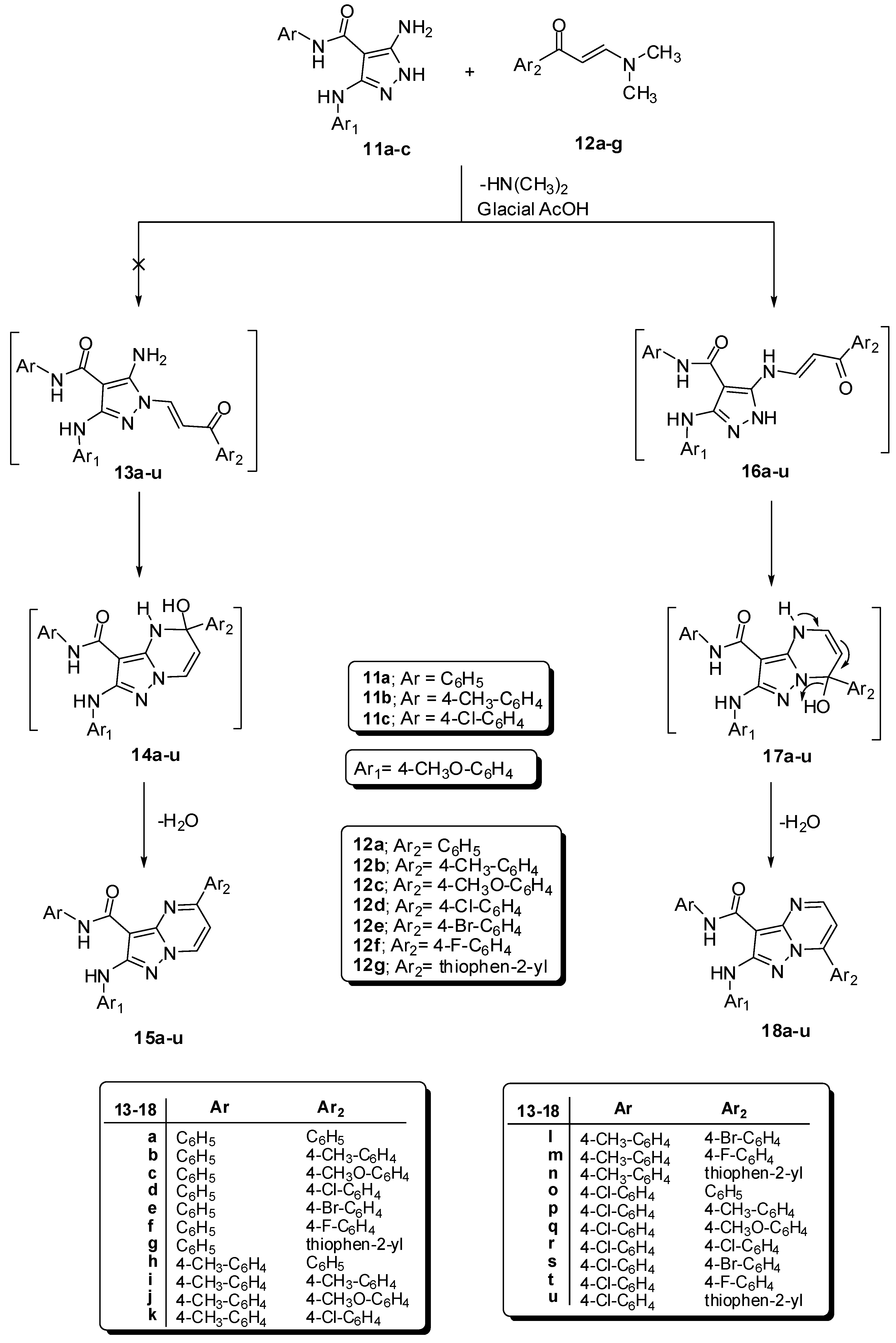
General Procedure for Synthesis of7-aryl-2-(arylamino)pyrazolo[1,5-a]pyrimidine-3-carboxamides18a–u. A mixture of compounds 11a–c (0.01 mol) with enaminones 12a–g {e.g., 3-(dimethylamino)-1-phenylprop-2-en-1-one (12a), 3-(dimethylamino)-1-(4-methylphenyl)prop-2-en-1-one (12b), 3-(dimethylamino)-1-(4-methoxyphenyl)prop-2-en-1-one (12c), 1-(4-chlorophenyl)-3-(dimethyl-amino)prop-2-en-1-one (12d), 1-(4-bromophenyl)-3-(dimethylamino)prop-2-en-1-one (12e), 3-(dimethylamino)-1-(4-fluorophenyl)prop-2-en-1-one (12f) or 3-(dimethylamino)-1-(thiophen-2-yl)prop-2-en-1-one (12g)} (0.01 mol) in glacial acetic acid (25 mL), the reaction mixture was refluxed for 1 h and then left to cool. The solid product was filtered off, washed with ethanol, dried and finally recrystallized from DMF/H2O to afford the corresponding pyrazolo[1,5-a]pyrimidine derivatives 18a–u.
2-(4-Methoxyphenylamino)-N,7-diphenylpyrazolo[1,5-a]pyrimidine-3-carboxamide (18a). Yellow crystals, m.p. 218–220 °C, yield (72%). IR (KBr) νmax/cm−1 3346 (NH), 1658 (C=O). 1H-NMR (CDCl3, 400 MHz, δ ppm): 3.80 (s, 3H, OCH3), 6.88 (d, 2H, J = 9.0 Hz,ArH), 6.96 (d, 1H, J = 4.8 Hz, pyrimidine), 7.12 (t, 1H, ArH), 7.36–7.42 (m, 5H, ArH), 7.62 (d, 2H, J = 9.0 Hz,ArH), 7.74 (d, 2H, J = 8.4 Hz,ArH), 8.11 (d, 2H, J = 8.3 Hz,ArH), 8.49 (d, 1H, J = 4.8 Hz, pyrimidine), 9.40 (s, 1H, NH), 10.05 (s, 1H, NH). 13C-NMR (CDCl3, 100 MHz, δ ppm): 55.7 (C, OCH3), 87.8 (C, C3-pyrazolopyrimidine), 107.0 (C, C6-pyrazolopyrimidine), 114.4, 119.2, 120.2, 123.7, 127.7, 129.1, 129.5, 129.6 (14C, Ar), 134.1 (C, C3a-pyrazolopyrimidine), 138.8, 142.4, 146.7 (3C, Ar), 147.9 (C, C7-pyrazolopyrimidine), 149.6 (C, Ar), 154.5 (C, C2-pyrazolopyrimidine), 157.8 (C, C5-pyrazolopyrimidine), 163.3 (C=O). MS (m/z, %): 435 (M+, 73.86). Anal. Calcd. (%) for C26H21N5O2 (435.48): C, 71.71; H, 4.86; N, 16.08. Found: C, 71.80; H, 4.81; N, 16.00%.
