Performance Comparisons of Polymer Semiconductors Synthesized by Direct (Hetero)Arylation Polymerization (DHAP) and Conventional Methods for Organic Thin Film Transistors and Organic Photovoltaics
Abstract
:1. Introduction
2. Polymer Semiconductors for Organic Thin Film Transistors
3. Polymer Semiconductors for Organic Photovoltaics
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Bao, Z.; Locklin, J. Organic Field-Effect Transistors; Optical Science and Engineering; Taylor & Francis: Abingdon, UK, 2007; ISBN 978-1-4200-0801-2. [Google Scholar]
- Arias, A.C.; MacKenzie, J.D.; McCulloch, I.; Rivnay, J.; Salleo, A. Materials and Applications for Large Area Electronics: Solution-Based Approaches. Chem. Rev. 2010, 110, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Dennler, G.; Scharber, M.C.; Brabec, C.J. Polymer-Fullerene Bulk-Heterojunction Solar Cells. Adv. Mater. 2009, 21, 1323–1338. [Google Scholar] [CrossRef]
- Lu, L.; Zheng, T.; Wu, Q.; Schneider, A.M.; Zhao, D.; Yu, L. Recent Advances in Bulk Heterojunction Polymer Solar Cells. Chem. Rev. 2015, 115, 12666–12731. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, Y.; Tan, H.S.; Guo, Y.; Di, C.-A.; Yu, G.; Liu, Y.; Lin, M.; Lim, S.H.; Zhou, Y.; et al. A stable solution-processed polymer semiconductor with record high-mobility for printed transistors. Sci. Rep. 2012, 2, 754. [Google Scholar] [CrossRef] [PubMed]
- Bucella, S.G.; Luzio, A.; Gann, E.; Thomsen, L.; McNeill, C.R.; Pace, G.; Perinot, A.; Chen, Z.; Facchetti, A.; Caironi, M. Macroscopic and high-throughput printing of aligned nanostructured polymer semiconductors for MHz large-area electronics. Nat. Commun. 2015, 6, 8394. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Kang, S.-J.; Dutta, G.K.; Han, Y.-K.; Shin, T.J.; Noh, Y.-Y.; Yang, C. A Thienoisoindigo-Naphthalene Polymer with Ultrahigh Mobility of 14.4 cm2/V·s That Substantially Exceeds Benchmark Values for Amorphous Silicon Semiconductors. J. Am. Chem. Soc. 2014, 136, 9477–9483. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Kyaw, A.K.K.; Perez, L.A.; Patel, S.; Wang, M.; Grimm, B.; Bazan, G.C.; Kramer, E.J.; Heeger, A.J. General Strategy for Self-Assembly of Highly Oriented Nanocrystalline Semiconducting Polymers with High Mobility. Nano Lett. 2014, 14, 2764–2771. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Li, S.; Yao, H.; Zhang, S.; Zhang, Y.; Yang, B.; Hou, J. Molecular Optimization Enables over 13% Efficiency in Organic Solar Cells. J. Am. Chem. Soc. 2017, 139, 7148–7151. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Xiong, Y.; Zhang, Q.; Li, S.; Wang, C.; Jiang, Z.; Hou, J.; You, W.; Ade, H. Solar Cells: Surpassing 10% Efficiency Benchmark for Nonfullerene Organic Solar Cells by Scalable Coating in Air from Single Nonhalogenated Solvent. Adv. Mater. 2018, 30, 1870054. [Google Scholar] [CrossRef]
- Lin, Y.; Zhao, F.; Prasad, S.K.K.; Chen, J.-D.; Cai, W.; Zhang, Q.; Chen, K.; Wu, Y.; Ma, W.; Gao, F.; et al. Balanced Partnership between Donor and Acceptor Components in Nonfullerene Organic Solar Cells with >12% Efficiency. Adv. Mater. 2018, 30, 1706363. [Google Scholar] [CrossRef] [PubMed]
- Bohra, H.; Wang, M. Direct C-H arylation: A “Greener” approach towards facile synthesis of organic semiconducting molecules and polymers. J. Mater. Chem. A 2017, 5, 11550–11571. [Google Scholar] [CrossRef]
- Burke, D.J.; Lipomi, D.J. Green chemistry for organic solar cells. Energy Environ. Sci. 2013, 6, 2053–2066. [Google Scholar] [CrossRef]
- Anastas, P.; Warner, J. Green Chemistry, Theory and Practice; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Organic Electronics for a Better Tomorrow: Innovation, Accessibility, Sustainability; Chemical Society and Sciences Summit (CS3): San Francisco, CA, USA, 2012.
