Indole Derivatives as Cyclooxygenase Inhibitors: Synthesis, Biological Evaluation and Docking Studies
Abstract
:1. Introduction
2. Results and Discussion
2.1. Anti-Inflammatory Activity
2.2. Analgesic Activity
2.3. Ulcerogenic Activity
2.4. Compound S3 Biological Characterization
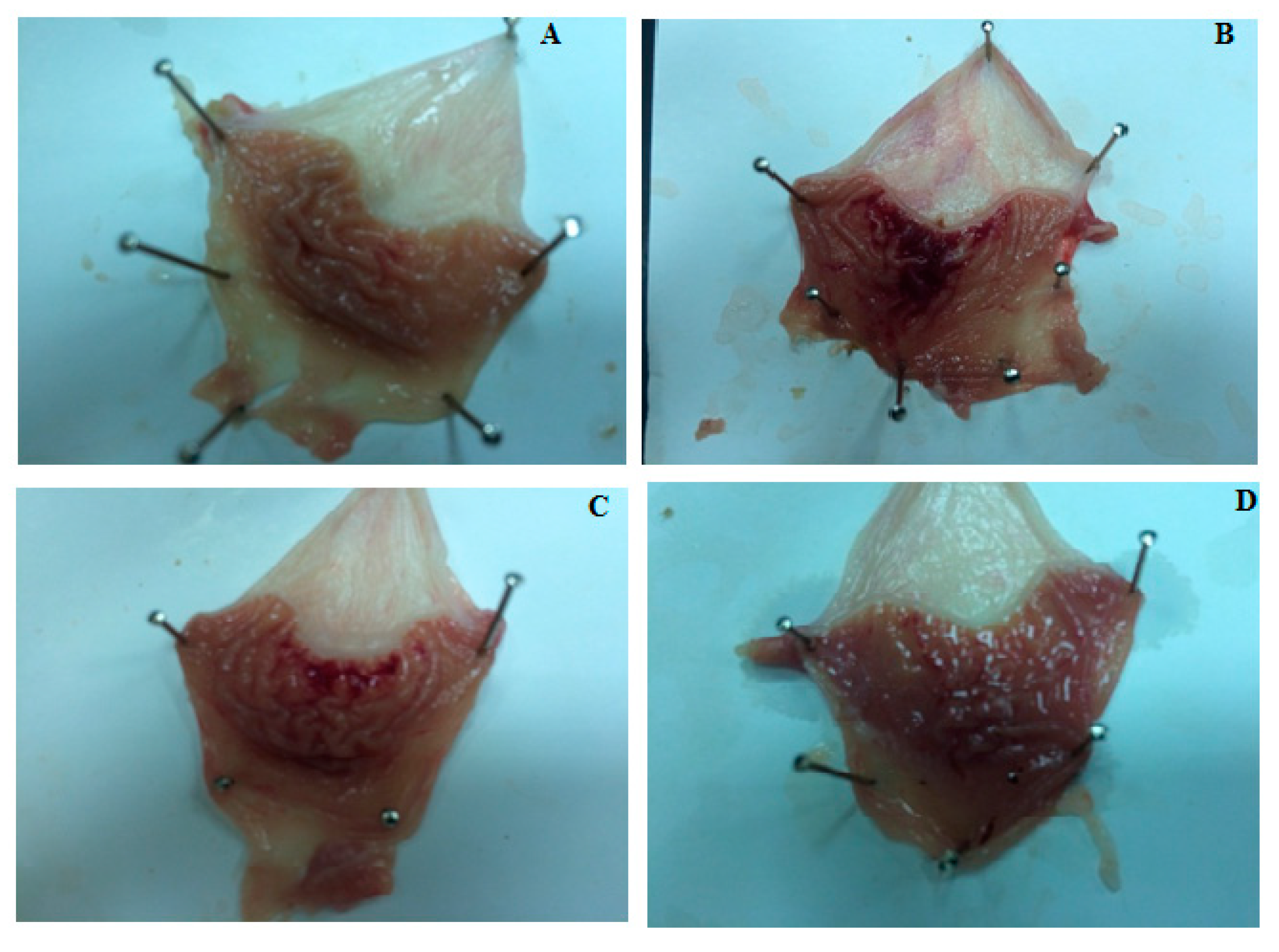
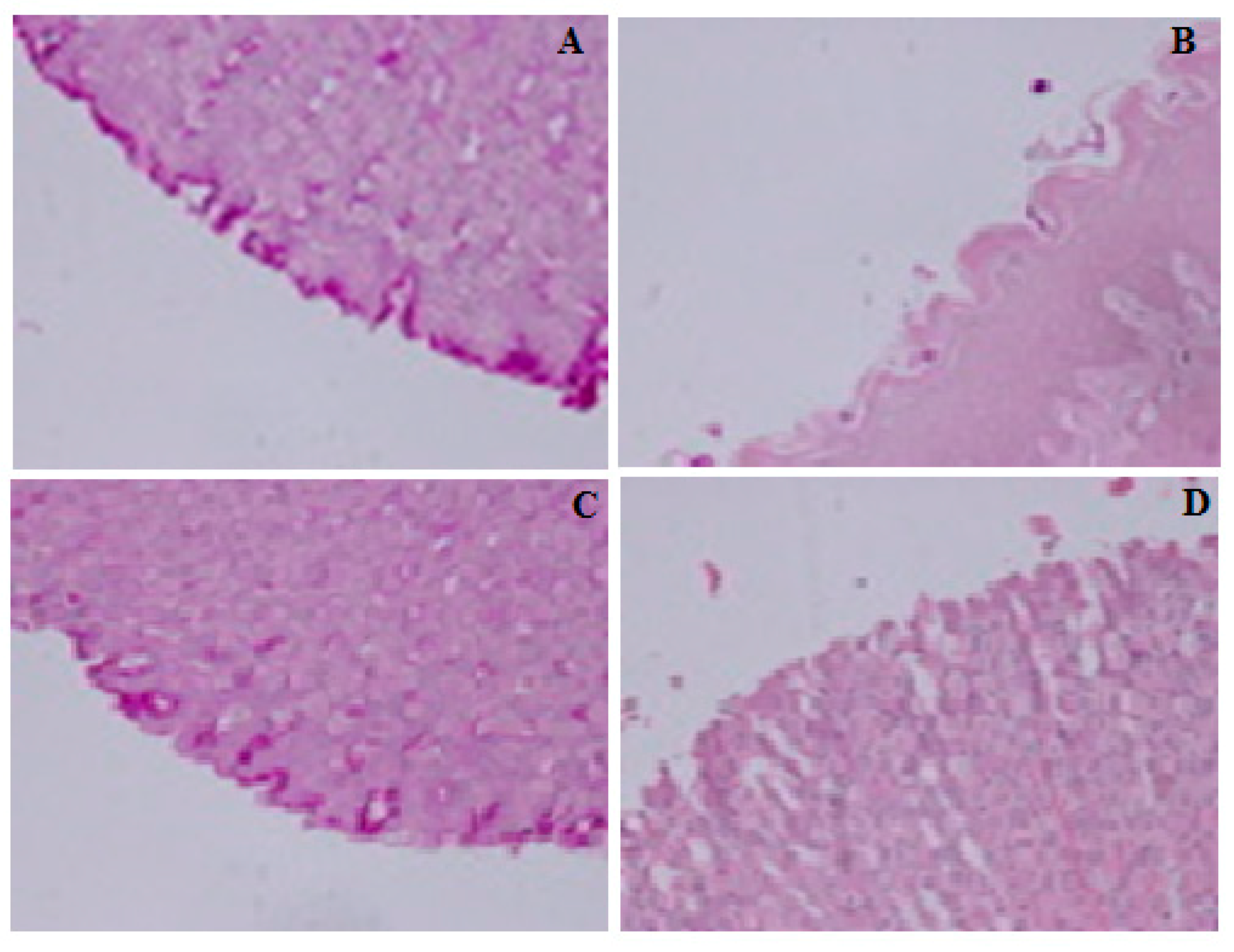
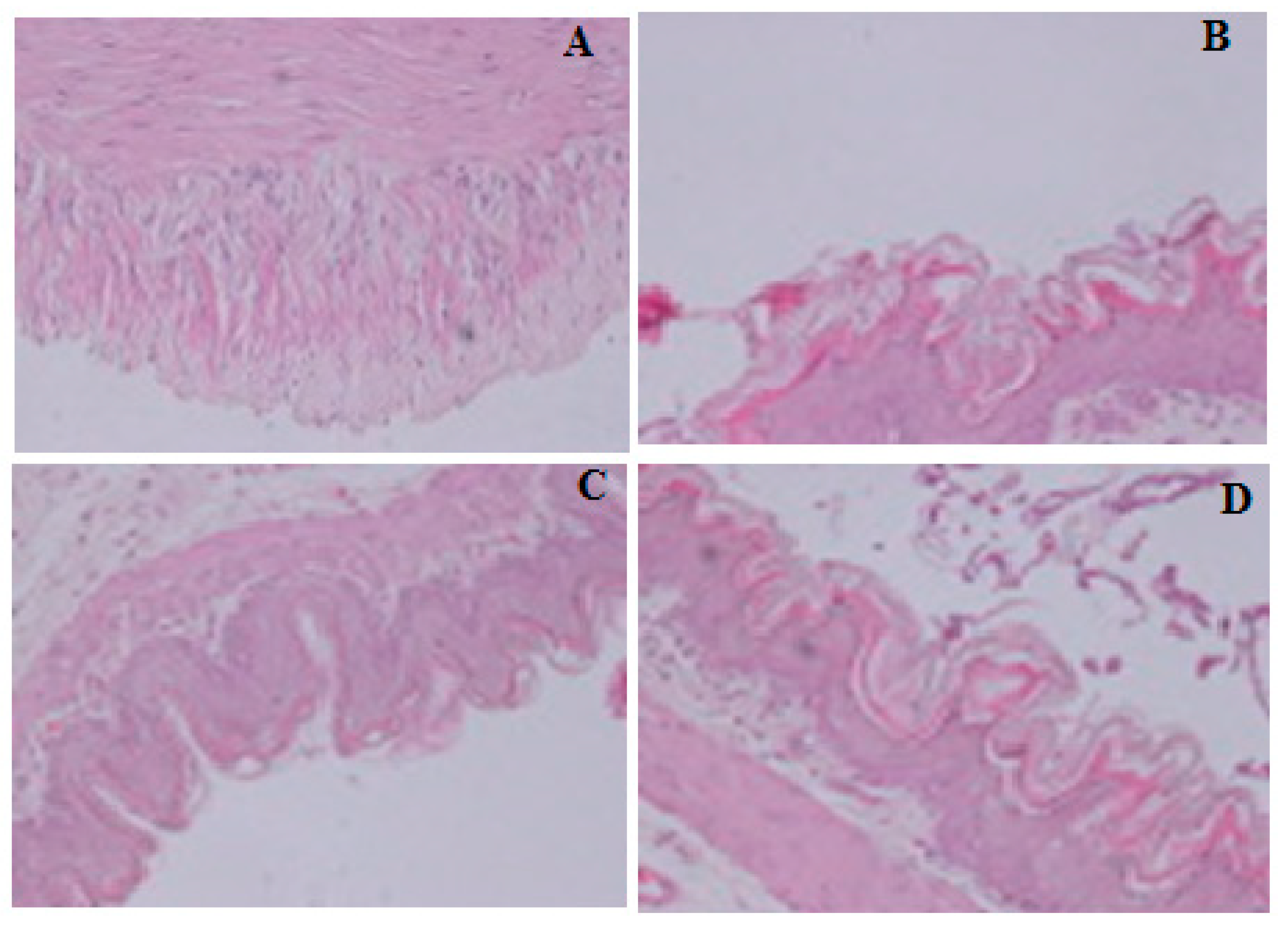
2.5. Gastro-Protective Effect of Compound S3
2.6. Toxicity of Compound S3
