Nutraceutical Oils Produced by Olives and Citrus Peel of Tuscany Varieties as Sources of Functional Ingredients
Abstract
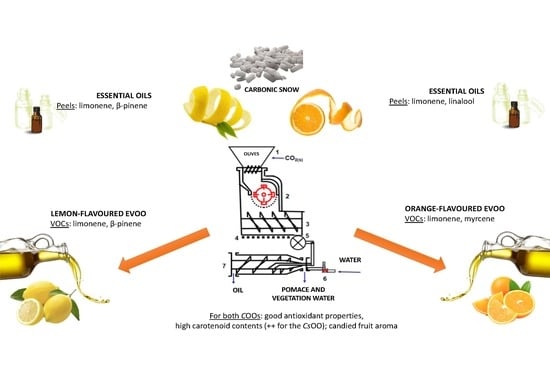
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition of the Essential Oils (EOs)
2.1.1. Lemon Essential Oils
2.1.2. Orange Essential Oils
2.2. The Citrus Olive Oils (COOs)
2.2.1. Volatiles Bouquet in the Headspace Emissions of the COOs
2.3. Chemical Characterization of the Citrus Olive Oil
2.3.1. Quality Parameters
2.3.2. Phenolic Content, Intensity of Bitterness and Antioxidant Capacity
2.3.3. Flavonoid Composition
2.3.4. Composition of Phenolic Alcohols, Aldehydes and Acids
2.3.5. Sensory Analysis
3. Materials and Methods
3.1. Plant Material
3.2.Phytochemical Analyses
3.2.1. Essential Oils (EOs) Hydrodistillation
3.2.2. Headspace Solid Phase Micro-Extraction (SPME) of the Citrus Olive Oils (COOs)
3.2.3. Gas Chromatography-Mass Spectrometry Analyses and Peak Identification
3.3. Citrus Olive Oil Extraction
3.4. COOs Chemical Analyses
3.4.1. Quality Parameters
3.4.2. Analysis of the Phenolic Content
3.4.3. Antioxidant Capacity Assay
3.4.4. Analysis of Flavonoid Contents
3.4.5. Analysis of Phenolic Alcohols, Aldehyde ad Acids
3.4.6. Intensity of Bitterness (IB) Determination
3.4.7. Carotenoids
3.4.8. Sensory Analysis
3.4.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Toscana, R. Prodotti Vetrina Toscana: Arancio Massese. Available online: http://www.vetrina.toscana.it/prodotti/arancio-massese/ (accessed on 12 April 2018).
- Tintori, O. Limone massese-Oscar Tintori-Gli Agrumi In Toscana-Sito Ufficiale e Online Shop. Available online: https://www.oscartintori.it/prodotto/limone-massese/ (accessed on 13 March 2018).
- Giampaoli, S. Appunti Sulla Coltivazione Degli Agrumi A Massa; Biblioteca civica Massa: Massa, Italy, 1976; p. 58. [Google Scholar]
- Toscana, R. Prodotti Agroalimentari Tradizionali Della Toscana—Scheda Identificativa Limone Massese. Available online: http://prodtrad.regione.toscana.it/LIB_ProdTrad/Prodotto.php?ID=116 (accessed on 13 March 2018).
- Toscana, R. Regione Toscana-Prodotti Vetrina Toscana: Limone Massese. Available online: http://www.vetrina.toscana.it/prodotti/limone-massese/ (accessed on 12 April 2018).
- Toscana, R. Prodotti Agroalimentari Tradizionali della Toscana—Scheda identificativa Arancio massese. Available online: http://prodtrad.regione.toscana.it/LIB_ProdTrad/Prodotto.php?ID=113 (accessed on 13 March 2018).
