Rapid Determination of Nutritional Parameters of Pasta/Sauce Blends by Handheld Near-Infrared Spectroscopy
Abstract
1. Introduction
2. Experimental Section
2.1. Experimental Set-Up
2.2. Instrumentation
2.3. Materials
2.4. Spectral Preprocessing Treatment
2.5. Chemometric Data Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sorak, D.; Herberholz, L.; Iwascek, S.; Altinpinar, S.; Pfeifer, F.; Siesler, H.W. New developments and applications of handheld raman, mid-infrared, and near-infrared spectrometers. Appl. Spectrosc. Rev. 2012, 47, 83–115. [Google Scholar]
- Crocombe, R.A. Portable Spectroscopy. Appl. Spectrosc. 2018, 72, 1701–1751. [Google Scholar] [CrossRef]
- Guillemain, A.; Dégardin, K.; Roggo, Y. Performance of NIR handheld spectrometers for the detection of counterfeit tablets. Talanta 2017, 165, 632–640. [Google Scholar] [PubMed]
- Soriano-Disla, J.M.; Janik, L.J.; McLaughlin, M.J. Assessment of cyanide contamination in soils with a handheld mid-infrared spectrometer. Talanta 2018, 178, 400–409. [Google Scholar] [CrossRef]
- Yakes, B.J.; Brückner, L.; Karunathilaka, S.R.; Mossoba, M.M.; He, K. First use of handheld Raman spectroscopic devices and on-board chemometric analysis for the detection of milk powder adulteration. Food Control 2018, 92, 137–146. [Google Scholar]
- Jentzsch, P.V.; Gualpa, F.; Ramos, L.A.; Ciobotă, V. Adulteration of clove essential oil: Detection using a handheld Raman spectrometer. Flavour Fragr. J. 2018, 33, 184–190. [Google Scholar] [CrossRef]
- Pügner, T.; Knobbe, J.; Grüger, H. Near-Infrared Grating Spectrometer for Mobile Phone Applications. Appl. Spectrosc. 2016, 70, 734–745. [Google Scholar] [CrossRef]
- BASF HertzstueckTM Smartphone NIR. Spectrosc. Eur. 2018, 30, 11.
- Lohumi, S.; Lee, S.; Lee, H.; Cho, B.K. A review of vibrational spectroscopic techniques for the detection of food authenticity and adulteration. Trends Food Sci. Technol. 2015, 46, 85–98. [Google Scholar] [CrossRef]
- Lohumi, S.; Mo, C.; Cho, B.-K.; Hong, S.-J.; Kang, J.-S. Nondestructive Evaluation for the Viability of Watermelon (Citrullus lanatus) Seeds Using Fourier Transform Near Infrared Spectroscopy. J. Biosyst. Eng. 2014, 38, 312–317. [Google Scholar] [CrossRef]
- Dos Santos, C.A.T.; Lopo, M.; Páscoa, R.N.M.J.; Lopes, J.A. A review on the applications of portable near-infrared spectrometers in the agro-food industry. Appl. Spectrosc. 2013, 67, 1215–1233. [Google Scholar] [CrossRef]
- Grassi, S.; Casiraghi, E.; Alamprese, C. Handheld NIR device: A non-targeted approach to assess authenticity of fish fillets and patties. Food Chem. 2018, 243, 382–388. [Google Scholar] [CrossRef]
- Karunathilaka, S.R.; Yakes, B.J.; He, K.; Chung, J.K.; Mossoba, M. Non-targeted NIR spectroscopy and SIMCA classification for commercial milk powder authentication: A study using eleven potential adulterants. Heliyon 2018, 4, e00806. [Google Scholar] [CrossRef] [PubMed]
- Fardin-Kia, A.R.; Mossoba, M.M.; Chung, J.K.; Srigley, C.; Karunathilaka, S.R. Rapid screening of commercial extra virgin olive oil products for authenticity: Performance of a handheld NIR device. NIR News 2017, 28, 9–14. [Google Scholar]
- Santos, P.M.; Pereira-Filho, E.R.; Rodrigues-Saona, L.E. Application of Hand-Held and Portable Infrared Spectrometers in Bovine Milk Analysis. J. Agric. Food Chem. 2015, 61, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Parra, H.A.; Pustjens, A.; Hettinga, K.; Mongondry, P.; van Ruth, S.M. Evaluation of portable near-infrared spectroscopy for organic milk authentication. Talanta 2018, 184, 128–135. [Google Scholar] [CrossRef]