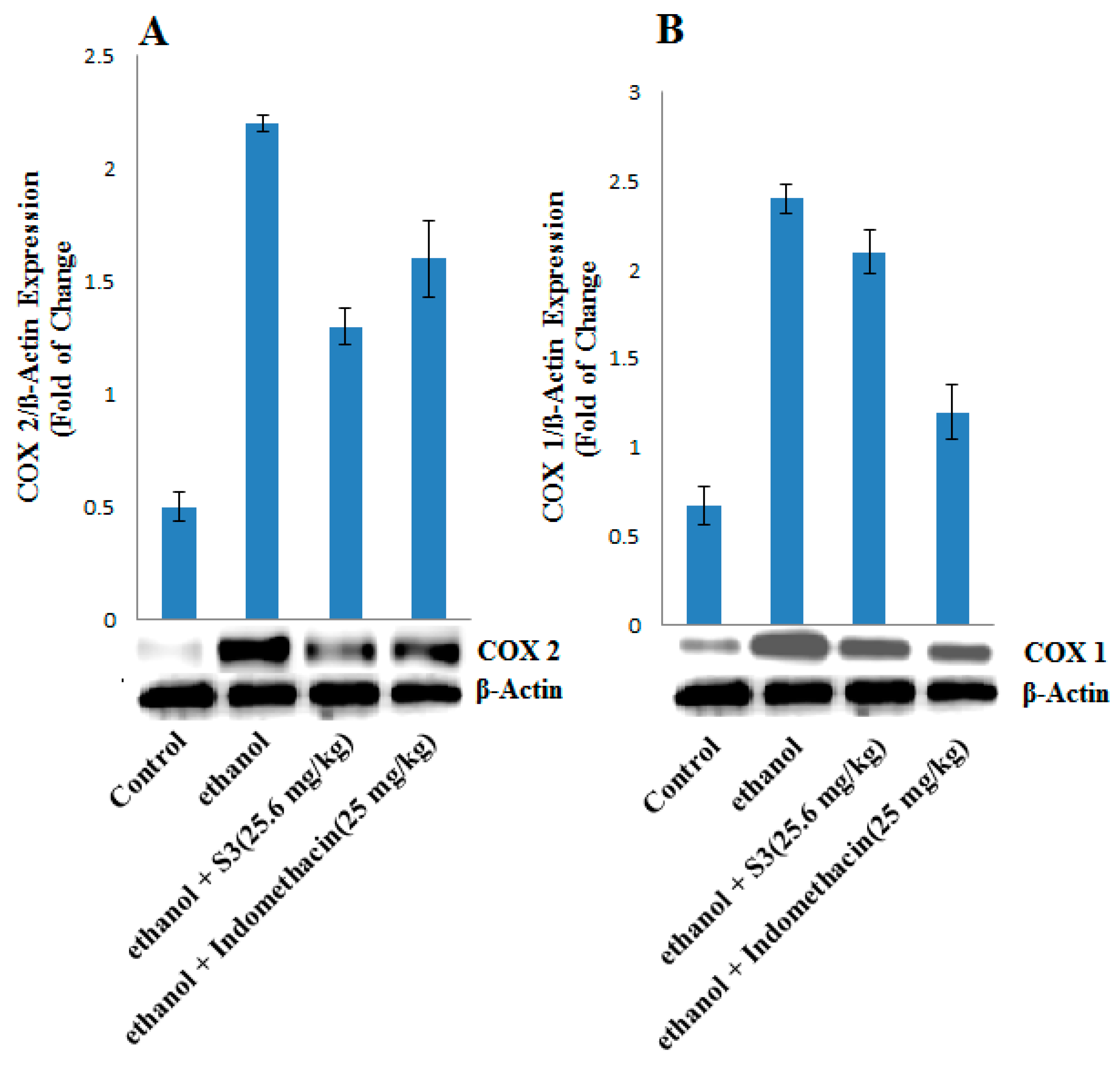
2.7. COX-1 and COX-2 Protein Expression
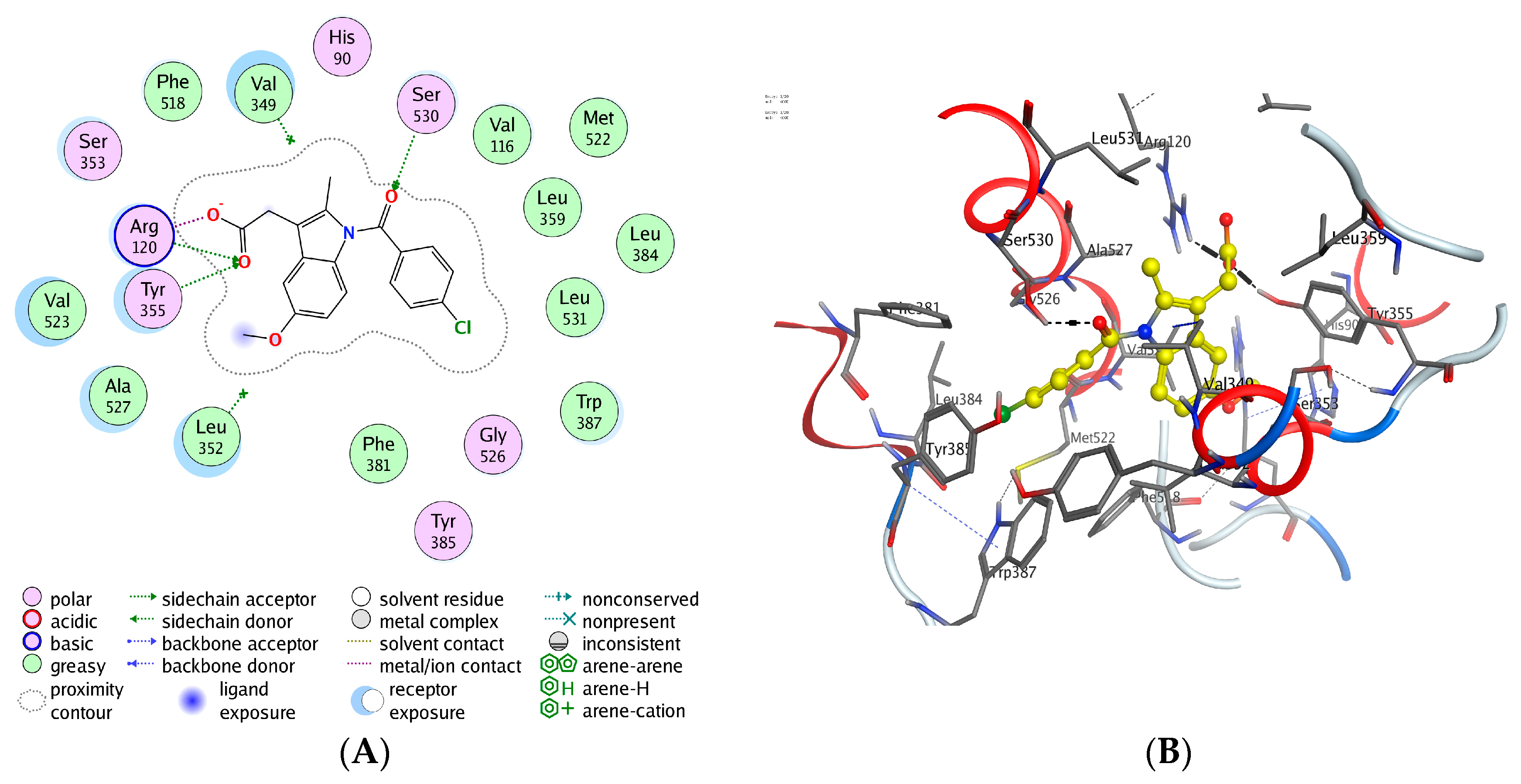
2.8. Docking Studies of Compounds (S1–S18) to the COX-1/COX-2
3. Material and Methods
3.1. Experimental Section
3.2. Synthesis of 2-(6-Methoxy-2-methyl-1H-indol-3-yl) Acetohydrazide (1)
3.3. General Procedure for the Synthesis of 2-(5-Methoxy-2-methyl-1-indol-3yl)-N-[(E)-Substituted Phenyl methylidine] Aceto Hydrazide Derivatives (S1–S18).