- Venturi, F.; Sanmartin, C.; Taglieri, I.; Andrich, G.; Zinnai, A. A simplified method to estimate Sc-CO2 extraction of bioactive compounds from different matrices: chili pepper vs. tomato by-products. Appl. Sci. 2017, 7, 361. [Google Scholar] [CrossRef]
- Venturi, F.; Sanmartin, C.; Taglieri, I.; Nari, A.; Andrich, G.; Terzuoli, E.; Donnini, S.; Nicolella, C.; Zinnai, A. Development of phenol-enriched olive oil with phenolic compounds extracted from wastewater produced by physical refining. Nutrients 2017, 9, 916. [Google Scholar] [CrossRef] [PubMed]
- Zinnai, A.; Venturi, F.; Andrich, G. Time evolution of phenols extractions from Sangiovese grapes with and without the addition of solid carbon dioxide. Agrochimica 2011, LV, 55. [Google Scholar] [CrossRef]
- Zinnai, A.; Venturi, F.; Sanmartin, C.; Taglieri, I.; Andrich, G. The utilization of solid carbon dioxide in the extraction of extra-virgin olive oil: VOO/EVOO yield and quality as a function of extraction conditions adopted. Agro Food Ind. Hi Tech. 2015, 26, 24–26. [Google Scholar]
- Papoutsis, K.; Vuong, Q.V.; Golding, J.B.; Hasperué, J.H.; Pristijono, P.; Bowyer, M.C.; Scarlett, C.J.; Stathopoulos, C.E. Pretreatment of citrus by-products affects polyphenol recovery: A review. Food Rev. Int. 2018, 34, 770–795. [Google Scholar] [CrossRef]
- Vekiari, S.A.; Protopapadakis, E.E.; Papadopoulou, P.; Papanicolaou, D.; Panou, C.; Vamvakias, M. Composition and seasonal variation of the essential oil from leaves and peel of a Cretan lemon variety. J. Agr. Food Chem. 2002, 50, 147–153. [Google Scholar] [CrossRef]
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Regulation, H. Commission Regulation (EEC) No 2568/91 of 11 July 1991 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis. OJEC 1991, 248, 1. [Google Scholar]
- Baiano, A.; Terracone, C.; Gambacorta, G.; Notte, E.L. Changes in Quality Indices, Phenolic Content and Antioxidant Activity of Flavored Olive Oils during Storage. J. Am. Oil Chem. Soc. 2009, 86, 1083. [Google Scholar] [CrossRef]
- Alquezar, B.; Rodrigo, M.J.; Zacarías, L. Regulation of carotenoid biosynthesis during fruit maturation in the red-fleshed orange mutant Cara Cara. Phytochemistry 2008, 69, 1997–2007. [Google Scholar] [CrossRef]
- Rodrigo, M.J.; Marcos, J.F.; Zacarías, L. Biochemical and molecular analysis of carotenoid biosynthesis in flavedo of orange (Citrus sinensis L.) during fruit development and maturation. J. Agr. Food Chem. 2004, 52, 6724–6731. [Google Scholar] [CrossRef]