2-(4-Methoxyphenylamino)-N-phenyl-7-(4-methylphenyl)-pyrazolo[1,5-a]pyrimidine-3-carboxamide (18b). Yellow crystals, m.p. 219–221 °C, yield (77%). IR (KBr) νmax/cm−1 3337 (NH), 1658 (C=O). 1H-NMR (CDCl3, 400 MHz, δ ppm): 2.49 (s, 3H, CH3), 3.80 (s, 3H, OCH3), 6.87 (d, 2H, J = 8.9 Hz, ArH), 6.91 (d, 1H, J = 4.7 Hz, pyrimidine), 7.12 (t, 1H, ArH), 7.36 (d, 2H, J = 8.3 Hz, ArH), 7.38 (t, 2H, ArH), 7.60 (d, 2H, J = 8.9 Hz, ArH), 7.73 (d, 2H, J = 7.6 Hz, ArH), 8.08 (d, 2H, J = 8.1 Hz,ArH), 8.43 (d, 1H, J = 4.7 Hz, pyrimidine), 9.38 (s, 1H, NH), 10.01 (s, 1H, NH). 13C-NMR (CDCl3, 100 MHz, δ ppm): 21.8 (C, CH3), 55.7 (C, OCH3), 87.7 (C, C3-pyrazolopyrimidine), 107.0 (C, C6-pyrazolopyrimidine), 114.4, 119.1, 120.1, 123.7, 127.6, 129.1, 129.4, 129.6 (14C, Ar), 134.1 (C, C3a-pyrazolopyrimidine), 138.8, 142.3, 146.6 (3C, Ar), 147.8 (C, C7-pyrazolopyrimidine), 149.6 (C, Ar), 154.4 (C, C2-pyrazolopyrimidine), 157.7 (C, C5-pyrazolopyrimidine), 163.3 (C=O). MS (m/z, %): 449 (M+, 67.43). Anal. Calcd. (%) for C27H23N5O2 (449.50): C, 72.14; H, 5.16; N, 15.58. Found: C, 72.10; H, 5.20; N, 15.60%.
7-(4-Methoxyphenyl)-2-(4-methoxyphenylamino)-N-phenylpyrazolo[1,5-a]pyrimidine-3-carboxamide (18c). Yellow crystals, m.p. 206–208 °C, yield (76%). IR (KBr) νmax/cm−1 3340 (NH), 1646 (C=O). 1H-NMR (CDCl3, 400 MHz, δ ppm): 3.80 (s, 3H, OCH3), 3.91 (s, 3H, OCH3), 6.87 (d, 2H, J = 8.9 Hz, ArH), 6.89 (d, 1H, J = 4.8 Hz, pyrimidine), 7.05 (d, 2H, J = 8.8 Hz, ArH), 7.11 (t, 1H, ArH), 7.37 (t, 2H, ArH), 7.60 (d, 2H, J = 8.9 Hz, ArH), 7.72 (d, 2H, J = 7.6 Hz, ArH), 8.18 (d, 2H, J = 8.8 Hz, ArH), 8.40 (d, 1H, J = 4.8 Hz, pyrimidine), 9.36 (s, 1H, NH), 10.02 (s, 1H, NH). 13C-NMR (CDCl3, 100 MHz, δ ppm): 55.5 (C, OCH3), 55.6 (C, OCH3), 87.4 (C, C3-pyrazolopyrimidine), 106.4 (C, C6-pyrazolopyrimidine), 113.9, 114.2, 119.0, 120.0, 122.4, 123.5, 128.9, 131.3 (14C, Ar), 134.0 (C, C3a-pyrazolopyrimidine), 138.6, 146.0 (2C, Ar), 147.7 (C, C7-pyrazolopyrimidine), 149.3 (C, Ar), 154.3 (C, C2-pyrazolopyrimidine), 157.5 (C, C5-pyrazolopyrimidine), 162.2 (C, Ar), 163.2 (C=O). MS (m/z, %): 465 (M+, 69.48). Anal. Calcd. (%) for C27H23N5O3 (465.50): C, 69.66; H, 4.98; N, 15.04. Found: C, 69.70; H, 4.95; N, 15.00%.
7-(4-Chlorophenyl)-2-(4-methoxyphenylamino)-N-phenylpyrazolo[1,5-a]pyrimidine-3-carboxamide (18d). Yellow crystals, m.p. 252–253 °C, yield (72%). IR (KBr) νmax/cm−1 3343 (NH), 1648 (C=O). 1H-NMR (CDCl3, 400 MHz, δ ppm): 3.81 (s, 3H, OCH3), 6.88 (d, 2H, J = 9.0 Hz, ArH), 6.94 (d, 1H, J = 4.7 Hz, pyrimidine), 7.13 (t, 1H, ArH), 7.39 (t, 2H, ArH), 7.58 (d, 4H, J = 8.8 Hz, ArH), 7.74 (d, 2H, J = 8.6 Hz, ArH), 8.15 (d, 2H, J = 8.7 Hz, ArH), 8.52 (d, 1H, J = 4.7 Hz, pyrimidine), 9.42 (s, 1H, NH), 9.99 (s, 1H, NH). 13C-NMR (CDCl3, 100 MHz, δ ppm): 55.7 (C, OCH3), 88.0 (C, C3-pyrazolopyrimidine), 107.0 (C, C6-pyrazolopyrimidine), 114.4, 119.2, 120.2, 123.8, 129.1, 129.1, 130.9, 131.8 (14C, Ar), 133.9 (C, C3a-pyrazolopyrimidine), 134.6, 138.0, 138.7 (3C, Ar), 145.3 (C, C7-pyrazolopyrimidine), 149.7 (C, Ar), 154.6 (C, C2-pyrazolopyrimidine), 157.9 (C, C5-pyrazolopyrimidine), 163.2 (C=O). MS (m/z, %): 469 (M+, 78.23). Anal. Calcd. (%) for C26H20ClN5O2 (469.92): C, 66.45; H, 4.29; N, 14.90. Found: C, 66.40; H, 4.30; N, 14.95%.