- Kimbrough, R.D. Toxicity and health effects of selected organotin compounds: A review. Environ. Health Perspect. 1976, 14, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.M.; Jolly, R.A.; Linder, R.J. Boronic Acids and Derivatives—Probing the Structure–Activity Relationships for Mutagenicity. Org. Process Res. Dev. 2015, 19, 1507–1516. [Google Scholar] [CrossRef]
- Lombeck, F.; Komber, H.; Gorelsky, S.I.; Sommer, M. Identifying Homocouplings as Critical Side Reactions in Direct Arylation Polycondensation. ACS Macro Lett. 2014, 3, 819–823. [Google Scholar] [CrossRef]
- Kuwabara, J.; Fujie, Y.; Maruyama, K.; Yasuda, T.; Kanbara, T. Suppression of Homocoupling Side Reactions in Direct Arylation Polycondensation for Producing High Performance OPV Materials. Macromolecules 2016, 49, 9388–9395. [Google Scholar] [CrossRef]
- Rudenko, A.E.; Thompson, B.C. Optimization of direct arylation polymerization (DArP) through the identification and control of defects in polymer structure. J. Polym. Sci. Part A Polym. Chem. 2014, 53, 135–147. [Google Scholar] [CrossRef]
- Chen, S.; Lee, K.C.; Zhang, Z.-G.; Kim, D.S.; Li, Y.; Yang, C. An Indacenodithiophene–Quinoxaline Polymer Prepared by Direct Arylation Polymerization for Organic Photovoltaics. Macromolecules 2016, 49, 527–536. [Google Scholar] [CrossRef]
- Homyak, P.; Liu, Y.; Liu, F.; Russel, T.P.; Coughlin, E.B. Systematic Variation of Fluorinated Diketopyrrolopyrrole Low Bandgap Conjugated Polymers: Synthesis by Direct Arylation Polymerization and Characterization and Performance in Organic Photovoltaics and Organic Field-Effect Transistors. Macromolecules 2015, 48, 6978–6986. [Google Scholar] [CrossRef]
- Marzano, G.; Kotowski, D.; Babudri, F.; Musio, R.; Pellegrino, A.; Luzzati, S.; Po, R.; Farinola, G.M. Tin-Free Synthesis of a Ternary Random Copolymer for BHJ Solar Cells: Direct (Hetero)arylation versus Stille Polymerization. Macromolecules 2015, 48, 7039–7048. [Google Scholar] [CrossRef]
- Pouliot, J.-R.; Sun, B.; Leduc, M.; Najari, A.; Li, Y.; Leclerc, M. A high mobility DPP-based polymer obtained via direct (hetero)arylation polymerization. Polym. Chem. 2015, 6, 278–282. [Google Scholar] [CrossRef]
- Rudenko, A.E.; Latif, A.A.; Thompson, B.C. Influence of β-linkages on the morphology and performance of DArP P3HT–PC 61 BM solar cells. Nanotechnology 2014, 25, 014005. [Google Scholar] [CrossRef] [PubMed]
- Lombeck, F.; Marx, F.; Strassel, K.; Kunz, S.; Lienert, C.; Komber, H.; Friend, R.; Sommer, M. To branch or not to branch: C-H selectivity of thiophene-based donor-acceptor-donor monomers in direct arylation polycondensation exemplified by PCDTBT. Polym. Chem. 2017, 8, 4738–4745. [Google Scholar] [CrossRef]
- Bura, T.; Beaupre, S.; Legare, M.-A.; Quinn, J.; Rochette, E.; Blaskovits, J.T.; Fontaine, F.-G.; Pron, A.; Li, Y.; Leclerc, M. Direct heteroarylation polymerization: Guidelines for defect-free conjugated polymers. Chem. Sci. 2017, 8, 3913–3925. [Google Scholar] [CrossRef] [PubMed]
- Pouliot, J.-R.; Grenier, F.; Blaskovits, J.T.; Beaupré, S.; Leclerc, M. Direct (Hetero)arylation Polymerization: Simplicity for Conjugated Polymer Synthesis. Chem. Rev. 2016, 116, 14225–14274. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, S.; Allard, S.; Zilberberg, K.; Riedl, T.; Scherf, U. Direct arylation polycondensation as simplified alternative for the synthesis of conjugated (co)polymers. Top. Issue Conduct. Polym. 2013, 38, 1805–1814. [Google Scholar] [CrossRef]