3.4. Anti-Inflammatory Activity
3.5. Analgesic Activity
3.6. Ulcerogenic Activity
3.7. Lipid Peroxidation
3.8. Ethanol Induced Ulcer Model
3.9. Determination of LD50
3.10. Western Blot
3.11. Docking Studies of Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Shuttleworth, S.J.; Bailey, S.G.; Townsend, P.A. Histone deacetylase inhibitors: New promise in the treatment of immune and inflammatory diseases. Curr. Drug Targets 2010, 11, 1430–1438. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Levy, B. Success of prostaglandin E2 in structure-function is a challenge for structure-based therapeutics. Proc. Natl. Acad. Sci. USA 2003, 100, 8609–8611. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.R.; Vyas, P.; Attur, M.; Leszczynska-Piziak, J.; Patel, I.R.; Weissmann, G.; Abramson, S.B. The mode of action of aspirin-like drugs: Effect on inducible nitric oxide synthase. Proc. Natl. Acad. Sci. USA 1995, 92, 7926–7930. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, M.; Kodela, R.; Duvalsaint, P.L.; Kashfi, K. Gastrointestinal safety, chemotherapeutic potential, and classic pharmacological profile of NOSH-naproxen (AVT-219) a dual NO- and H2S-releasing hybrid. Pharmacol. Res. Perspect. 2016, 4, e00224. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.I. Non-steroidal anti-inflammatory drugs and gastrointestinal damage-problems and solutions. Postgrad. Med. J. 2001, 77, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Gierse, J.; Kurumbail, R.; Walker, M.; Hood, B.; Monahan, J.; Pawlitz, J.; Stegeman, R.; Stevens, A.; Kiefer, J.; Koboldt, C.; et al. Mechanism of inhibition of novel COX-2 inhibitors. Adv. Exp. Med. Biol. 2002, 507, 365–369. [Google Scholar] [PubMed]
- Almansa, C.; Alfon, J.; de Arriba, A.F.; Cavalcanti, F.L.; Escamilla, I.; Gómez, L.A.; Miralles, A.; Soliva, R.; Bartrolí, J.; Carceller, E.; et al. Synthesis and structure-activity relationship of a new series of COX-2 selective inhibitors: 1,5-diarylimidazoles. J. Med. Chem. 2003, 46, 3463–3475. [Google Scholar] [CrossRef] [PubMed]
- Amir, M.; Kumar, S. Anti-inflammatory and gastro sparing activity of some new indomethacin derivatives. Arch. Pharm. 2005, 338, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Abuelizz, H.A.; Al-Salahi, R.; Al-Asri, J.; Mortier, J.; Marzouk, M.; Ezzeldin, E.; Ali, A.A.; Khalil, M.G.; Wolber, G.; Ghabbour, H.A.; et al. Synthesis, crystallographic characterization, molecular docking and biological activity of isoquinoline derivatives. Chem. Cent. J. 2017, 11, 103–121. [Google Scholar] [CrossRef] [PubMed]
- Abuelizz, H.A.; Hassane, A.E.; Marzouk, M.; Ezzeldin, E.; Ali, A.A.; Al-Salahi, R. Molecular modeling, enzyme activity, anti-inflammatory and antiarthritic activities of newly synthesized quinazoline derivatives. Future Med. Chem. 2017, 9, 1995–2009. [Google Scholar] [CrossRef] [PubMed]
- Lamie, P.F.; Ali, W.A.M.; Bazgier, V.; Rárová, L. Novel N-substituted indole Schiff bases as dual inhibitors of cyclooxygenase-2 and 5-lipoxygenase enzymes: Synthesis, biological activities in vitro and docking study. Eur. J. Med. Chem. 2016, 123, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Abdellatif, K.R.; Lamie, P.F.; Omar, H.A. 3-methyl-2-phenyl-1-substituted-indole derivatives as indomethacin analogs: Design, synthesis and biological evaluation as potential anti-inflammatory and analgesic agents. J. Enzyme Inhib. Med Chem. 2016, 31, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Rani, P.; Srivastava, V.K.; Kumar, A. Synthesis and antiinflammatory activity of heterocyclic indole derivatives. Eur. J. Med. Chem. 2004, 39, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.A.; Al-Omar, M.A.; Ansari, M.A.; Zoheir, K.M.A.; Imam, F.; Attia, S.M.; Bakheet, S.A.; Nadeem, A.; Korashy, H.M.; Voronkov, A.; et al. Design and synthesis of N-aryl-phthalimides as inhibitors of glucocorticoid-induced TNF receptor-related protein, pro-inflammatory mediators and cytokines in carrageenan-induced lung inflammation. J. Med. Chem. 2015, 58, 8850–8867. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.A.; Al-Omar, M.A. Synthesis, characterization and in vitro anti Mycobacterium tuberculosis activity of terpene schiff bases. Med. Chem. Res. 2013, 22, 4522–4528. [Google Scholar] [CrossRef]
- Bhat, M.A.; Al-Omar, M.A. Synthesis, characterization and in vivo anticonvulsant screening of schiff bases of phthalimide. Acta Pol. Pharm. Drug Res. 2011, 68, 375–380. [Google Scholar]
- Siddiqui, N.; Bhat, M.A.; Khan, S.A.; Ahsan, W.; Alam, M.S. Synthesis and in vivo anticonvulsant screening of coumarin incorporated Schiff bases of 1,3,4-oxadiazoles. J. Chin. Chem. Soc. 2008, 55, 1326–1331. [Google Scholar] [CrossRef]
- Bhat, M.A.; Al-Omar, M.A.; Raish, M.; Ansari, M.A.; Abuelizz, H.A. Cyclooxygenase Inhibitors. U.S. patent 9,808,443 B1, 7 November 2017. [Google Scholar]
- Winter, C.A.; Risley, E.A.; Nuss, G.W. Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc. Soc. Exp. Biol. Med. 1962, 111, 544–547. [Google Scholar] [CrossRef] [PubMed]
- Eddy, N.B.; Leimbach, D.J. Synthetic analgesics. II. Dithie-nylbutenyl- and dithienylbutylamines. J. Pharmacol. Exper. Therap. 1953, 107, 385–393. [Google Scholar]
- Cioli, V.; Putzolu, S.; Rossi, V.; Scorza Barcellona, P.; Corradino, C. The role of direct tissue contact in the production of gastrointestinal ulcers by anti-inflammatory drugs in rats. Toxicol. Appl. Pharmacol. 1979, 50, 283–289. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Rafatullah, S.; Tariq, M.; Al-Yahya, M.A.; Mossa, J.S.; Ageel, A.M. Evaluation of turmeric (Curcuma longa) for gastric and duodenal antiulcer activity in rats. J. Ethnopharmacol. 1990, 29, 25–34. [Google Scholar] [CrossRef]
- Kauffman, G.L., Jr.; Grossman, M.I. Prostaglandin and cimetidine inhibit the formation of ulcers produced by parenteral salicylates. Gastroenterology 1978, 75, 1099–1102. [Google Scholar] [PubMed]
- Karber, G. Beitrag zur kollektiven behandlung pharmakologischer reihenversuche. Arch. Exptl. Pathol. Pharmakol. 1931, 162, 480–483. [Google Scholar] [CrossRef]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Biotechnology 1992, 24, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Kurumbail, R.G.; Stevens, A.M.; Gierse, J.K.; McDonald, J.J.; Stegeman, A.R.; Pak, Y.G.; Gildehaus, D.; Iyashiro, M.J.; Penning, T.D.; Seibert, K.; et al. Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature 1996, 384, 644–648. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds (S1–S18) in pure form are available from authors. |


| Treatments | Increase in Paw Volume (mm) | % Inhibition | Potency | ||
|---|---|---|---|---|---|
| After 2 h | After 3 h | After 2 h | After 3 h | ||
| S1 | 0.53 ± 0.04 | 0.53 ± 0.04 *** | 43.95 | 44.31 | 0.57 |
| S2 | 0.81 ± 0.03 | 0.79 ± 0.02 *** | 14.71 | 17.93 | 0.21 |