7-(4-Bromophenyl)-2-(4-methoxyphenylamino)-N-phenylpyrazolo[1,5-a]pyrimidine-3-carboxamide (18e). Yellow crystals, m.p. 278–280 °C, yield (69%). IR (KBr) νmax/cm−1 3365 (NH), 1650 (C=O). 1H-NMR (DMSO-d6, 400 MHz, δ ppm): 3.73 (s, 3H, OCH3), 6.93 (d, 2H, J = 9.0 Hz, ArH), 7.12 (t, 1H, ArH), 7.39 (t, 2H, ArH), 7.41 (d, 1H, J = 4.8 Hz, pyrimidine), 7.59 (d, 2H, J = 9.0 Hz, ArH), 7.73 (d, 2H, J = 7.6 Hz, ArH), 7.90 (d, 2H, J = 8.7 Hz, ArH), 8.20 (d, 2H, J = 8.7 Hz, ArH), 8.74 (d, 1H, J = 4.8 Hz, pyrimidine), 9.26 (s, 1H, NH), 10.03 (s, 1H, NH). 13C-NMR (DMSO-d6, 100 MHz, δ ppm): 55.7 (C, OCH3), 87.6 (C, C3-pyrazolopyrimidine), 106.9 (C, C6-pyrazolopyrimidine), 114.4, 119.1, 120.5, 123.3, 129.4, 129.8, 131.0, 131.6 (14C, Ar), 133.7 (C, C3a-pyrazolopyrimidine), 133.4, 136.1, 138.7 (3C, Ar), 145.2 (C, C7-pyrazolopyrimidine), 149.5 (C, Ar), 154.8 (C, C2-pyrazolopyrimidine), 157.1 (C, C5-pyrazolopyrimidine), 163.7 (C=O). MS (m/z, %): 514 (M+, 81.26). Anal. Calcd. (%) for C26H20BrN5O2 (514.37): C, 60.71; H, 3.92; N, 13.62. Found: C, 60.65; H, 3.97; N, 13.65%.
7-(4-Fluorophenyl)-2-(4-methoxyphenylamino)-N-phenylpyrazolo[1,5-a]pyrimidine-3-carboxamide (18f). Yellow crystals, m.p. 237–239 °C, yield (70%). IR (KBr) νmax/cm−1 3343 (NH), 1647 (C=O). 1H-NMR (DMSO-d6, 400 MHz, δ ppm): 3.73 (s, 3H, OCH3), 6.91 (d, 2H, J = 9.0 Hz, ArH), 7.11 (t, 1H, ArH), 7.37 (d, 1H, J = 4.9 Hz, pyrimidine), 7.39 (d, 2H, J = 7.6 Hz, ArH), 7.52 (t, 2H, ArH), 7.58 (d, 2H, J = 9.0 Hz, ArH), 7.71 (d, 2H, J = 8.6 Hz, ArH), 8.31 (d, 2H, J = 8.9 Hz, ArH), 8.71 (d, 1H, J = 4.8 Hz, pyrimidine), 9.23 (s, 1H, NH), 10.01 (s, 1H, NH). 13C-NMR (DMSO-d6, 100 MHz, δ ppm): 55.2 (C, OCH3), 86.7 (C, C3-pyrazolopyrimidine), 108.3 (C, C6-pyrazolopyrimidine), 114.3, 115.7, 115.9, 118.8, 119.4, 123.5, 126.4, 129.1 (14C, Ar), 132.4 (C, C3a-pyrazolopyrimidine), 133.3, 138.4 (2C, Ar), 145.0 (C, C7-pyrazolopyrimidine), 147.1 (C, Ar), 151.1 (C, C2-pyrazolopyrimidine), 154.1 (C, C5-pyrazolopyrimidine), 156.6 (C, Ar), 162.2 (C=O). MS (m/z, %): 453 (M+, 87.33). Anal. Calcd. (%) for C26H20FN5O2 (453.47): C, 68.86; H, 4.45; N, 15.44. Found: C, 68.95; H, 4.40; N, 15.50%.
2-(4-Methoxyphenylamino)-N-phenyl-7-(thiophen-2-yl)pyrazolo[1,5-a]pyrimidine-3-carboxamide (18g). Yellow crystals, m.p. 233–235 °C, yield (71%). IR (KBr) νmax/cm−1 3356 (NH), 1652 (C=O). 1H-NMR (DMSO-d6, 400 MHz, δ ppm): 3.78 (s, 3H, OCH3), 7.03 (d, 2H, J = 8.4 Hz, ArH), 7.12 (t, 1H, ArH), 7.40 (t, 2H, ArH), 7.47 (t, 1H, J = 4.9 Hz, thiophene), 7.74 (d, 2H, J = 7.8 Hz, ArH), 7.84 (d, 2H, J = 8.5 Hz, ArH), 7.90 (d, 1H, J = 4.6 Hz, pyrimidine), 8.28 (d, 1H, J = 4.4 Hz, thiophene), 8.58 (d, 1H, J = 2.8 Hz, thiophene), 8.71 (d, 1H, J = 4.4 Hz, pyrimidine), 9.44 (s, 1H, NH), 10.07 (s, 1H, NH). 13C-NMR (DMSO-d6, 100 MHz, δ ppm): 55.7 (C, OCH3), 86.8 (C, C3-pyrazolopyrimidine), 107.3 (C, C6-pyrazolopyrimidine), 114.8, 119.2, 120.8, 126.3 (7C, Ar), 128.1, 129.8 (2C, thiophene), 130.1 (2C, Ar), 133.2 (C, thiophene), 133.9 (C, C3a-pyrazolopyrimidine), 134.5, 137.1 (2C, Ar), 139.4 (C, thiophene), 147.3 (C, Ar), 150.8 (C, C2-pyrazolopyrimidine), 154.4 (C, C5-pyrazolopyrimidine), 156.3 (C, C7-pyrazolopyrimidine), 162.9 (C=O). MS (m/z, %): 441 (M+, 100). Anal. Calcd. (%) for C24H19N5O2S (441.50): C, 65.29; H, 4.34; N, 15.86. Found: C, 65.35; H, 4.30; N, 15.90%.
2-(4-Methoxyphenylamino)-7-phenyl-N-(4-methylphenyl)-pyrazolo[1,5-a]pyrimidine-3-carboxamide (18h). Yellow crystals, m.p. 251–253 °C, yield (76%). IR (KBr) νmax/cm−1 3374 (NH), 1660 (C=O). 1H-NMR (CDCl3, 400 MHz, δ ppm): 2.35 (s, 3H, CH3), 3.80 (s, 3H, OCH3), 6.88 (d, 2H, J = 9.0 Hz, ArH), 6.95 (d, 1H, J = 4.8 Hz, pyrimidine), 7.18 (d, 2H, J = 8.2 Hz, ArH), 7.40 (d, 2H, J = 8.2 Hz, ArH), 7.60–7.64 (m, 5H, ArH), 8.11 (d, 2H, J = 8.2 Hz, ArH), 8.48 (d, 1H, J = 4.8 Hz, pyrimidine), 9.42 (s, 1H, NH), 9.97 (s, 1H, NH).13C-NMR (CDCl3, 100 MHz, δ ppm): 21.0 (C, CH3), 55.7 (C, OCH3), 87.8 (C, C3-pyrazolopyrimidine), 106.9 (C, C6-pyrazolopyrimidine), 114.4, 119.1, 120.2, 127.7, 129.4, 129.6, 133.3 (14C, Ar), 134.2 (C, C3a-pyrazolopyrimidine), 136.2, 142.4, 146.6 (3C, Ar), 147.8 (C, C7-pyrazolopyrimidine), 149.6 (C, Ar), 154.4 (C, C2-pyrazolopyrimidine), 157.8 (C, C5-pyrazolo-pyrimidine), 163.2 (C=O). MS (m/z, %): 449 (M+, 92.11). Anal. Calcd. (%) for C27H23N5O2 (449.50): C, 72.14; H, 5.16; N, 15.58. Found: C, 72.20; H, 5.11; N, 15.50%.