- Coyne, T.; Ibiebele, T.I.; Baade, P.D.; Dobson, A.; McClintock, C.; Dunn, S.; Leonard, D.; Shaw, J. Diabetes mellitus and serum carotenoids: findings of a population-based study in Queensland, Australia. Am. J. Clin. Nutr. 2005, 82, 685–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, D.A.; Eldridge, A.L.; Peters, J.C. Dietary carotenoids and certain cancers, heart disease, and age-related macular degeneration: a review of recent research. Nutr. Rev. 1999, 57, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Peres, F.; Martins, L.L.; Mourato, M.; Vitorino, C.; Antunes, P.; Ferreira-Dias, S. Phenolic compounds of ‘Galega Vulgar’ and ‘Cobrançosa’ olive oils along early ripening stages. Food Chem. 2016, 211, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Franco, M.N.; Galeano-Díaz, T.; López, Ó.; Fernández-Bolaños, J.G.; Sánchez, J.; De Miguel, C.; Gil, M.V.; Martín-Vertedor, D. Phenolic compounds and antioxidant capacity of virgin olive oil. Food Chem. 2014, 163, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Tripoli, E.; Guardia, M.L.; Giammanco, S.; Majo, D.D.; Giammanco, M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007, 104, 466–479. [Google Scholar] [CrossRef]
- Karim, N.; Jia, Z.; Zheng, X.; Cui, S.; Chen, W. A recent review of citrus flavanone naringenin on metabolic diseases and its potential sources for high yield-production. Trends Food Sci. Tech. 2018, 79, 35–54. [Google Scholar] [CrossRef]
- Alam, M.A.; Subhan, N.; Rahman, M.M.; Uddin, S.J.; Reza, H.M.; Sarker, S.D. Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Adv. Nutr. 2014, 5, 404–417. [Google Scholar] [CrossRef]
- Ameer, B.; Weintraub, RA. Drug interactions with grapefruit juice. Clin. Pharmacokinet. 1997, 33, 103–121. [Google Scholar] [CrossRef]
- Fuhr, U.; Klittich, K.; Staib, AH. Inhibitory effect of grapefruit juice and its bitter principal, naringenin, on CYP1A2 dependent metabolism of caffeine in man. Br. J. Clin. Pharmacol. 1993, 35, 431–436. [Google Scholar] [CrossRef]
- Masaki, H.; Okamoto, N.; Sakaki, S.; Sakurai, H. Protective effects of hydroxybenzoic acids and their esters on cell damage induced by hydroxyl radicals and hydrogen peroxides. Biol. Pharm. Bull. 1997, 20, 304–308. [Google Scholar] [CrossRef]
- Maga, J.A. Simple phenol and phenolic compounds in food flavor. Crit. Rev. Food Sci. Nutr. 1978, 10, 323–372. [Google Scholar] [CrossRef]
- Mallek-Ayadi, S.; Bahloul, N.; Kechaou, N. Characterization, phenolic compounds and functional properties of Cucumis melo L. peels. Food Chem. 2017, 221, 1691–1697. [Google Scholar] [CrossRef]
- Karoui, I.J.; Marzouk, B. Characterization of bioactive compounds in tunisian bitter orange (Citrus aurantium L.) peel and juice and determination of their antioxidant activities. BioMed. Res. Int. 2013, 2013, 345415. [Google Scholar]
- Covas, M.I.; Gutiérrez, R.V.; De la Torre, R.; Kafatos, A.; Raventós, L.R.M.; Osada, J.; Owen, R.W.; Visioli, F. Minor components of olive oil: Evidence to date of health benefits in humans. Nutr. Rev. 2006, 64, S20–S30. [Google Scholar] [CrossRef]