- Yu, S.; Liu, F.; Yu, J.; Zhang, S.; Cabanetos, C.; Gao, Y.; Huang, W. Eco-friendly direct (hetero)-arylation polymerization: Scope and limitation. J. Mater. Chem. C 2017, 5, 29–40. [Google Scholar] [CrossRef]
- Kleinschmidt, A.T.; Root, S.E.; Lipomi, D.J. Poly(3-hexylthiophene) (P3HT): Fruit fly or outlier in organic solar cell research? J. Mater. Chem. A 2017, 5, 11396–11400. [Google Scholar] [CrossRef]
- Sugimoto, R.; Takeda, S.; Gu, H.B.; Yoshino, K. Preparation of Soluble Polythiophene Derivatives Utilizing Transition Metal Halides as Catalysts and Their Property. Chem. Express 1986, 1, 635–638. [Google Scholar]
- Mao, H.; Xu, B.; Holdcroft, S. Synthesis and structure-property relationships of regioirregular poly(3-hexylthiophenes). Macromolecules 1993, 26, 1163–1169. [Google Scholar] [CrossRef]
- Stein, P.C.; Botta, C.; Bolognesi, A.; Catellani, M. NMR study of the structural defects in poly(3-alkylthiophene)s: Influence of the polymerization method. Proc. Int. Conf. Sci. Technol. Synth. Met. 1995, 69, 305–306. [Google Scholar] [CrossRef]
- Leclerc, M.; Martinez, D.F.; Wegner, G. Structural analysis of poly(3-alkylthiophene)s. Makromol. Chem. 2003, 190, 3105–3116. [Google Scholar] [CrossRef]
- Sirringhaus, H.; Brown, P.J.; Friend, R.H.; Nielsen, M.M.; Bechgaard, K.; Langeveld-Voss, B.M.W.; Spiering, A.J.H.; Janssen, R.A.J.; Meijer, E.W.; Herwig, P.; et al. Two-dimensional charge transport in self-organized, high-mobility conjugated polymers. Nature 1999, 401, 685. [Google Scholar] [CrossRef]
- McCullough, R.D.; Lowe, R.D. Enhanced electrical conductivity in regioselectively synthesized poly(3-alkylthiophenes). J. Chem. Soc. Chem. Commun. 1992, 70–72. [Google Scholar] [CrossRef]
- McCullough, R.D.; Lowe, R.D.; Jayaraman, M.; Anderson, D.L. Design, synthesis, and control of conducting polymer architectures: Structurally homogeneous poly(3-alkylthiophenes). J. Org. Chem. 1993, 58, 904–912. [Google Scholar] [CrossRef]
- Chen, T.A.; Rieke, R.D. The first regioregular head-to-tail poly(3-hexylthiophene-2,5-diyl) and a regiorandom isopolymer: Nickel versus palladium catalysis of 2(5)-bromo-5(2)-(bromozincio)-3-hexylthiophene polymerization. J. Am. Chem. Soc. 1992, 114, 10087–10088. [Google Scholar] [CrossRef]
- Chen, T.-A.; Wu, X.; Rieke, R.D. Regiocontrolled Synthesis of Poly(3-alkylthiophenes) Mediated by Rieke Zinc: Their Characterization and Solid-State Properties. J. Am. Chem. Soc. 1995, 117, 233–244. [Google Scholar] [CrossRef]
- Guillerez, S.; Bidan, G. New convenient synthesis of highly regioregular poly(3-octylthiophene) based on the Suzuki coupling reaction. Synth. Met. 1998, 93, 123–126. [Google Scholar] [CrossRef]
- Iraqi, A.; Barker, W.G. Synthesis and characterisation of telechelic regioregular head-to-tail poly(3-alkylthiophenes). J. Mater. Chem. 1998, 8, 25–29. [Google Scholar] [CrossRef]
- Loewe, R.S.; Khersonsky, S.M.; McCullough, R.D. A Simple Method to Prepare Head-to-Tail Coupled, Regioregular Poly(3-alkylthiophenes) Using Grignard Metathesis. Adv. Mater. 1999, 11, 250–253. [Google Scholar] [CrossRef]
- Loewe, R.S.; Ewbank, P.C.; Liu, J.; Zhai, L.; McCullough, R.D. Regioregular, Head-to-Tail Coupled Poly(3-alkylthiophenes) Made Easy by the GRIM Method: Investigation of the Reaction and the Origin of Regioselectivity. Macromolecules 2001, 34, 4324–4333. [Google Scholar] [CrossRef]