2-(4-Methoxyphenylamino)-N,7-di-(4-methylphenyl)pyrazolo[1,5-a]pyrimidine-3-carboxamide (18i). Yellow crystals, m.p. 261 °C, yield (74%). IR (KBr) νmax/cm−1 3293 (NH), 1642 (C=O). 1H-NMR (CDCl3, 400 MHz, δ ppm): 2.35 (s, 3H, CH3), 2.49 (s, 3H, CH3), 3.80 (s, 3H, OCH3), 6.87 (d, 2H, J = 9.0 Hz, ArH), 6.91 (d, 1H, J = 4.8 Hz, pyrimidine), 7.18 (d, 2H, J = 8.2 Hz, ArH), 7.37 (d, 2H, J = 8.0 Hz, ArH), 7.60 (d, 2H, J = 9.0 Hz, ArH), 7.61 (d, 2H, J = 8.5 Hz, ArH), 8.08 (d, 2H, J = 8.3 Hz, ArH), 8.43 (d, 1H, J = 4.8 Hz, pyrimidine), 9.40 (s, 1H, NH), 9.94 (s, 1H, NH). 13C-NMR (CDCl3, 100 MHz, δ ppm): 21.0 (C, CH3), 21.8 (C, CH3), 55.7 (C, OCH3), 87.7 (C, C3-pyrazolopyrimidine), 106.9 (C, C6-pyrazolopyrimidine), 114.4, 119.1, 120.1, 127.6, 129.4, 129.5, 129.6, 133.2 (14C, Ar), 134.1 (C, C3a-pyrazolopyrimidine), 136.2, 142.3, 146.5 (3C, Ar), 147.7 (C, C7-pyrazolopyrimidine), 149.5 (C, Ar), 154.4 (C, C2-pyrazolopyrimidine), 157.7 (C, C5-pyrazolopyrimidine), 163.2 (C=O). MS (m/z, %): 463 (M+, 100). Anal. Calcd. (%) for C28H25N5O2 (463.53): C, 72.55; H, 5.44; N, 15.11. Found: C, 72.55; H, 5.44; N, 15.11%.
7-(4-Methoxyphenyl)-2-(4-methoxyphenylamino)-N-(4-methylphenyl)pyrazolo[1,5-a]pyrimidine-3-carboxamide (18j). Yellow crystals, m.p. 244–245 °C, yield (75%). IR (KBr) νmax/cm−1 3368 (NH), 1649 (C=O). 1H-NMR (CDCl3, 400 MHz, δ ppm): 2.35 (s, 3H, CH3), 3.80 (s, 3H, OCH3), 3.94 (s, 3H, OCH3), 6.88 (d, 2H, J = 9.0 Hz, ArH), 6.93 (d, 1H, J = 4.8 Hz, pyrimidine), 7.10 (d, 2H, J = 9.0 Hz, ArH), 7.18 (d, 2H, J = 8.2 Hz, ArH), 7.61–7.64 (m, 4H, ArH), 8.22 (d, 2H, J = 8.9 Hz, ArH), 8.45 (d, 1H, J = 4.8 Hz, pyrimidine), 9.42 (s, 1H, NH), 9.98 (s, 1H, NH). 13C-NMR (CDCl3, 100 MHz, δ ppm): 21.0 (C, CH3), 55.7 (C, OCH3), 55.7 (C, OCH3), 87.7 (C, C3-pyrazolopyrimidine), 106.5 (C, C6-pyrazolopyrimidine), 114.1, 114.4, 119.1, 120.2, 122.7, 129.6, 131.5, 133.2 (14C, Ar), 134.2 (C, C3a-pyrazolopyrimidine), 136.2, 146.2 (2C, Ar), 147.9 (C, C7-pyrazolopyrimidine), 149.5 (C, Ar), 154.4 (C, C2-pyrazolopyrimidine), 157.8 (C, C5-pyrazolopyrimidine), 162.3 (C, Ar), 163.3 (C=O). MS (m/z, %): 479 (M+, 92.77). Anal. Calcd. (%) for C28H25N5O3 (479.53): C, 70.13; H, 5.25; N, 14.60. Found: C, 70.05; H, 5.30; N, 14.55%.
7-(4-Chlorophenyl)-2-(4-methoxyphenylamino)-N-(4-methylphenyl)pyrazolo[1,5-a]pyrimidine-3-carboxamide (18k). Yellow crystals, m.p. 267–269 °C, yield (71%). IR (KBr) νmax/cm−1 3315 (NH), 1662 (C=O). 1H-NMR (CDCl3, 400 MHz, δ ppm): 2.35 (s, 3H, CH3), 3.81 (s, 3H, OCH3), 6.88 (d, 2H, J = 9.0 Hz, ArH), 6.93 (d, 1H, J = 4.7 Hz, pyrimidine), 7.19 (d, 2H, J = 8.2 Hz, ArH), 7.57–7.62 (m, 6H, ArH), 8.14 (d, 2H, J = 8.7 Hz, ArH), 8.50 (d, 1H, J = 4.7 Hz, pyrimidine), 9.43 (s, 1H, NH), 9.91 (s, 1H, NH). 13C-NMR (CDCl3, 100 MHz, δ ppm): 21.0 (C, CH3), 55.7 (C, OCH3), 88.0 (C, C3-pyrazolopyrimidine), 107.0 (C, C6-pyrazolopyrimidine), 114.4, 119.2, 120.2, 129.1, 129.6, 130.9, 131.7, 133.4 (14C, Ar), 134.1 (C, C3a-pyrazolopyrimidine), 134.4, 136.0, 137.9 (3C, Ar), 146.1 (C, C7-pyrazolopyrimidine), 149.7 (C, Ar), 154.6 (C, C2-pyrazolopyrimidine), 159.4 (C, C5-pyrazolopyrimidine), 163.1 (C=O). MS (m/z, %): 483 (M+, 87.08). Anal. Calcd. (%) for C27H22ClN5O2 (483.95): C, 67.01; H, 4.58; N, 14.47. Found: C, 67.10; H, 4.50; N, 14.50%.