- Fernández-Mar, M.I.; Mateos, R.; García-Parrilla, M.C.; Puertas, B.; Cantos-Villar, E. Bioactive compounds in wine: Resveratrol, hydroxytyrosol and melatonin: A review. Food Chem. 2012, 130, 797–813. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Morató, J.; Xicota, L.; Fitó, M.; Farré, M.; Dierssen, M.; De la Torre, R. Potential role of olive oil phenolic compounds in the prevention of neurodegenerative diseases. Molecules 2015, 20, 4655–4680. [Google Scholar] [CrossRef]
- Bocco, A.; Cuvelier, M.E.; Richard, H.; Berset, C. Antioxidant activity and phenolic composition of citrus peel and seed extracts. J. Agr. Food Chem. 1998, 46, 2123–2129. [Google Scholar] [CrossRef]
- Zang, L.Y.; Cosma, G.; Gardner, H.; Shi, X.; Castranova, V.; Vallyathan, V. Effect of antioxidant protection by p-coumaric acid on low-density lipoprotein cholesterol oxidation. Am. J. Phys. 2000, 279, C954–C960. [Google Scholar] [CrossRef]
- Kanski, J.; Aksenova, M.; Stoyanova, A.; Butterfield, D.A. Ferulic acid antioxidant protection against hydroxyl and peroxyl radical oxidation in synaptosomal and neuronal cell culture systems in vitro: Structure-activity studies. J. Nutr. Biochem. 2002, 13, 273–281. [Google Scholar] [CrossRef]
- Wang, A.Y.; Zhou, M.Y.; Lin, W.C. Antioxidative and anti-inflammatory properties of Citrus sulcata extracts. Food Chem. 2011, 124, 958–963. [Google Scholar] [CrossRef]
- Uceda, M; Frias, L. Harvest dates:Evolution of the fruit oil content, oil composition and oil quality. In Proceedings of the II Seminario Oleícola Internacional, I.O.O.C., Cordoba, Spain, 6 October 1975; pp. 125–130. [Google Scholar]
- Adams, R.P.; Zanoni, T.A.; Lara, A.; Barrero, A.F.; Cool, L.G. Comparisons among Cupressus arizonica Greene, C. benthamii Endl., C. lindleyi Klotz, ex Endl. and C. lusitanica Mill, using leaf essential oils and DNA fingerprinting. J. Essent. Oil Res. 1997, 9, 303–309. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of essential oil components by gas chromatography/mass spectroscopy. J. Am. Soc. Mass Spectrom. 1997, 8, 671–672. [Google Scholar]
- Davies, N. Gas chromatographic retention indices of monoterpenes and sesquiterpenes on methyl silicon and Carbowax 20M phases. J. Chromatogr. A 1990, 503, 1–24. [Google Scholar] [CrossRef]
- Jennings, W.; Shibamoto, T. Qualitative Analysis of Flavor and Fragrance Volatiles by Glass Capillary Gas Chromatography; Academic Press: New York, NY, USA, 1980. [Google Scholar]
- Masada, Y. Analysis of Essential Oil by Gas Chromatography and Mass Spectrometry; John Wiley & Sons, Inc.: New York, NY, USA, 1976. [Google Scholar]
- Swigar, A.A.; Silverstein, R.M. Monoterpenes; Aldrich Chemical Company: Milwaukee, WI, USA, 1981. [Google Scholar]
- Zinnai, A.; Venturi, F.; Quartacci, M.F.; Sanmartin, C.; Favati, F.; Andrich, G. Solid carbon dioxide to promote the extraction of extra-virgin olive oil. Grasas Aceites 2016, 67, e121. [Google Scholar] [CrossRef]