- Wang, Q.; Takita, R.; Kikuzaki, Y.; Ozawa, F. Palladium-Catalyzed Dehydrohalogenative Polycondensation of 2-Bromo-3-hexylthiophene: An Efficient Approach to Head-to-Tail Poly(3-hexylthiophene). J. Am. Chem. Soc. 2010, 132, 11420–11421. [Google Scholar] [CrossRef] [PubMed]
- Se’vignon, M.; Papillon, J.; Schulz, E.; Lemaire, M. New synthetic method for the polymerization of alkylthiophenes. Tetrahedron Lett. 1999, 40, 5873–5876. [Google Scholar] [CrossRef]
- Campeau, L.-C.; Fagnou, K. Palladium-catalyzed direct arylation of simple arenes in synthesis of biaryl molecules. Chem. Commun. 2006, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Pouliot, J.-R.; Wakioka, M.; Ozawa, F.; Li, Y.; Leclerc, M. Structural Analysis of Poly(3-hexylthiophene) Prepared via Direct Heteroarylation Polymerization. Macromol. Chem. Phys. 2016, 217, 1493–1500. [Google Scholar] [CrossRef]
- Yan, H.; Chen, Z.; Zheng, Y.; Newman, C.; Quinn, J.R.; Dotz, F.; Kastler, M.; Facchetti, A. A high-mobility electron-transporting polymer for printed transistors. Nature 2009, 457, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Matsidik, R.; Komber, H.; Luzio, A.; Caironi, M.; Sommer, M. Defect-free Naphthalene Diimide Bithiophene Copolymers with Controlled Molar Mass and High Performance via Direct Arylation Polycondensation. J. Am. Chem. Soc. 2015, 137, 6705–6711. [Google Scholar] [CrossRef] [PubMed]
- Schuettfort, T.; Thomsen, L.; McNeill, C.R. Observation of a Distinct Surface Molecular Orientation in Films of a High Mobility Conjugated Polymer. J. Am. Chem. Soc. 2013, 135, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Lin, H.; Lou, S.J.; Yu, X.; Guo, P.; Manley, E.F.; Loser, S.; Hartnett, P.; Huang, H.; Wasielewski, M.R.; et al. Morphology-Performance Relationships in High-Efficiency All-Polymer Solar Cells. Adv. Energy Mater. 2013, 4, 1300785. [Google Scholar] [CrossRef]
- Deshmukh, K.D.; Qin, T.; Gallaher, J.K.; Liu, A.C.Y.; Gann, E.; O’Donnell, K.; Thomsen, L.; Hodgkiss, J.M.; Watkins, S.E.; McNeill, C.R. Performance, morphology and photophysics of high open-circuit voltage, low band gap all-polymer solar cells. Energy Environ. Sci. 2014, 8, 332–342. [Google Scholar] [CrossRef]
- Mu, C.; Liu, P.; Ma, W.; Jiang, K.; Zhao, J.; Zhang, K.; Chen, Z.; Wei, Z.; Yi, Y.; Wang, J.; et al. High-Efficiency All-Polymer Solar Cells Based on a Pair of Crystalline Low-Bandgap Polymers. Adv. Mater. 2014, 26, 7224–7230. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Uddin, M.A.; Lee, C.; Kim, K.-H.; Nguyen, T.L.; Lee, W.; Li, Y.; Wang, C.; Woo, H.Y.; Kim, B.J. Determining the Role of Polymer Molecular Weight for High-Performance All-Polymer Solar Cells: Its Effect on Polymer Aggregation and Phase Separation. J. Am. Chem. Soc. 2015, 137, 2359–2365. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Ying, L.; Zhu, P.; Pan, F.; Liu, F.; Chen, J.; Huang, F.; Cao, Y. All-Polymer Solar Cells Based on a Conjugated Polymer Containing Siloxane-Functionalized Side Chains with Efficiency over 10%. Adv. Mater. 2017, 29, 1703906. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhang, Z.; Xue, L.; Min, J.; Zhang, J.; Wei, Z.; Li, Y. All-Polymer Solar Cells Based on Absorption-Complementary Polymer Donor and Acceptor with High Power Conversion Efficiency of 8.27%. Adv. Mater. 2015, 28, 1884–1890. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Watson, M.D. Conjugated Polymers from Naphthalene Bisimide. Org. Lett. 2008, 10, 5333–5336. [Google Scholar] [CrossRef] [PubMed]