7-(4-Bromophenyl)-2-(4-methoxyphenylamino)-N-(4-methylphenyl)pyrazolo[1,5-a]pyrimidine-3-carboxamide (18l). Yellow crystals, m.p. 278–279 °C, yield (68%). IR (KBr) νmax/cm−1 3325 (NH), 1649 (C=O). 1H-NMR (DMSO-d6, 400 MHz, δ ppm): 2.30 (s, 3H, CH3), 3.74 (s, 3H, OCH3), 6.95 (d, 2H, J = 9.3 Hz, ArH), 7.20 (d, 2H, J = 8.6 Hz, ArH), 7.44 (d, 1H, J = 4.1 Hz, pyrimidine), 7.62 (d, 2H, J = 8.4 Hz, ArH), 7.63 (d, 2H, J = 7.8 Hz, ArH), 7.93 (d, 2H, J = 8.3 Hz, ArH), 8.22 (d, 2H, J = 8.3 Hz, ArH), 8.77 (d, 1H, J = 4.3 Hz, pyrimidine), 9.31 (s, 1H, NH), 9.98 (s, 1H, NH). 13C-NMR (DMSO-d6, 100 MHz, δ ppm): 21.0 (C, CH3), 55.7 (C, OCH3), 88.0 (C, C3-pyrazolopyrimidine), 107.0 (C, C6-pyrazolopyrimidine), 114.9, 119.2, 120.5, 125.2, 129.7, 129.4, 131.0, 133.5 (14C, Ar), 134.2 (C, C3a-pyrazolopyrimidine), 134.4, 135.9, 137.9 (3C, Ar), 146.0 (C, C7-pyrazolopyrimidine), 149.7 (C, Ar), 154.5 (C, C2-pyrazolopyrimidine), 159.3 (C, C5-pyrazolopyrimidine), 163.1 (C=O). MS (m/z, %): 528 (M+, 26.25). Anal. Calcd. (%) for C27H22BrN5O2 (528.40): C, 61.37; H, 4.20; N, 13.25. Found: C, 61.45; H, 4.16; N, 13.30%.
7-(4-Fluorophenyl)-2-(4-methoxyphenylamino)-N-(4-methylphenyl)pyrazolo[1,5-a]pyrimidine-3-carboxamide (18m). Yellow crystals, m.p. 255–257 °C, yield (69%). IR (KBr) νmax/cm−1 3329 (NH), 1652 (C=O). 1H-NMR (DMSO-d6, 400 MHz, δ ppm): 2.30 (s, 3H, CH3), 3.73 (s, 3H, OCH3), 6.93 (d, 2H, J = 9.0 Hz, ArH), 7.19 (d, 2H, J = 8.3 Hz, ArH), 7.39 (d, 1H, J = 4.8 Hz, pyrimidine), 7.54 (d, 2H, J = 8.9 Hz, ArH), 7.60 (d, 2H, J = 9.0 Hz, ArH), 7.62 (d, 2H, J = 8.4 Hz, ArH), 8.32 (d, 2H, J = 9.0 Hz, ArH), 8.74 (d, 1H, J = 4.8 Hz, pyrimidine), 9.27 (s, 1H, NH), 9.97 (s, 1H, NH). 13C-NMR (DMSO-d6, 100 MHz, δ ppm): 21.0 (C, CH3), 55.8 (C, OCH3), 87.9 (C, C3-pyrazolopyrimidine), 107.0 (C, C6-pyrazolopyrimidine), 114.6, 116.1, 119.5, 120.5, 129.5, 130.9, 131.7 (13C, Ar), 134.2 (C, C3a-pyrazolopyrimidine), 134.2, 136.1, 138.0 (3C, Ar), 146.2 (C, C7-pyrazolopyrimidine), 149.6 (C, Ar), 154.3 (C, C2-pyrazolopyrimidine), 159.0 (C, C5-pyrazolopyrimidine), 160.1 (C, Ar), 162.9 (C=O). MS (m/z, %): 467 (M+, 45.13). Anal. Calcd. (%) for C27H22FN5O2 (467.49): C, 69.37; H, 4.74; N, 14.98. Found: C, 69.30; H, 4.80; N, 15.05%.
2-(4-Methoxyphenylamino)-7-(thiophen-2-yl)-N-(4-methylphenyl)pyrazolo[1,5-a]pyrimidine-3-carboxamide (18n). Yellow crystals, m.p. 278–279 °C, yield (70%). IR (KBr) νmax/cm−1 3345 (NH), 1652 (C=O). 1H-NMR (DMSO-d6, 400 MHz, δ ppm): 2.30 (s, 3H, CH3), 3.78 (s, 3H, OCH3), 7.03 (d, 2H, J = 8.9 Hz, ArH), 7.20 (d, 2H, J = 8.1 Hz, ArH), 7.47 (t, 1H, thiophene), 7.62 (d, 2H, J = 8.0 Hz, ArH), 7.83 (d, 2H, J = 8.6 Hz, ArH), 7.90 (d, 1H, J = 3.6 Hz, pyrimidine), 8.28 (d, 1H, J = 4.7 Hz, thiophene), 8.59 (d, 1H, J = 2.3 Hz, thiophene), 8.70 (d, 1H, J = 4.8 Hz, pyrimidine), 9.46 (s, 1H, NH), 10.00 (s, 1H, NH). 13C-NMR (DMSO-d6, 100 MHz, δ ppm): 21.0 (C, CH3), 55.7 (C, OCH3), 87.5 (C, C3-pyrazolopyrimidine), 107.1 (C, C6-pyrazolopyrimidine), 114.1, 119.5, 120.3 (6C, Ar), 127.6, 128.1, 129.8 (3C, thiophene), 130.0 (2C, Ar), 133.6 (C, C3a-pyrazolopyrimidine), 133.5, 134.5, 137.1 (3C, Ar), 139.8 (C, thiophene), 147.8 (C, Ar), 151.5 (C, C2-pyrazolopyrimidine), 154.1 (C, C5-pyrazolopyrimidine), 157.2 (C, C7-pyrazolopyrimidine), 163.0 (C=O). MS (m/z, %): 455 (M+, 65.71). Anal. Calcd. (%) for C25H21N5O2S (455.53): C, 65.92; H, 4.65; N, 15.37. Found: C, 66.00; H, 4.60; N, 15.31%.