- Sanmartin, C.; Venturi, F.; Macaluso, M.; Nari, A.; Quartacci, M.F.; Sgherri, C.; Flamini, G.; Taglieri, I.; Ascrizzi, R.; Andrich, G.; et al. Preliminary results about the use of argon and carbon dioxide in the extra virgin olive oil (EVOO) storage to extend oil shelf life: Chemical and sensorial point of view. Eur. J. Lipid Sci. Tech. 2018, 120, 1800156. [Google Scholar] [CrossRef]
- Sanmartin, C.; Venturi, F.; Sgherri, C.; Nari, A.; Macaluso, M.; Flamini, G.; Quartacci, M.F.; Taglieri, I.; Andrich, G.; Zinnai, A. The effects of packaging and storage temperature on the shelf-life of extra virgin olive oil. Heliyon 2018, 4, e00888. [Google Scholar] [CrossRef]
- Sgherri, C.; Micaelli, F.; Andreoni, N.; Baldanzi, M.; Ranieri, A. Retention of phenolic compounds and antioxidant properties in potato bread obtained from a dough enriched with a powder from the purple cv. Vitelotte. Agrochimica 2016, 60, 312–328. [Google Scholar]
- Fellegrini, N.; Ke, R.; Yang, M.; Rice-Evans, C. Screening of dietary carotenoids and carotenoid-rich fruit extracts for antioxidant activities applying 2,2′-azinobis(3-ethylenebenzothiazoline-6-sulfonic acid radical cation decolorization assay. In Methods in Enzymology; Academic Press: New York, NY, USA, 1999; Volume 299, pp. 379–389. [Google Scholar]
- Sgherri, C.; Perez-Lopez, U.; Micaelli, F.; Miranda-Apodaca, J.; Mena-Petite, A.; Munoz-Rueda, A.; Quartacci, M.F. Elevated CO2 and salinity are responsible for phenolics-enrichment in two differently pigmented lettuces. Plant Physiol. Biochem. 2017, 115, 269–278. [Google Scholar] [CrossRef]
- Gutiérrez Rosales, F.; Perdiguero, S.; Gutiérrez, R.; Olias, J.M. Evaluation of the bitter taste in virgin olive oil. J. Am. Oil Chem. Soc. 1992, 69, 394–395. [Google Scholar] [CrossRef]
- Isabel Minguez-Mosquera, M.; Rejano-Navarro, L.; Gandul-Rojas, B.; Sanchez Gomez, A.H.; Garrido-Fernandez, J. Color-pigment correlation in virgin olive oil. J. Am. Oil Chem. Soc. 1991, 68, 332–336. [Google Scholar] [CrossRef]
- Venturi, F.; Sanmartin, C.; Taglieri, I.; Xiaoguo, Y.; Andrich, G.; A, Z. The influence of packaging on the sensorial evolution of white wine as a function of the operating conditions adopted during storage. Agrochimica 2016, 60, 150–160. [Google Scholar]
- Bendini, A.; Valli, E.; Barbieri, S.; Toschi, T.G. Sensory analysis of olive oil-standard glass for oil tasting. In Olive Oil—Constituents, Quality, Health Properties and Bioconversions; Boskou, D., Ed.; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Venturi, F.; Sanmartin, C.; Taglieri, I.; Xiaoguo, Y.; Andrich, G.; Zinnai, A. A kinetic approach to describe the time evolution of red wine as a function of packaging and storage conditions. Acta Aliment. 2017, 46, 336–345. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds are not available from the authors. |


| Constituents | l.r.i. 1 | Relative Abundance (%) | |
|---|---|---|---|
| Peels EO | Squeezed Peels VF 2 | ||
| α-Thujene | 931 | 0.4 ± 0.0 | - 3 |
| α-Pinene | 941 | 1.6 ± 0.0 | 2.3 ± 0.1 |
| Sabinene | 976 | 2.0 ± 0.0 | 2.5 ± 0.1 |