- Luzio, A.; Fazzi, D.; Nübling, F.; Matsidik, R.; Straub, A.; Komber, H.; Giussani, E.; Watkins, S.E.; Barbatti, M.; Thiel, W.; et al. Structure–Function Relationships of High-Electron Mobility Naphthalene Diimide Copolymers Prepared Via Direct Arylation. Chem. Mater. 2014, 26, 6233–6240. [Google Scholar] [CrossRef]
- Li, Y.; Sonar, P.; Murphy, L.; Hong, W. High mobility diketopyrrolopyrrole (DPP)-based organic semiconductor materials for organic thin film transistors and photovoltaics. Energy Environ. Sci. 2013, 6, 1684–1710. [Google Scholar] [CrossRef]
- Li, Y.; Singh, S.P.; Sonar, P. A High Mobility P-Type DPP-Thieno[3,2-b]thiophene Copolymer for Organic Thin-Film Transistors. Adv. Mater. 2010, 22, 4862–4866. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sonar, P.; Singh, S.P.; Soh, M.S.; van Meurs, M.; Tan, J. Annealing-Free High-Mobility Diketopyrrolopyrrole−Quaterthiophene Copolymer for Solution-Processed Organic Thin Film Transistors. J. Am. Chem. Soc. 2011, 133, 2198–2204. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Guo, Y.; Yu, G.; Zhao, Y.; Zhang, J.; Gao, D.; Liu, H.; Liu, Y. Highly π-Extended Copolymers with Diketopyrrolopyrrole Moieties for High-Performance Field-Effect Transistors. Adv. Mater. 2012, 24, 4618–4622. [Google Scholar] [CrossRef] [PubMed]
- Kang, I.; Yun, H.-J.; Chung, D.S.; Kwon, S.-K.; Kim, Y.-H. Record High Hole Mobility in Polymer Semiconductors via Side-Chain Engineering. J. Am. Chem. Soc. 2013, 135, 14896–14899. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, X.; Tian, H.; Zhang, J.; Yan, D.; Geng, Y.; Wang, F. High Mobility Ambipolar Diketopyrrolopyrrole-Based Conjugated Polymer Synthesized Via Direct Arylation Polycondensation. Adv. Mater. 2015, 27, 6753–6759. [Google Scholar] [CrossRef] [PubMed]
- Broll, S.; Nübling, F.; Luzio, A.; Lentzas, D.; Komber, H.; Caironi, M.; Sommer, M. Defect Analysis of High Electron Mobility Diketopyrrolopyrrole Copolymers Made by Direct Arylation Polycondensation. Macromolecules 2015, 48, 7481–7488. [Google Scholar] [CrossRef]
- Guo, C.; Quinn, J.; Sun, B.; Li, Y. Dramatically different charge transport properties of bisthienyl diketopyrrolopyrrole-bithiazole copolymers synthesized via two direct (hetero)arylation polymerization routes. Polym. Chem. 2016, 7, 4515–4524. [Google Scholar] [CrossRef]
- Fu, B.; Wang, C.-Y.; Rose, B.D.; Jiang, Y.; Chang, M.; Chu, P.-H.; Yuan, Z.; Fuentes-Hernandez, C.; Kippelen, B.; Brédas, J.-L.; et al. Molecular Engineering of Nonhalogenated Solution-Processable Bithiazole-Based Electron-Transport Polymeric Semiconductors. Chem. Mater. 2015, 27, 2928–2937. [Google Scholar] [CrossRef]
- Gobalasingham, N.S.; Pankow, R.M.; Ekiz, S.; Thompson, B.C. Evaluating structure-function relationships toward three-component conjugated polymers via direct arylation polymerization (DArP) for Stille-convergent solar cell performance. J. Mater. Chem. A 2017, 5, 14101–14113. [Google Scholar] [CrossRef]
- Kuwabara, J.; Yasuda, T.; Choi, S.J.; Lu, W.; Yamazaki, K.; Kagaya, S.; Han, L.; Kanbara, T. Direct Arylation Polycondensation: A Promising Method for the Synthesis of Highly Pure, High-Molecular-Weight Conjugated Polymers Needed for Improving the Performance of Organic Photovoltaics. Adv. Funct. Mater. 2014, 24, 3226–3233. [Google Scholar] [CrossRef]
- Kuwabara, J.; Yasuda, T.; Takase, N.; Kanbara, T. Effects of the Terminal Structure, Purity, and Molecular Weight of an Amorphous Conjugated Polymer on Its Photovoltaic Characteristics. ACS Appl. Mater. Interfaces 2016, 8, 1752–1758. [Google Scholar] [CrossRef] [PubMed]