N-(4-Chlorophenyl)-2-(4-methoxyphenylamino)-7-phenylpyrazolo[1,5-a]pyrimidine-3-carboxamide (18o). Yellow crystals, m.p. 252–254 °C, yield (73%). IR (KBr) νmax/cm−1 3336 (NH), 1650 (C=O). 1H-NMR (DMSO-d6, 400 MHz, δ ppm): 3.72 (s, 3H, OCH3), 6.90 (d, 2H, J = 9.0 Hz, ArH), 7.40 (d, 1H, J = 4.8 Hz, pyrimidine), 7.44 (d, 2H, J = 8.8 Hz, ArH), 7.61 (d, 2H, J = 9.0 Hz, ArH), 7.68–7.70 (m, 3H, ArH), 7.78 (d, 2H, J = 8.9 Hz, ArH), 8.23 (d, 2H, J = 7.2 Hz, ArH), 8.75 (d, 1H, J = 4.8 Hz, pyrimidine), 9.20 (s, 1H, NH), 10.11 (s, 1H, NH). 13C-NMR (DMSO-d6, 100 MHz, δ ppm): 55.7 (C, OCH3), 87.9 (C, C3-pyrazolopyrimidine), 107.0 (C, C6-pyrazolopyrimidine), 114.5, 119.1, 120.2, 124.0, 128.4, 129.1, 130.9, 131.8 (13C, Ar), 134.0 (C, C3a-pyrazolopyrimidine), 134.6, 135.9, 138.0, 138.7 (4C, Ar), 145.6 (C, C7-pyrazolopyrimidine), 149.7 (C, Ar), 154.8 (C, C2-pyrazolopyrimidine), 158.0 (C, C5-pyrazolopyrimidine), 163.8 (C=O). MS (m/z, %): 469 (M+, 29.83). Anal. Calcd. (%) for C26H20ClN5O2 (469.92): C, 66.45; H, 4.29; N, 14.90. Found: C, 66.40; H, 4.35; N, 14.85%.
N-(4-Chlorophenyl)-2-(4-methoxyphenylamino)-7-(4-methylphenyl)pyrazolo[1,5-a]pyrimidine-3-carboxamide (18p). Yellow crystals, m.p. 261 °C, yield (75%). IR (KBr) νmax/cm−1 3322 (NH), 1658 (C=O). 1H-NMR (DMSO-d6, 400 MHz, δ ppm): 2.32 (s, 3H, CH3), 3.74 (s, 3H, OCH3), 6.93 (d, 2H, J = 7.6 Hz, ArH), 7.42 (d, 1H, J = 4.8 Hz, pyrimidine), 7.45 (d, 2H, J = 7.7 Hz, ArH), 7.51 (d, 2H, J = 8.6 Hz, ArH), 7.64 (d, 2H, J = 7.8 Hz, ArH), 7.79 (d, 2H, J = 8.4 Hz, ArH), 8.19 (d, 2H, J = 7.5 Hz, ArH), 8.74 (d, 1H, J = 4.8 Hz, pyrimidine), 9.23 (s, 1H, NH), 10.14 (s, 1H, NH). 13C-NMR (DMSO-d6, 100 MHz, δ ppm): 21.0 (C, CH3), 55.7 (C, OCH3), 87.5 (C, C3-pyrazolopyrimidine), 107.0 (C, C6-pyrazolopyrimidine), 114.4, 119.3, 120.9, 129.0, 129.6, 131.0, 131.7, 133.4 (14C, Ar), 134.0 (C, C3a-pyrazolopyrimidine), 134.3, 136.0, 137.9 (3C, Ar), 146.1 (C, C7-pyrazolopyrimidine), 149.7 (C, Ar), 154.5 (C, C2-pyrazolopyrimidine), 159.4 (C, C5-pyrazolopyrimidine), 163.1 (C=O). MS (m/z, %): 483 (M+, 22.71). Anal. Calcd. (%) for C27H22ClN5O2 (483.95): C, 67.01; H, 4.58; N, 14.47. Found: C, 67.10; H, 4.50; N, 14.55%.
N-(4-Chlorophenyl)-7-(4-methoxyphenyl)-2-(4-methoxyphenylamino)pyrazolo[1,5-a]pyrimidine-3-carboxamide (18q). Yellow crystals, m.p. 266–267 °C, yield (74%). IR (KBr) νmax/cm−1 3365 (NH), 1661 (C=O). 1H-NMR (DMSO-d6, 400 MHz, δ ppm): 3.74 (s, 3H, OCH3), 3.92 (s, 3H, OCH3), 6.95 (d, 2H, J = 8.8 Hz, ArH), 7.24 (d, 2H, J = 8.7 Hz, ArH), 7.40 (d, 1H, J = 4.7 Hz, pyrimidine), 7.44 (d, 2H, J = 8.7 Hz, ArH), 7.65 (d, 2H, J = 8.8 Hz, ArH), 7.78 (d, 2H, J = 8.6 Hz, ArH), 8.31 (d, 2H, J = 8.6 Hz, ArH), 8.70 (d, 1H, J = 4.7 Hz, pyrimidine), 9.22 (s, 1H, NH), 10.15 (s, 1H, NH). 13C-NMR (DMSO-d6, 100 MHz, δ ppm): 55.6 (C, OCH3), 55.7 (C, OCH3), 87.8 (C, C3-pyrazolopyrimidine), 106.4 (C, C6-pyrazolopyrimidine), 114.2, 114.6, 119.0, 120.5, 122.8, 129.5, 131.6 (13C, Ar), 133.1 (C, C3a-pyrazolopyrimidine), 134.8, 135.0, 136.1 (3C, Ar), 147.9 (C, C7-pyrazolopyrimidine), 149.4 (C, Ar), 154.5 (C, C2-pyrazolopyrimidine), 157.8 (C, C5-pyrazolopyrimidine), 161.5 (C, Ar), 163.2 (C=O). MS (m/z, %): 499 (M+, 18.46). Anal. Calcd. (%) for C27H22ClN5O3 (499.95): C, 64.86; H, 4.44; N, 14.01. Found: C, 64.95; H, 4.40; N, 14.05%.
N,7-bis(4-Chlorophenyl)-2-(4-methoxyphenylamino)pyrazolo[1,5-a]pyrimidine-3-carboxamide (18r). Yellow crystals, m.p. 282–284 °C, yield (70%). IR (KBr) νmax/cm−1 3317 (NH), 1653 (C=O). 1H-NMR (DMSO-d6, 400 MHz, δ ppm): 3.74 (s, 3H, OCH3), 6.94 (d, 2H, J = 8.8 Hz, ArH), 7.44 (d, 2H, J = 8.6 Hz, ArH), 7.45 (d, 1H, J = 3.8 Hz, pyrimidine), 7.61 (d, 2H, J = 8.8 Hz, ArH), 7.78 (d, 4H, J = 8.4 Hz, ArH), 8.29 (d, 2H, J = 8.6 Hz, ArH), 8.76 (d, 1H, J = 4.7 Hz, pyrimidine), 9.23 (s, 1H, NH), 10.10 (s, 1H, NH). 13C-NMR (DMSO-d6, 100 MHz, δ ppm): 55.8 (C, OCH3), 87.4 (C, C3-pyrazolopyrimidine), 106.9 (C, C6-pyrazolopyrimidine), 115.0, 119.3, 120.4, 129.1, 129.7, 129.9, 131.6 (13C, Ar), 133.1 (C, C3a-pyrazolopyrimidine), 133.8, 134.3, 136.0, 137.9 (4C, Ar), 146.0 (C, C7-pyrazolopyrimidine), 149.8 (C, Ar), 154.5 (C, C2-pyrazolopyrimidine), 159.4 (C, C5-pyrazolopyrimidine), 162.9 (C=O). MS (m/z, %): 504 (M+, 22.87). Anal. Calcd. (%) for C26H19Cl2N5O2 (504.37): C, 61.91; H, 3.80; N, 13.89. Found: C, 62.00; H, 3.75; N, 13.80%.