| β-Pinene | 982 | 10.6 ± 0.4 | 14.9 ± 0.3 |
| Myrcene | 993 | 1.6 ± 0.1 | 0.5 ± 0.7 |
| Octanal | 1001 | 0.2 ± 0.0 | - |
| α-Terpinene | 1018 | 0.3 ± 0.0 | - |
| p-Cymene | 1027 | 0.3 ± 0.0 | - |
| Limonene | 1032 | 50.2 ± 0.3 | 70.3 ± 0.6 |
| (E)-β-Ocimene | 1052 | 0.2 ± 0.0 | - |
| γ-Terpinene | 1062 | 9.6 ± 0.6 | 9.6 ± 0.3 |
| cis-Sabinene hydrate | 1070 | 0.2 ± 0.0 | - |
| Terpinolene | 1088 | 0.6 ± 0.0 | - |
| Linalool | 1101 | 1.0 ± 0.1 | - |
| Nonanal | 1102 | 0.5 ± 0.1 | - |
| Camphor | 1143 | 0.1 ± 0.1 | - |
| Citronellal | 1155 | 0.4 ± 0.0 | - |
| Isoneral | 1171 | 0.2 ± 0.0 | - |
| 4-Terpineol | 1178 | 0.6 ± 0.1 | - |
| Isogeranial | 1184 | 0.2 ± 0.0 | - |
| α-Terpineol | 1191 | 2.0 ± 0.1 | - |
| Nerol | 1230 | 0.8 ± 0.1 | - |
| Neral | 1240 | 5.7 ± 0.1 | - |
| Geraniol | 1257 | 0.8 ± 0.3 | - |
| Geranial | 1271 | 7.1 ± 0.0 | - |
| Neryl acetate | 1366 | 0.6 ± 0.1 | - |
| Geranyl acetate | 1385 | 0.4 ± 0.1 | - |
| β-Caryophyllene | 1420 | 0.3 ± 0.0 | - |
| trans-α-Bergamotene | 1438 | 0.4 ± 0.0 | - |
| Valencene | 1492 | 0.2 ± 0.0 | - |
| Bicyclogermacrene | 1495 | 0.2 ± 0.0 | - |
| β-Bisabolene | 1509 | 0.7 ± 0.1 | - |
| Valerianol | 1656 | 0.1 ± 0.0 | - |
| Monoterpene hydrocarbons | 77.3 ± 1.1 | 100.0 ± 0.0 | |
| Oxygenated monoterpenes | 0.7 ± 0.1 | - | |
| Sesquiterpene hydrocarbons | 20.1 ± 0.9 | - | |
| Oxygenated sesquiterpenes | 1.8 ± 0.1 | - | |
| Non-terpene derivatives | 0.1 ± 0.0 | - | |
| Extraction yield (% w/w) | 0.57 | - | |
| Total identified (%) | 100.0 ± 0.0 | 100.0 ± 0.0 | |
| Constituents | l.r.i. 1 | Relative Abundance (%) | |
|---|---|---|---|
| Peels EO | Squeezed Peels VF 2 | ||
| α-Pinene | 941 | 0.6 ± 0 | 0.9 ± 0.0 |
| Sabinene | 976 | 2.0 ± 0.1 | 3.2 ± 0.0 |
| Myrcene | 993 | 2.2 ± 0.1 | 2.6 ± 0.0 |
| Octanal | 1001 | 2.0 ± 0.0 | 0.5 ± 0.0 |
| Limonene | 1032 | 85.7 ± 0.2 | 91.4 ± 0.1 |
| (E)-β-Ocimene | 1052 | - 3 | 0.1 ± 0.0 |
| n-Octanol | 1071 | 0.3 ± 0.0 | - |
| Linalool | 1101 | 3.5 ± 0.2 | 0.5 ± 0.1 |
| Nonanal | 1102 | - | 0.2 ± 0.0 |
| trans-Limonene oxide | 1141 | 0.1 ± 0.0 | - |
| Citronellal | 1155 | 0.1 ± 0.0 | - |
| 4-Terpineol | 1178 | 0.3 ± 0.0 | - |
| α-Terpineol | 1189 | 0.5 ± 0.0 | - |
| Decanal | 1204 | 0.6 ± 0.0 | 0.3 ± 0.0 |
| Citronellol | 1230 | 0.1 ± 0.0 | - |
| Neral | 1240 | 0.4 ± 0.0 | - |
| Geranial | 1271 | 0.6 ± 0.0 | - |
| Valencene | 1492 | 0.9 ± 0.1 | 0.5 ± 0.1 |
| Valerianol | 1656 | 0.1 ± 0.1 | - |
| Monoterpene hydrocarbons | 90.5 ± 0.1 | 98.1 ± 0.2 | |
| Oxygenated monoterpenes | 5.6 ± 0.0 | 0.5 ± 0.1 | |
| Sesquiterpene hydrocarbons | 0.9 ± 0.1 | 0.5 ± 0.1 | |
| Oxygenated sesquiterpenes | 0.1 ± 0.1 | - | |
| Non-terpene derivatives | 2.9 ± 0.1 | 0.9 ± 0.0 | |
| Extraction yield (% w/w) | 0.35 | - | |
| Total identified (%) | 100.0 ± 0.0 | 100.0 ± 0.0 | |
| Constituents | l.r.i. 1 | Relative Abundance (%) | Aroma Contribution 2 | ||
|---|---|---|---|---|---|
| (EVOO control) | ClOO | CsOO | |||