| S3 | 0.36 ± 0.04 *** | 0.37 ±0.04 *** | 61.99 | 61.20 | 0.79 |
| S4 | 0.43 ± 0.01 *** | 0.43 ± 0.02 *** | 54.46 | 55 | 0.71 |
| S5 | 0.82 ± 0.07 | 0.85 ± 0.06 | 13.48 | 11.89 | 0.16 |
| S6 | 0.46 ± 0.04 *** | 0.46 ± 0.05 *** | 51.31 | 51.89 | 0.66 |
| S7 | 0.36 ± 0.02 *** | 0.36 ± 02 *** | 61.47 | 62.24 | 0.80 |
| S8 | 0.91 ± 0.04 | 0.95 ± 0.03 | 4.20 | 0 | 0.02 |
| S9 | 0.57 ± 0.02 *** | 0.51 ± 0.03 *** | 39.40 | 46.37 | 0.55 |
| S10 | 0.68 ± 0.02 *** | 0.69 ± 0.01 *** | 28.19 | 27.75 | 0.36 |
| S11 | 0.45 ± 0.02 *** | 0.43 ± 0.03 *** | 52.18 | 55 | 0.69 |
| S12 | 0.81 ± 0.03 * | 0.79 ± 0.03 ** | 14.71 | 18.27 | 0.21 |
| S13 | 0.76 ± 0.33 ** | 0.82 ± 0.04 * | 19.96 | 14.65 | 0.22 |
| S14 | 0.35 ± 0.02 *** | 0.34 ± 0.02 *** | 62.69 | 63.69 | 0.82 |
| S15 | 0.88 ± 0.01 | 0.89 ± 0.09 | 7.35 | 7.06 | 0.09 |
| S16 | 0.78 ± 0.04 * | 0.79 ± 0.04 * | 17.68 | 17.41 | 0.22 |
| S17 | 0.49 ± 0.02 *** | 0.48 ± 0.02 *** | 47.81 | 50.34 | 0.63 |
| S18 | 0.69 ± 0.12 | 0.62 ± 0.05 *** | 27.49 | 35.68 | 0.40 |
| indomethacin | 0.20 ± 0.02 *** | 0.22 ± 0.02 *** | 77.23 | 76.89 | 1.00 |
| Control | 0.95 ± 0.02 | 0.96 ± 0.02 | - | - | - |
| Treatments | Pretreatment (0 h) | Post Treatment (3 h) | % Inhibition | Potency |
|---|---|---|---|---|
| Mean ± SE (Second) | Mean ± SE (Second) | |||
| S1 | 8.33 ± 0.49 | 10.83 ± 0.79 * | 30 | 0.35 |
| S3 | 7.33 ± 0.42 | 11.83 ± 0.65 *** | 61.36 | 0.72 |
| S4 | 6.83 ± 0.30 | 7.33 ± 0.40 | 7.31 | 0.08 |
| S6 | 7.33 ± 0.33 | 8.33 ± 0.49 | 13.63 | 0.16 |
| S7 | 7.33 ± 0.42 | 10.83 ± 0.47 *** | 47.72 | 0.56 |
| S9 | 7.16 ± 0.30 | 11.16 ± 0.60 *** | 55.81 | 0.66 |
| S10 | 6.66 ± 0.33 | 10.83 ± 0.47 *** | 62.50 | 0.74 |
| S11 | 7.16 ± 0.47 | 10.16 ± 0.65 ** | 41.86 | 0.49 |
| S14 | 6.16 ± 0.30 | 10.50 ± 0.42 *** | 70.27 | 0.83 |
| S17 | 6.50 ± 0.22 | 10.50 ± 0.42 *** | 61.53 | 0.73 |
| S18 | 6.16 ± 0.30 | 7.66 ± 0.33 ** | 24.32 | 0.28 |
| indomethacin | 7.33 ± 0.42 | 13.50 ± 0.42 *** | 84.09 | 1.00 |
| Treatments | Ulcerogenic Activity (Index) | Nanomoles of MDA Content (Liver tissue) | Nanomoles of MDA Content (Kidney Tissue) | |||
|---|---|---|---|---|---|---|
| Mean ± SE | % Inhibition | Mean ± SEM/100 mg Tissue | % Change | Mean ± SEM/100 mg Tissue | % Change | |
| S1 | 0.582 ± 0.17 | 38.6 | 7.00 ± 0.25 * | 14.13 | 4.78 ± 0.14 *** | 28.66 |
| S2 | 0.362 ± 0.17 | 61.81 | 5.94 ± 0.25 *** | 27.22 | 5.89 ±0.14 ** | 12.10 |
| S3 | 0.116 ± 0.07 ** | 87.76 | 5.55 ± 0.18 *** | 31.93 | 6.83 ± 0.17 | 1.91 |
| S4 | 0.00 | 100 | 4.70 ± 5.75 *** | 40.32 | 4.01 ± 0.14 *** | 40.12 |
| S5 | 0.532 ± 0.08 | 43.88 | 6.08 ± 0.18 *** | 18.84 | 4.40 ± 0.27 *** | 40.12 |
| S6 | 0.00 | 100 | 4.35 ± 0.16 *** | 45.59 | 3.80 ± 0.12 *** | 43.31 |
| S7 | 0.432 ± 0.04 * | 54.30 | 6.32 ± 0.14 *** | 22.51 | 5.64 ± 0.20 ** | 15.92 |
| S8 | 0.132 ± 0.08 ** | 86.07 | 6.88 ± 0.37 * | 15.70 | 5.94 ± 0.18 * | 11.46 |
| S9 | 0.064 ± 0.03 ** | 93.24 | 7.82 ± 0.28 | 4.18 | 6.02 ± 0.19 * | 10.19 |
| S10 | 0.948 ± 0.17 | 0 | 8.58 ± 0.38 | 5.23 | 7.17 ± 0.20 | 7.00 |
| S11 | 0.00 | 100 | 4.74 ±0.28 *** | 41.88 | 4.14 ± 0.18 *** | 38.21 |
| S12 | 0.316 ± 0.09 * | 66.66 | 5.85 ± 0.18 *** | 28.27 | 5.29 ± 0.28 ** | 21.09 |
| S13 | 0.696 ± 0.09 | 26.58 | 5.29 ± 0.17 *** | 35.07 | 5.12 ± 0.16 *** | 23.56 |
| S14 | 0.616 ± 0.11 | 35.2 | 5.59 ± 0.19 *** | 41.36 | 5.68 ± 0.12 ** | 15.28 |
| S15 | 0.00 | 100 | 4.78 ± 0.06 *** | 41.36 | 4.01 ± 0.23 *** | 40.12 |