7-(4-Bromophenyl)-N-(4-chlorophenyl)-2-(4-methoxyphenylamino)pyrazolo[1,5-a]pyrimidine-3-carboxamide (18s). Yellow crystals, m.p. 275–277 °C, yield (67%). IR (KBr) νmax/cm−1 3327 (NH), 1648 (C=O). 1H-NMR (DMSO-d6, 400 MHz, δ ppm): 3.74 (s, 3H, OCH3), 6.94 (d, 2H, J = 7.3 Hz, ArH), 7.44 (d, 2H, J = 7.2 Hz, ArH), 7.45 (d, 1H, J = 4.4 Hz, pyrimidine), 7.61 (d, 2H, J = 7.1 Hz, ArH), 7.79 (d, 2H, J = 7.9 Hz, ArH), 7.92 (d, 2H, J = 7.3 Hz, ArH), 8.21 (d, 2H, J = 7.8 Hz, ArH), 8.76 (d, 1H, J = 4.3 Hz, pyrimidine), 9.23 (s, 1H, NH), 10.11 (s, 1H, NH). 13C-NMR (DMSO-d6, 100 MHz, δ ppm): 55.7 (C, OCH3), 87.6 (C, C3-pyrazolopyrimidine), 106.9 (C, C6-pyrazolopyrimidine), 114.4, 119.2, 120.2, 123.2, 129.1, 129.6, 130.7, 131.4 (14C, Ar), 132.7 (C, C3a-pyrazolopyrimidine), 134.2, 136.0, 137.5 (3C, Ar), 146.0 (C, C7-pyrazolopyrimidine), 149.8 (C, Ar), 154.5 (C, C2-pyrazolopyrimidine), 159.5 (C, C5-pyrazolopyrimidine), 163.2 (C=O). MS (m/z, %): 548 (M+, 20.55). Anal. Calcd. (%) for C26H19BrClN5O2 (548.82): C, 56.90; H, 3.49; N, 12.76. Found: C, 57.00; H, 3.40; N, 12.80%.
N-(4-Chlorophenyl)-7-(4-fluorophenyl)-2-(4-methoxyphenylamino)pyrazolo[1,5-a]pyrimidine-3-carboxamide (18t). Yellow crystals, m.p. 251–252 °C, yield (67%). IR (KBr) νmax/cm−1 3339 (NH), 1651 (C=O). 1H-NMR (DMSO-d6, 400 MHz, δ ppm): 3.74 (s, 3H, OCH3), 6.93 (d, 2H, J = 7.8 Hz, ArH), 7.44 (d, 2H, J = 8.7 Hz, ArH), 7.45 (d, 1H, J = 4.2 Hz, pyrimidine), 7.56 (d, 2H, J = 8.0 Hz, ArH), 7.62 (d, 2H, J = 8.0 Hz, ArH), 7.78 (d, 2H, J = 7.4 Hz, ArH), 8.33 (d, 2H, J = 8.1 Hz, ArH), 8.75 (d, 1H, J = 4.5 Hz, pyrimidine), 9.21 (s, 1H, NH), 10.10 (s, 1H, NH). 13C-NMR (DMSO-d6, 100 MHz, δ ppm): 55.8 (C, OCH3), 86.7 (C, C3-pyrazolopyrimidine), 108.3 (C, C6-pyrazolopyrimidine), 114.3, 115.9, 119.0, 119.4, 124.6, 128.1, 129.1 (13C, Ar), 132.3 (C, C3a-pyrazolopyrimidine), 133.2, 134.3, 138.4 (3C, Ar), 145.0 (C, C7-pyrazolopyrimidine), 147.3 (C, Ar), 151.1 (C, C2-pyrazolopyrimidine), 154.0 (C, C5-pyrazolopyrimidine), 156.6 (C, Ar), 162.3 (C=O). MS (m/z, %): 487 (M+, 21.30). Anal. Calcd. (%) for C26H19ClFN5O2 (487.91): C, 64.00; H, 3.93; N, 14.35. Found: C, 64.10; H, 4.00; N, 14.30%.
N-(4-Chlorophenyl)-2-(4-methoxyphenylamino)-7-(thiophen-2-yl)pyrazolo[1,5-a]pyrimidine-3-carboxamide (18u). Yellow crystals, m.p. 289–291 °C, yield (71%). IR (KBr) νmax/cm−1 3293 (NH), 1644 (C=O). 1H-NMR (DMSO-d6, 400 MHz, δ ppm): 3.78 (s, 3H, OCH3), 7.04 (d, 2H, J = 9.0 Hz, ArH), 7.33 (d, 2H, J = 9.2 Hz, ArH), 7.47 (t, 1H, thiophene), 7.79 (d, 2H, J = 9.1 Hz, ArH), 7.84 (d, 2H, J = 8.9 Hz, ArH), 7.91 (d, 1H, J = 4.8 Hz, pyrimidine), 8.29 (d, 1H, J = 5.1 Hz, thiophene), 8.60 (d, 1H, J = 2.9 Hz, thiophene), 8.71 (d, 1H, J = 5.7 Hz, pyrimidine), 9.39 (s, 1H, NH), 10.14 (s, 1H, NH). 13C-NMR (DMSO-d6, 100 MHz, δ ppm): 55.7 (C, OCH3), 87.8 (C, C3-pyrazolopyrimidine), 107.1 (C, C6-pyrazolopyrimidine), 114.0, 119.5, 120.4, (6C, Ar), 127.6, 128.2, 129.7 (3C, thiophene), 130.0 (2C, Ar), 133.1 (C, C3a-pyrazolopyrimidine), 133.6, 134.5, 136.9 (3C, Ar), 139.7 (C, thiophene), 148.0 (C, Ar), 151.4 (C, C2-pyrazolopyrimidine), 154.2 (C, C5-pyrazolopyrimidine), 157.2 (C, C7-pyrazolopyrimidine), 162.9 (C=O). MS (m/z, %): 475 (M+, 74.59). Anal. Calcd. (%) for C24H18ClN5O2S (475.95): C, 60.56; H, 3.81; N, 14.71. Found: C, 60.50; H, 3.90; N, 14.80%.