- Bijleveld, J.C.; Gevaerts, V.S.; Di Nuzzo, D.; Turbiez, M.; Mathijssen, S.G.J.; de Leeuw, D.M.; Wienk, M.M.; Janssen, R.A.J. Efficient Solar Cells Based on an Easily Accessible Diketopyrrolopyrrole Polymer. Adv. Mater. 2010, 22, E242–E246. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, K.H.; Heintges, G.H.L.; Gevaerts, V.S.; Wienk, M.M.; Janssen, R.A.J. High-Molecular-Weight Regular Alternating Diketopyrrolopyrrole-based Terpolymers for Efficient Organic Solar Cells. Angew. Chem. Int. Ed. 2013, 52, 8341–8344. [Google Scholar] [CrossRef] [PubMed]
- Bijleveld, J.C.; Zoombelt, A.P.; Mathijssen, S.G.J.; Wienk, M.M.; Turbiez, M.; de Leeuw, D.M.; Janssen, R.A.J. Poly(diketopyrrolopyrrole−terthiophene) for Ambipolar Logic and Photovoltaics. J. Am. Chem. Soc. 2009, 131, 16616–16617. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, K.H.; Li, W.; Heintges, G.H.L.; van Pruissen, G.W.P.; Wienk, M.M.; Janssen, R.A.J. Homocoupling Defects in Diketopyrrolopyrrole-Based Copolymers and Their Effect on Photovoltaic Performance. J. Am. Chem. Soc. 2014, 136, 11128–11133. [Google Scholar] [CrossRef] [PubMed]
- Boland, P.; Lee, K.; Namkoong, G. Device optimization in PCPDTBT:PCBM plastic solar cells. Sol. Energy Mater. Sol. Cells 2010, 94, 915–920. [Google Scholar] [CrossRef]
- Zhu, Z.; Waller, D.; Gaudiana, R.; Morana, M.; Mühlbacher, D.; Scharber, M.; Brabec, C. Panchromatic Conjugated Polymers Containing Alternating Donor/Acceptor Units for Photovoltaic Applications. Macromolecules 2007, 40, 1981–1986. [Google Scholar] [CrossRef]
- Peet, J.; Kim, J.Y.; Coates, N.E.; Ma, W.L.; Moses, D.; Heeger, A.J.; Bazan, G.C. Efficiency enhancement in low-bandgap polymer solar cells by processing with alkane dithiols. Nat. Mater. 2007, 6, 497. [Google Scholar] [CrossRef] [PubMed]
- Tsao, H.N.; Cho, D.M.; Park, I.; Hansen, M.R.; Mavrinskiy, A.; Yoon, D.Y.; Graf, R.; Pisula, W.; Spiess, H.W.; Müllen, K. Ultrahigh Mobility in Polymer Field-Effect Transistors by Design. J. Am. Chem. Soc. 2011, 133, 2605–2612. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-W.; Waters, H.; Kettle, J.; Kuo, Z.-R.; Li, C.-H.; Yu, C.-Y.; Horie, M. Pd-Catalysed Direct Arylation Polymerisation for Synthesis of Low-Bandgap Conjugated Polymers and Photovoltaic Performance. Macromol. Rapid Commun. 2012, 33, 1927–1932. [Google Scholar] [CrossRef] [PubMed]
- Livi, F.; Gobalasingham, N.S.; Thompson, B.C.; Bundgaard, E. Analysis of diverse direct arylation polymerization (DArP) conditions toward the efficient synthesis of polymers converging with stille polymers in organic solar cells. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 2907–2918. [Google Scholar] [CrossRef]
- Iizuka, E.; Wakioka, M.; Ozawa, F. Mixed-Ligand Approach to Palladium-Catalyzed Direct Arylation Polymerization: Effective Prevention of Structural Defects Using Diamines. Macromolecules 2016, 49, 3310–3317. [Google Scholar] [CrossRef]
- Dudnik, A.S.; Aldrich, T.J.; Eastham, N.D.; Chang, R.P.H.; Facchetti, A.; Marks, T.J. Tin-Free Direct C–H Arylation Polymerization for High Photovoltaic Efficiency Conjugated Copolymers. J. Am. Chem. Soc. 2016, 138, 15699–15709. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ye, L.; Zhao, W.; Liu, D.; Yao, H.; Hou, J. Side Chain Selection for Designing Highly Efficient Photovoltaic Polymers with 2D-Conjugated Structure. Macromolecules 2014, 47, 4653–4659. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, S.; Zhao, W.; Yao, H.; Hou, J. Highly Efficient 2D-Conjugated Benzodithiophene-Based Photovoltaic Polymer with Linear Alkylthio Side Chain. Chem. Mater. 2014, 26, 3603–3605. [Google Scholar] [CrossRef]