| n-Hexanal | 802 | 2.7 ± 0.4 | - 3 | - | Green, fruity |
| (E)-2-Hexenal | 856 | 82.7 ± 2.3 | 0.3 ± 0.1 | 0.1 ± 0.0 | Sweet, fruity, fragrant |
| p-Xylene | 870 | 1.5 ± 0.1 | - | - | |
| 1-Hexanol | 871 | - | - | 0.2 ± 0.0 | |
| o-Xylene | 897 | 1.6 ± 0.2 | - | - | |
| 3-Ethyl-1,5-octadiene (isomer 1) | 898 | 0.7 ± 0.0 | - | - | |
| 3-Ethyl-1,5-octadiene (isomer 2) | 901 | 0.5 ± 0.0 | - | - | |
| α-Thujene | 931 | - | 0.7 ± 0.1 | - | |
| α-Pinene | 941 | - | 2.9 ± 0.3 | 1.2 ± 0.2 | Pine-, turpentine-like |
| 1-Ethyl-4-methylbenzene | 965 | 0.3 ± 0.4 | - | - | |
| Sabinene | 976 | - | 2.6 ± 0.0 | 1.5 ± 0.2 | |
| β-Pinene | 982 | - | 13.6 ± 0.6 | 0.9 ± 0.1 | Dry, woody, resinous |
| Myrcene | 993 | - | 2.6 ± 0.1 | 3.0 ± 0.2 | Sweet, balsamic |
| n-Octanal | 1001 | - | - | 0.5 ± 0.1 | Citrus, honey-like |
| α-Terpinene | 1018 | - | 0.3 ± 0.0 | - | |
| 1,2,4-Trimethylbenzene | 1025 | 0.2 ± 0.2 | - | - | |
| Limonene | 1032 | 1.0 ± 0.7 | 67.1 ± 0.4 | 91.3 ± 0.2 | Pleasant, lemon-like |
| (E)-β-Ocimene | 1052 | 2.0 ± 0.3 | 0.1 ± 0.0 | - | Warm herbaceous |
| γ-Terpinene | 1062 | - | 8.1 ± 0.0 | 0.4 ± 0.1 | Citrus, woody, bitter |
| Terpinolene | 1088 | - | 0.5 ± 0.0 | - | Citrus, pine-like |
| Linalool | 1101 | - | 0.1 ± 0.0 | 0.6 ± 0.4 | Pleasant, floral |
| n-Nonanal | 1102 | 0.5 ± 0.8 | - | 0.1 ± 0.1 | Citrus, rose-like |
| (E)-4,8-Dimethylnona-1,3,7-Triene | 1116 | 0.8 ± 0.1 | - | - | |
| n-Decanal | 1204 | - | - | 0.1 ± 0.1 | |
| (E)-2-Dodecene | 1205 | 0.4 ± 0.5 | - | - | |
| Neral | 1240 | - | 0.2 ± 0.0 | - | |
| Geranial | 1271 | - | 0.3 ± 0.0 | - | |
| Cyclosativene | 1368 | 0.2 ± 0.3 | - | - | |
| α-Copaene | 1376 | 2.3 ± 0.2 | - | - | |
| Valencene | 1492 | 1.3 ± 0.0 | - | - | |
| (E,E)-α-Farnesene | 1507 | 0.4 ± 0.5 | - | - | |
| Liguloxide | 1532 | 0.7 ± 0.1 | - | - | |
| Monoterpene hydrocarbons | 3.0 ± 0.4 | 99.1 ± 0.1 | 98.3 ± 0.8 | ||
| Oxygenated monoterpenes | - | 0.6 ± 0.0 | 0.6 ± 0.4 | ||
| Sesquiterpene hydrocarbons | 4.2 ± 0.5 | - | - | ||
| Oxygenated sesquiterpenes | 0.7 ± 0.1 | - | - | ||
| Non-terpene derivatives | 92.1 ± 0.1 | 0.3 ± 0.0 | 1.1 ± 0.4 | ||
| Total identified (%) | 99.9 ± 0.1 | 100.0 ± 0.0 | 100.0 ± 0.0 | ||
| Reference Extra-Virgin Olive Oil (EEC Reg/2568/91 l.m.i.) | Control EVOO | ClOO | CsOO | |
|---|---|---|---|---|
| Free Fatty Acidity (g oleic acid/kg oil) | ≤0.80 | 0.18 a | 0.18 a | 0.18 a |
| Peroxide Value (meq O2/kg oil) | ≤20.00 | 5.00 a | 5.10 a | 5.00 a |
| K232 | ≤2.50 | 1.48 a | 1.60 a | 1.52 a |
| K270 | ≤0.22 | 0.12 a | 0.13 a | 0.16 a |
| ΔK | ≤0.10 | 0.00 a | 0.00 a | 0.00 a |
| Control EVOO | ClOO | CsOO | |
|---|---|---|---|
| Total Phenol Content (TPC) (ppm gallic acid) | 398 a ** | 242 b ** | 219 c ** |
| Intensity of Bitterness (IB) | 5.38 a ** | 2.19 c ** | 2.29 b ** |
| Antioxidant capacity (AC) (μmol TEAC/mL) | 0.27 a | 0.11 b | 0.12 b |
| Total Carotenoid (TC) (mg/kg lutein) | 0.98 b *** | 0.94 b *** | 5.88 a *** |