General Procedure for Synthesis of N-aryl-2-(arylamino)-8,8-dimethyl-6-oxo-6,7,8,9-tetrahydropyrazolo[1,5-a]quinazoline-3-carboxamides25a–c. A mixture of compounds 11a–c (0.01 mol) with 2-((dimethyl-amino)methylene)-5,5-dimethylcyclohexane-1,3-dione (19, 0.01 mol, 1.95 g) in glacial acetic acid (25 mL), the reaction mixture was refluxed for 1 h and then left to cool. The solid product was filtered off, washed with ethanol, dried and finally recrystallized from DMF/H2O to afford the corresponding pyrazolo[1,5-a]quinazolines 25a–c.
2-(4-Methoxyphenylamino)-8,8-dimethyl-6-oxo-N-phenyl-6,7,8,9-tetrahydropyrazolo[1,5-a]quinazoline-3-carboxamide (25a). Yellow crystals, m.p. 270–272 °C, yield (73%). IR (KBr) νmax/cm−1 3302 (NH), 1655 (C=O). 1H-NMR (CDCl3, 400 MHz, δ ppm): 1.24 (s, 6H, 2CH3), 2.58 (s, 2H, CH2), 3.31 (s, 2H, CH2), 3.82 (s, 3H, OCH3), 6.95 (d, 2H, J = 9.0 Hz, ArH), 7.14 (t, 1H, ArH), 7.39 (t, 2H, ArH), 7.64 (d, 2H, J = 9.0 Hz, ArH), 7.71 (d, 2H, J = 7.5 Hz, ArH), 8.99 (s, 1H, quinazoline), 9.48 (s, 1H, NH), 9.88 (s, 1H, NH). 13C-NMR (CDCl3, 100 MHz, δ ppm): 28.7 (2C, 2CH3), 32.7 (C, C8-quinazoline), 37.7 (C, CH2), 51.1 (C, CH2), 55.7 (C, OCH3), 90.9 (C, C3-quinazoline), 113.8 (C, C5a-quinazoline), 114.5, 119.6, 120.3, 124.2, 129.2 (9C, Ar), 133.4 (C, C3a-quinazoline), 138.2, 147.1, 148.6 (3C, Ar), 151.5 (C, C2-quinazoline), 155.0 (C, C5-quinazoline), 159.1 (C=O), 162.6 (C, C9a-quinazoline), 193.9 (C=O). MS (m/z, %): 455 (M+, 71.06). Anal. Calcd. (%) for C26H25N5O3 (455.51): C, 68.56; H, 5.53; N, 15.37. Found: C, 68.50; H, 5.55; N, 15.40%.
2-(4-Methoxyphenylamino)-8,8-dimethyl-6-oxo-N-(4-methylphenyl)-6,7,8,9-tetrahydropyrazolo[1,5-a]quinazoline-3-carboxamide (25b). Yellow crystals, m.p. 266–268 °C, yield (77%). IR (KBr) νmax/cm−1 3316 (NH), 1659 (C=O). 1H-NMR (CDCl3, 400 MHz, δ ppm): 1.19 (s, 6H, 2CH3), 2.34 (s, 3H, CH3), 2.52 (s, 2H, CH2), 3.22 (s, 2H, CH2), 3.81 (s, 3H, OCH3), 6.91 (d, 2H, J = 9.0 Hz, ArH), 7.16 (d, 2H, J = 8.2 Hz, ArH), 7.54 (d, 2H, J = 8.3 Hz, ArH), 7.58 (d, 2H, J = 9.0 Hz, ArH), 8.90 (s, 1H, quinazoline), 9.41 (s, 1H, NH), 9.72 (s, 1H, NH). 13C-NMR (CDCl3, 100 MHz, δ ppm): 21.0 (C, CH3), 28.7 (2C, 2CH3), 32.6 (C, C8-quinazoline), 37.5 (C, CH2), 50.9 (C, CH2), 55.6 (C, OCH3), 90.7 (C, C3-quinazoline), 113.7 (C, C5a-quinazoline), 114.4, 119.4, 120.1, 129.6 (8C, Ar), 133.4 (C, C3a-quinazoline), 133.7, 135.7, 146.8, 148.5 (4C, Ar), 151.5 (C, C2-quinazoline), 154.8 (C, C5-quinazoline), 158.8 (C=O), 162.3 (C, C9a-quinazoline), 194.0 (C=O). MS (m/z, %): 469 (M+, 93.88). Anal. Calcd. (%) for C27H27N5O3 (469.53): C, 69.07; H, 5.80; N, 14.92. Found: C, 69.15; H, 5.75; N, 15.00%.
N-(4-Chlorophenyl)-2-(4-methoxyphenylamino)-8,8-dimethyl-6-oxo-6,7,8,9-tetrahydropyrazolo[1,5-a]quinazoline-3-carboxamide (25c). Yellow crystals, m.p. 291–293 °C, yield (72%). IR (KBr) νmax/cm−1 3299 (NH), 1662 (C=O). 1H-NMR (DMSO-d6, 400 MHz, δ ppm): 1.16 (s, 6H, 2CH3), 2.59 (s, 2H, CH2), 3.36 (s, 2H, CH2), 3.76 (s, 3H, OCH3), 6.98 (d, 2H, J = 8.8 Hz, ArH), 7.45 (d, 2H, J = 8.6 Hz, ArH), 7.72 (d, 2H, J = 8.6 Hz, ArH), 7.76 (d, 2H, J = 8.8 Hz, ArH), 8.95 (s, 1H, quinazoline), 9.30 (s, 1H, NH), 10.01 (s, 1H, NH). 13C-NMR (DMSO-d6, 100 MHz, δ ppm): 28.7 (2C, 2CH3), 32.6 (C, C8-quinazoline), 37.6 (C, CH2), 50.0 (C, CH2), 55.7 (C, OCH3), 90.8 (C, C3-quinazoline), 113.7 (C, C5a-quinazoline), 114.4, 119.3, 120.1, 129.3 (8C, Ar), 133.4 (C, C3a-quinazoline), 133.8, 136.3, 146.9, 148.1 (4C, Ar), 151.5 (C, C2-quinazoline), 154.8 (C, C5-quinazoline), 158.9 (C=O), 162.4 (C, C9a-quinazoline), 193.9 (C=O). MS (m/z, %): 489 (M+, 63.07). Anal. Calcd. (%) for C26H24ClN5O3 (489.95): C, 63.74; H, 4.94; N, 14.29. Found: C, 63.80; H, 5.00; N, 14.20%.