- Mateker, W.R.; Douglas, J.D.; Cabanetos, C.; Sachs-Quintana, I.T.; Bartelt, J.A.; Hoke, E.T.; El Labban, A.; Beaujuge, P.M.; Frechet, J.M.J.; McGehee, M.D. Improving the long-term stability of PBDTTPD polymer solar cells through material purification aimed at removing organic impurities. Energy Environ. Sci. 2013, 6, 2529–2537. [Google Scholar] [CrossRef] [Green Version]
- Marzano, G.; Carulli, F.; Babudri, F.; Pellegrino, A.; Po, R.; Luzzati, S.; Farinola, G.M. PBDTTPD for plastic solar cells via Pd(PPh3)4-catalyzed direct (hetero)arylation polymerization. J. Mater. Chem. A 2016, 4, 17163–17170. [Google Scholar] [CrossRef]
- Kim, H.; Lee, B.H.; Lee, K.C.; Kim, G.; Yu, J.Y.; Kim, N.; Lee, S.H.; Lee, K. Role of the Side Chain in the Phase Segregation of Polymer:Fullerene Bulk Heterojunction Composites. Adv. Energy Mater. 2013, 3, 1575–1580. [Google Scholar] [CrossRef]
- Najari, A.; Beaupré, S.; Allard, N.; Ouattara, M.; Pouliot, J.-R.; Charest, P.; Besner, S.; Simoneau, M.; Leclerc, M. Thieno, Furo, and Selenopheno[3,4-c]pyrrole-4,6-dione Copolymers: Air-Processed Polymer Solar Cells with Power Conversion Efficiency up to 7.1%. Adv. Energy Mater. 2015, 5, 1501213. [Google Scholar] [CrossRef]
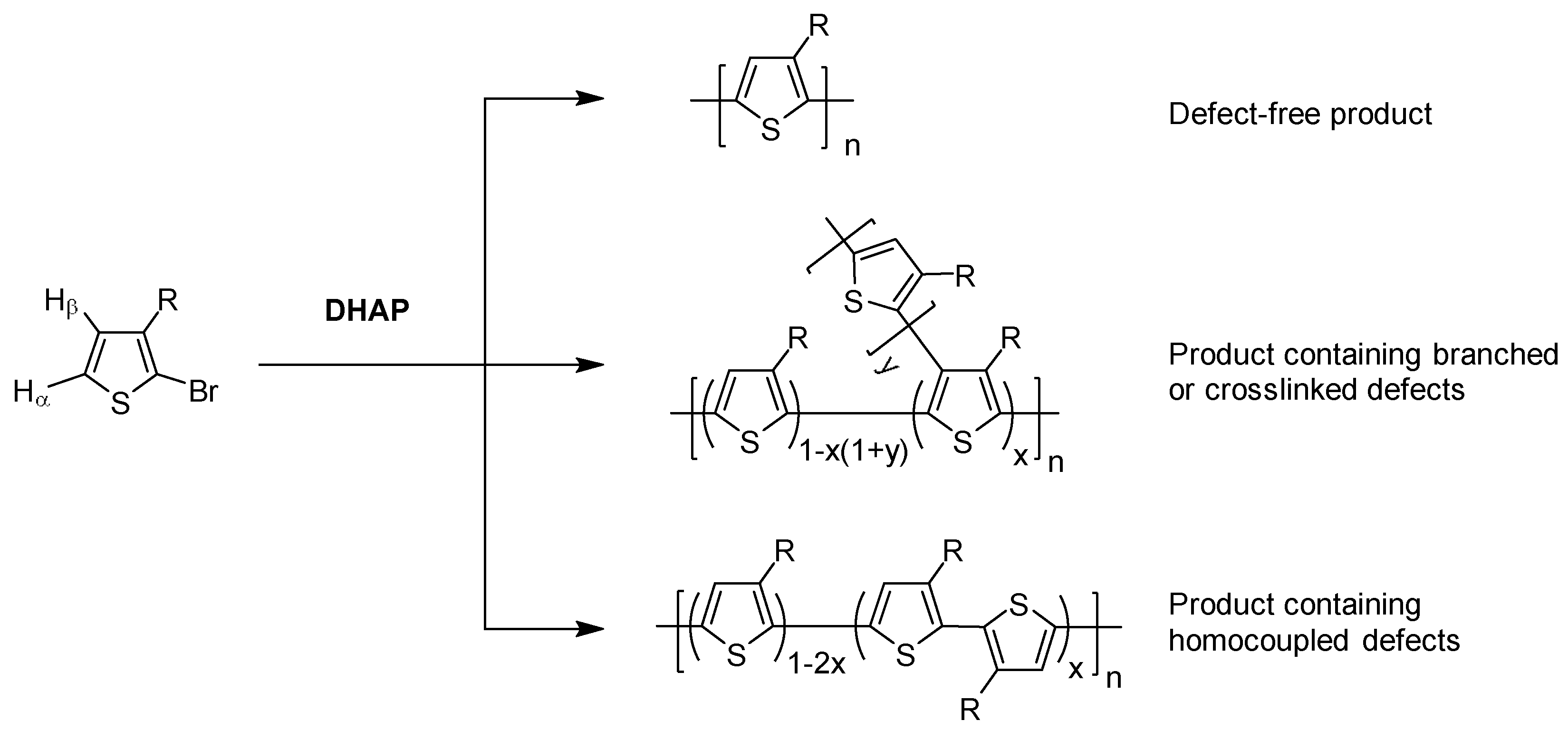




| Synthesis | OTFT Performance | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| ID | Name | Mn, kDa (HT mol %) | Mw/Mn | Yield, % | Device | uh/ue, cm2 V−1 s−1 | Ion/Ioff | Vth, V | Ref. |
| P1DHAP1 | P3HT | 33 (>99.5) | 1.8 | 96 | BGBC | 0.19/- | 1000/- | -/- | 2016 [48] |
| P1GRIM | P3HT | 88 (98.0) | 1.5 | NA | BGBC | 0.11/- | 1000/- | -/- | 2016 [48] |
| P1Rieke | P3HT | 25 (95.5) | 1.9 | NA | BGBC | 0.02/- | 1000/- | -/- | 2016 [48] |
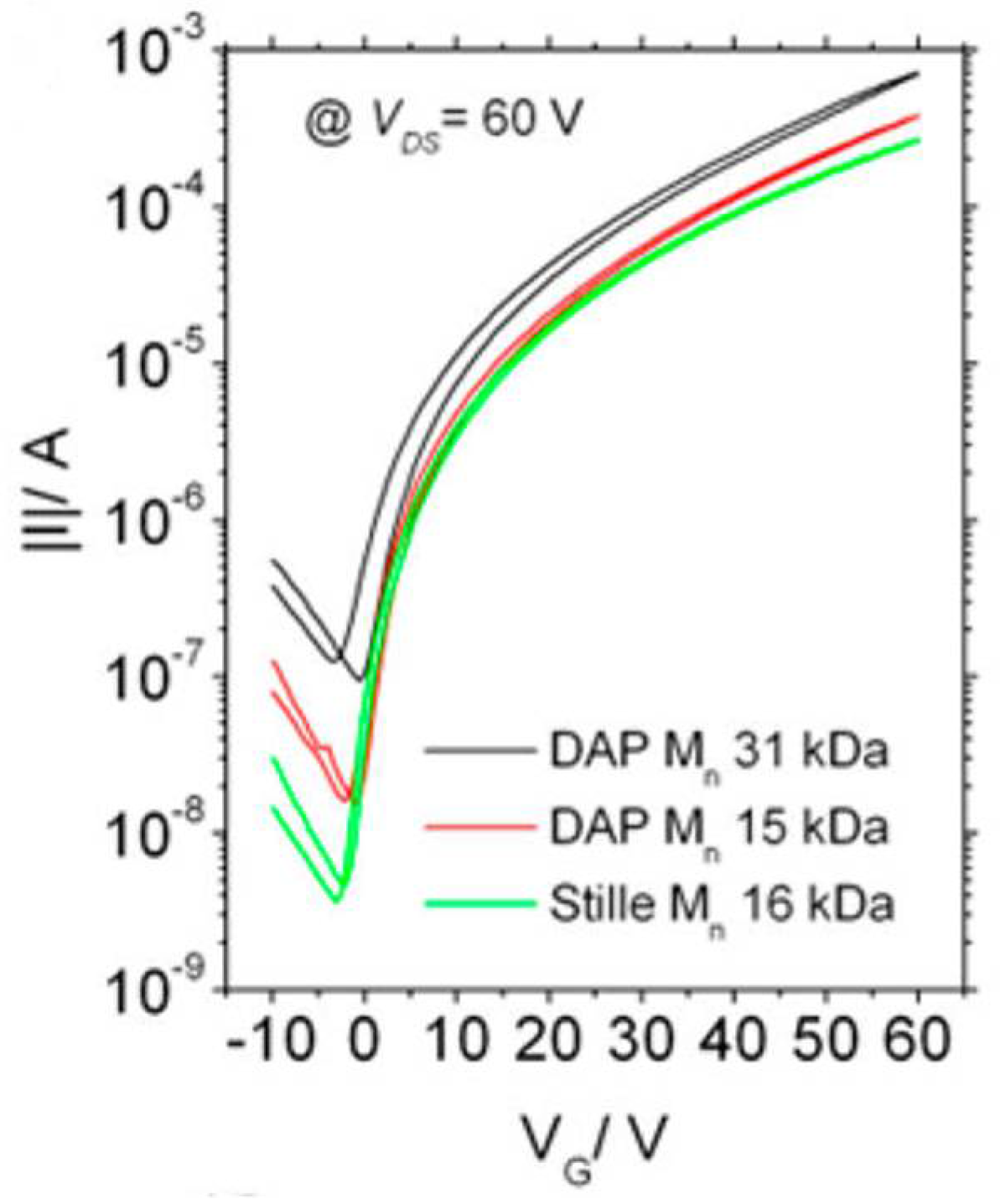
| P2DHAP | N2200 | 31 | 2.9 | 99 | TGBC | -/2.9 | -/>1000 | -/- | 2015 [50] |
| P2Stille | N2200 | 32 | 5.4 | 100 | TGBC | -/3.2 | -/>1000 | -/- | 2015 [50] |
| P3DHAP | P(ThNDIThF4) | 7.8 | 1.7 | - | TGBC | -/1.3 | -/~105 | -/- | 2014 [59] |
| P4DHAP | PDPP-4FTVT | 60 | 4.9 | 93 | BGTC | 3.4/5.9 | >105/>10 | −1~−15/40~55 | 2015 [65] |
| P5DHAP | PDPPTh2F4 | 30 | 2.4 | 75 | TGBC | -/0.60 | -/~104 | -/24.5 | 2015 [66] |
| P6DHAP | PDBTz-24 | 18 | 3.8 | 66 | TGBC | 0.06/0.53 | ~106/~105 | -/- | 2016 [67] |
| P6Stille | PDBTz-27 | 64 | 3.6 | 90 | BGTC | -/0.31 | -/105 | -/4 | 2015 [68] |
| P7DHAP | PDPP | 46 | 2.5 | 84 | BGBC | 1.2/- | ~103/- | 0/- | 2015 [24] |
| Synthesis | Solar Cells | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ID | Name | Mn, kDa (HT %) | Mw/Mn | Yield, % | Acceptor | PCE (Best Reported) | JSC, mA cm−2 | VOC, V | FF, % | Ref. |
| P1DHAP2 a | P3HT | 19 (90.0) | 2.0 | - | PC61BM | 2.70 | 8.70 | 0.62 | 50.0 | 2013 [25] |
| P1Stille1 b | P3HT | 19 (93.0) | 2.7 | - | PC61BM | 2.30 | 8.37 | 0.61 | 45.0 | 2013 [25] |
| P1DHAP3 a | P3HT | 20 (96.2) | 2.1 | 74 | PC61BM | 3.28 | 9.40 | 0.59 | 59.1 | 2017 [69] |
| P1Stille2 b | P3HT | 18 (92.9) | 2.4 | 68 | PC61BM | 2.86 | 8.34 | 0.59 | 58.1 | 2017 [69] |
| P8DHAP | PEDOTF | 150 | 2.89 | 89 | PC71BM | 4.08 | 9.41 | 0.83 | 52.0 | 2014 [70] |
| P8Suzuki | PEDOTF | 17 | 2.08 | 85 | PC71BM | 0.48 | 2.58 | 0.59 | 31.0 | 2014 [70] |
| P9DHAP | PDPP-TPT | 14 | 1.8 | 29 | PC71BM | 4.37 | 13.3 | 0.77 | 41.5 | 2015 [22] |
| P9Suzuki1 c | PDPP-TPT | 65 a | - | 69 | PC71BM | 5.50 | 10.8 | 0.80 | 65.0 | 2010 [72] |