| S16 | 0.966 ± 0.16 | 0 | 7.56 ± 0.43 | 7.32 | 7.17 ± 0.22 | 7.00 |
| S17 | 0.00 | 100 | 4.65 ± 0.21 *** | 42.93 | 4.44 ± 0.17 *** | 33.75 |
| S18 | 0.598 ± 0.11 | 36.91 | 5.81 ± 0.41 *** | 28.79 | 5.64 ± 0.18 ** | 15.92 |
| indomethacin | 0.948 ± 0.21 | 8.16 ± 0.28 *** | 114.60 | 6.70 ± 0.20 *** | 98.73 | |
| Control | 0.00 | 100 | 3.80 ± 0.18 | - | 3.37 ± 0.12 | |
| Animal Groups | Treatment (5 mL/kg Dose) | Ulcer Index (mm2) |
|---|---|---|
| Mean ± SEM | ||
| 1 | normal control | 0 |
| 2 | ethanol group | 7.83 ± 0.33 |
| 3 | S3 (25.6 mg/kg) | 2.83 ± 0.87 |
| 4 | Indomethacin (25 mg/kg) | 12.34 ± 0.73 |
| Compd. No. | Amino Acid Residues | Interaction Type | Distance (Å) | Total Binding Energy (kcal·mol−1) | RMSD |
|---|---|---|---|---|---|
| indomethacin | SER 530 | H-acceptor | 2.92 | −8.86 | 0.748 |
| ARG 120 | H-acceptor | 2.84 | |||
| TYR 355 | H-acceptor | 2.84 | |||
| ARG 120 | ionic | 2.42 | |||
| ARG 120 | ionic | 3.04 | |||
| S1 | ARG 120 | H-acceptor | 2.87 | −7.12 | 2.008 |
| TYR 355 | H-acceptor | 2.87 | |||
| S2 | ARG 120 | H-acceptor | 2.78 | −7.73 | 2.008 |
| TYR 355 | H-acceptor | 3.04 | |||
| S3 | ARG 120 | H-acceptor | 2.80 | −7.80 | 2.0 |
| TYR 355 | H-acceptor | 3.08 | |||
| LEU 93 | Pi-H | 4.46 | |||
| S4 | ARG 120 | H-acceptor | 2.82 | −7.86 | 1.322 |
| TYR 355 | H-acceptor | 2.97 | |||
| S5 | ARG 120 | H-acceptor | 2.87 | −7.79 | 1.480 |
| TYR 355 | H-acceptor | 2.88 | |||
| S6 | ARG 120 | H-acceptor | 2.83 | −7.80 | 1.645 |
| TYR 355 | H-acceptor | 2.94 | |||
| S7 | TYR 355 | H-acceptor | 2.75 | −7.47 | 1.058 |
| S8 | ARG 120 | H-acceptor | 2.88 | −7.997 | 1.365 |
| TYR 355 | H-acceptor | 3.06 | |||
| S9 | ARG 120 | H-acceptor | 2.88 | −7.31 | 1.606 |
| TYR 355 | H-acceptor | 2.99 | |||
| S10 | ARG 120 | H-acceptor | 2.85 | −7.52 | 1.851 |
| TYR 355 | H-acceptor | 2.96 | |||
| S11 | ARG 120 | H-acceptor | 2.79 | −7.16 | 0.941 |
| VAL 523 | pi-H | 4.61 | |||
| S12 | ARG 120 | H-acceptor | 2.87 | −7.73 | 2.050 |
| TYR 355 | H-acceptor | 2.87 | |||
| S13 | TYR 355 | pi-H | 3.46 | −7.48 | 1.474 |
| VAL 523 | pi-H | 4.77 | |||
| S14 | ARG 120 | H-acceptor | 2.83 | −8.38 | 1.357 |
| TYR 355 | H-acceptor | 2.92 | |||
| S15 | ARG 120 | H-acceptor | 2.87 | −8.49 | 2.301 |
| TYR 355 | H-acceptor | 2.87 | |||
| LEU 93 | pi-H | 4.56 | |||
| S16 | ARG 120 | H-acceptor | 2.82 | −8.22 | 2.279 |
| TYR 355 | H-acceptor | 2.90 | |||
| S17 | TYR 355 | H-acceptor | 3.12 | −7.71 | 2.563 |
| S18 | ARG 120 | H-acceptor | 3.35 | −7.60 | 1.363 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhat, M.A.; Al-Omar, M.A.; Raish, M.; Ansari, M.A.; Abuelizz, H.A.; Bakheit, A.H.; Naglah, A.M. Indole Derivatives as Cyclooxygenase Inhibitors: Synthesis, Biological Evaluation and Docking Studies. Molecules 2018, 23, 1250. https://doi.org/10.3390/molecules23061250
Bhat MA, Al-Omar MA, Raish M, Ansari MA, Abuelizz HA, Bakheit AH, Naglah AM. Indole Derivatives as Cyclooxygenase Inhibitors: Synthesis, Biological Evaluation and Docking Studies. Molecules. 2018; 23(6):1250. https://doi.org/10.3390/molecules23061250
Chicago/Turabian StyleBhat, Mashooq Ahmad, Mohamed A. Al-Omar, Mohammad Raish, Mushtaq Ahmad Ansari, Hatem A. Abuelizz, Ahmed H. Bakheit, and Ahmed M. Naglah. 2018. "Indole Derivatives as Cyclooxygenase Inhibitors: Synthesis, Biological Evaluation and Docking Studies" Molecules 23, no. 6: 1250. https://doi.org/10.3390/molecules23061250