| EVOO Control | ClOO | CsOO | |
|---|---|---|---|
| Luteolin-7-O-glucoside | 1.10 ± 0.02 b | 2.92 ± 0.07 a | 1.13 ± 0.09 b |
| Rutin | 0.05 ± 0.00 b | n.d. | n.d. |
| Quercetin-3-O-glucoside | 0.11 ± 0.00 a | n.d. | n.d. |
| Apigenin-7-O-glucoside | 3.45 ± 0.04 a | 0.04 ± 0.00 b | 0.01 ± 0.00 b |
| Quercitrin | 4.30 ± 0.07 a | 2.13 ± 0.09 c | 2.72 ± 0.08 b |
| Quercetin-3-O-glucuronide | 2.22 ± 0.08 a | 0.18 ± 0.00 b | 0.07 ± 0.00 c |
| Quercetin | 1.23 ± 0.03 a | 0.01 ± 0.00 c | 0.08 ± 0.00 b |
| Luteolin | 18.88 ± 0.32 b | 22.72 ± 0.30 a | 18.14 ± 0.67 b |
| Kaempferol | 20.37 ± 0.69 a | 2.70 ± 0.13 b | 0.18 ± 0.03 c |
| Naringenin | n.d. | 10.53 ± 0.49 a | 6.11 ± 0.59 b |
| Total | 51.71 ± 0.37 a | 41.22 ± 0.63 b | 28.45 ± 1.46 c |
| EVOO Control | ClOO | CsOO | |
|---|---|---|---|
| PHENOLIC ALCOHOLS | |||
| Hydroxytyrosol | 0.99 ± 0.004 b | 1.39 ± 0.04 a | 1.47± 0.03 a |
| Tyrosol | 28.36 ± 3.30 b | 98.05 ± 8.36 a | 93.46 ± 0.94 a |
| PHENOLIC ALDEHYDES | |||
| Vanillin | 0.98 ± 0.05 a | 1.01 ± 0.04 a | 0.99 ± 0.01 a |
| PHENOLIC ACIDS | |||
| Chlorogenic acid | n.d. | 0.20 ± 0.01 a | 0.18 ± 0.01 a |
| Vanillic acid | 0.23 ± 0.01 c | 1.29 ± 0.04 a | 0.74 ± 0.02 b |
| Caffeic acid | 0.12 ± 0.003 a | 0.13 ± 0.01 a | 0.12 ± 0.00 a |
| Syringic acid | 0.06 ± 0.002 a | 0.08 ± 0.01 a | 0.03 ± 0.01 b |
| p-coumaric acid | 0.14 ± 0.004 c | 0.50 ± 0.02 a | 0.40 ± 0.01 b |
| Ferulic acid | 0.68 ± 0.02 b | 9.42 ± 1.38 a | 2.50 ± 0.10 b |
| Rosmarinic acid | n.d. | 0.43 ± 0.02 b | 0.59 ± 0.04 a |
| TOTAL | 31.56 ± 3.31 b | 112.50 ± 7.12 a | 100.50 ± 1.03 a |
| Ripeness Index (0:7) [38] | 3.5 ± 0.2 |
| Average Weight (g) | 1.56 ± 0.02 |
| Average Volume (cm3) | 1.67 ± 0.1 |
| Water Content (%) | 50.31 ± 0.03 |
| Dry Matter (%) | 49.69 ± 0.03 |
| Oil Content (% d.m.) | 32.20 ± 0.04 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ascrizzi, R.; Taglieri, I.; Sgherri, C.; Flamini, G.; Macaluso, M.; Sanmartin, C.; Venturi, F.; Quartacci, M.F.; Pistelli, L.; Zinnai, A. Nutraceutical Oils Produced by Olives and Citrus Peel of Tuscany Varieties as Sources of Functional Ingredients. Molecules 2019, 24, 65. https://doi.org/10.3390/molecules24010065
Ascrizzi R, Taglieri I, Sgherri C, Flamini G, Macaluso M, Sanmartin C, Venturi F, Quartacci MF, Pistelli L, Zinnai A. Nutraceutical Oils Produced by Olives and Citrus Peel of Tuscany Varieties as Sources of Functional Ingredients. Molecules. 2019; 24(1):65. https://doi.org/10.3390/molecules24010065
Chicago/Turabian StyleAscrizzi, Roberta, Isabella Taglieri, Cristina Sgherri, Guido Flamini, Monica Macaluso, Chiara Sanmartin, Francesca Venturi, Mike Frank Quartacci, Luisa Pistelli, and Angela Zinnai. 2019. "Nutraceutical Oils Produced by Olives and Citrus Peel of Tuscany Varieties as Sources of Functional Ingredients" Molecules 24, no. 1: 65. https://doi.org/10.3390/molecules24010065