| P9Suzuki2 c | PDPP-TPT | 72 | 1.98 | 93 | PC71BM | 7.40 | 14.0 | 0.80 | 67.0 | 2013 [73] |
| P10DHAP | PDPP-3T | 29 | 3.8 | 45 | PC71BM | 4.01 | 10.3 | 0.71 | 56.2 | 2015 [22] |
| P10Suzuki | PDPP-3T | 54 | 3.15 | 84 | PC71BM | 4.69 | 11.8 | 0.66 | 60.0 | 2009 [74] |
| P10Stille | PDPP-3T | 150 | 2.72 | 85 | PC71BM | 7.10 | 15.4 | 0.67 | 69.0 | 2013 [73] |
| P11DHAP | PCPDTBT | 72 | 4.52 | 76 | PC71BM | 3.98 | 13.9 | 0.63 | 45.5 | 2012 [80] |
| P11Suzuki | PCPDTBT | 15 | 2.1 | 83 | PC71BM | 3.74 | 12.7 | 0.64 | 43.8 | 2012 [80] |
| P11Stille | PCPDTBT | 28 | 1.5 | 61 | PC61BM | 3.50 | 11.8 | 0.65 | 46.0 | 2007 [77] |
| P12DHAP | PPDTBT | 15 | 2.1 | 78 | PC61BM | 3.40 | 10.5 | 0.72 | 45.0 | 2016 [81] |
| P12Stille1-HMW d | PPDTBT | 59 | 3.3 | 79 | PC61BM | 3.80 | 11.5 | 0.73 | 45.0 | 2016 [81] |
| P12Stille2-LMW d | PPDTBT | 16 | 2.1 | 70 | PC61BM | 2.90 | 8.88 | 0.72 | 46.0 | 2016 [81] |
| P13DHAP | - | 10 | 7.6 | 70 | PC71BM | 2.80 | 5.58 | 0.89 | 56.0 | 2015 [23] |
| P13Stille | - | 20 | 3.1 | 85 | PC71BM | 4.80 | 9.89 | 0.81 | 60.0 | 2015 [23] |
| P14DHAP | PBDTT-FTTE | 25 | 2.2 | 98 | PC71BM | 8.36 | 15.5 | 0.78 | 68.8 | 2016 [83] |
| P14Stille | PBDTT-FTTE | 25 | 2.2 | - | PC71BM | 8.40 | 14.9 | 0.78 | 72.2 | 2016 [83] |
| P15DHAP | PBDTT-TPD | 30 | 2.7 | 76 | PC71BM | 5.84 | 10.0 | 0.99 | 57.9 | 2016 [83] |
| P15Stille | PBDTT-TPD | 15 | 2.4 | 69 | PC71BM | 5.20 | 9.10 | 0.99 | 58.7 | 2016 [83] |
| P16DHAP | PTPD3T | 19 | 2.0 | 83 | PC71BM | 7.20 | 13.3 | 0.82 | 66.0 | 2016 [83] |
| P16Stille | PTPD3T | 30 | 1.8 | 94 | PC71BM | 7.38 | 13.2 | 0.78 | 71.1 | 2016 [83] |
| P17DHAP | PBDTTPD | 12 | - | 80 | PC71BM | 5.31 | 10.41 | 0.92 | 56.0 | 2016 [87] |
| P17Stille | PBDTTPD | 10 | - | 90 | PC71BM | 4.82 | 9.17 | 0.93 | 57.0 | 2016 [87] |
| P18DHAP | - | 25 | 1.9 | 82 | PC71BM | 6.80 | 13.8 | 0.91 | 53.5 | 2016 [19] |
| P18Stille | - | 15 | 1.2 | 52 | PC71BM | 5.20 | 10.0 | 0.88 | 59.0 | 2013 [88] |
| P19DHAP | PSePD3T | 29 | 1.6 | 57 | PC61BM | 7.13 | 13.2 | 0.85 | 64.0 | 2015 [89] |
| P20DHAP | IDT-TQ | 27 | 1.6 | 71 | PC71BM | 5.10 | 10.8 | 0.89 | 53.4 | 2016 [21] |
| P20Stille | IDT-TQ | 23 | 1.5 | 64 | PC71BM | 4.82 | 10.4 | 0.89 | 52.1 | 2016 [21] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hendsbee, A.D.; Li, Y. Performance Comparisons of Polymer Semiconductors Synthesized by Direct (Hetero)Arylation Polymerization (DHAP) and Conventional Methods for Organic Thin Film Transistors and Organic Photovoltaics. Molecules 2018, 23, 1255. https://doi.org/10.3390/molecules23061255
Hendsbee AD, Li Y. Performance Comparisons of Polymer Semiconductors Synthesized by Direct (Hetero)Arylation Polymerization (DHAP) and Conventional Methods for Organic Thin Film Transistors and Organic Photovoltaics. Molecules. 2018; 23(6):1255. https://doi.org/10.3390/molecules23061255
Chicago/Turabian StyleHendsbee, Arthur D., and Yuning Li. 2018. "Performance Comparisons of Polymer Semiconductors Synthesized by Direct (Hetero)Arylation Polymerization (DHAP) and Conventional Methods for Organic Thin Film Transistors and Organic Photovoltaics" Molecules 23, no. 6: 1255. https://doi.org/10.3390/molecules23061255
APA StyleHendsbee, A. D., & Li, Y. (2018). Performance Comparisons of Polymer Semiconductors Synthesized by Direct (Hetero)Arylation Polymerization (DHAP) and Conventional Methods for Organic Thin Film Transistors and Organic Photovoltaics. Molecules, 23(6), 1255. https://doi.org/10.3390/molecules23061255