- De Lima, G.F.; Andrade, S.A.C.; da Silva, V.H.; Honorato, F.A. Multivariate Classification of UHT Milk as to the Presence of Lactose Using Benchtop and Portable NIR Spectrometers. Food Anal. Methods 2018, 11, 2699–2706. [Google Scholar] [CrossRef]
- Modroño, S.; Soldado, A.; Martínez-Fernández, A.; de la Roza-Delgado, B. Handheld NIRS sensors for routine compound feed quality control: Real time analysis and field monitoring. Talanta 2017, 162, 597–603. [Google Scholar] [CrossRef] [PubMed]
- González Arrojo, A.; Cuevas Valdés, M.; Garrido-Varo, A.; Maroto, F.; Pérez-Marín, D.; Soldado, A.; de la Roza-Delgado, B. Matching portable NIRS instruments for in situ monitoring indicators of milk composition. Food Control 2017, 76, 74–81. [Google Scholar]
- Wiedemair, V.; Huck, C.W. Evaluation of the performance of three hand-held near-infrared spectrometer through investigation of total antioxidant capacity in gluten-free grains. Talanta 2018, 189, 233–240. [Google Scholar] [CrossRef]
- Pérez-Marín, D.; Paz, P.; Guerrero, J.E.; Garrido-Varo, A.; Sánchez, M.T. Miniature handheld NIR sensor for the on-site non-destructive assessment of post-harvest quality and refrigerated storage behavior in plums. J. Food Eng. 2010, 99, 294–302. [Google Scholar] [CrossRef]
- Basri, K.N.; Hussain, M.N.; Bakar, J.; Sharif, Z.; Khir, M.F.A.; Zoolfakar, A.S. Classification and quantification of palm oil adulteration via portable NIR spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 335–342. [Google Scholar] [CrossRef]
- Basri, K.N.; Laili, A.R.; Tuhaime, N.A.; Hussain, M.N.; Bakar, J.; Sharif, Z.; Abdul Khir, M.F.; Zoolfakar, A.S. FT-NIR, MicroNIR and LED-MicroNIR for detection of adulteration in palm oil via PLS and LDA. Anal. Methods 2018, 10, 4143–4151. [Google Scholar] [CrossRef]
- Rodrigues, R.R.T.; Tosato, F.; Correia, R.M.; Domingos, E.; Aquino, L.F.M.; Romão, W.; Lacerda, V.; Filgueiras, P.R. Portable near infrared spectroscopy applied to quality control of Brazilian coffee. Talanta 2017, 176, 59–68. [Google Scholar]
- Sánchez, M.T.; Entrenas, J.A.; Torres, I.; Vega, M.; Pérez-Marín, D. Monitoring texture and other quality parameters in spinach plants using NIR spectroscopy. Comput. Electron. Agric. 2018, 155, 446–452. [Google Scholar] [CrossRef]
- Yan, H.; Siesler, H.W. Hand-held near-infrared spectrometers: State-of-the-art instrumentation and practical applications. NIR News 2018, 29, 8–12. [Google Scholar] [CrossRef]
- Yan, H.; Siesler, H.W. Handheld Raman, Mid-Infrared and Near Infrared Spectrometers: State-of-the-Art Instrumentation and Useful Applications. Spectroscopy 2018, 33, 6–16. [Google Scholar]
- Kolomiets, O.; Hoffmann, U.; Geladi, P.; Siesler, H.W. Quantitative Determination of Pharmaceutical Drug Formulations by Near-Infrared Spectroscopic Imaging. Appl. Spectrosc. 2008, 62, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Hudak, S.J.; Haber, K.; Sando, G.; Kidder, L.H.; Lewis, E.N. Practical limits of spatial resolution in diffuse reflectance NIR chemical imaging. NIR News 2007, 18, 6–8. [Google Scholar] [CrossRef]
- Bashkatov, A.N.; Genina, E.A.; Kochubey, V.I.; Tuchin, V.V. Optical properties of human skin, subcutaneous and mucous tissues in the wavelength range from 400 to 2000 nm. J. Phys. D Appl. Phys. 2005, 38, 2543–2555. [Google Scholar] [CrossRef]
- Sjöström, M.; Eriksson, L.; Wold, S. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar]
- Geladi, P.; Kowalski, B.R. Partial Least-Squares regression: A tutorial. Analytica Chimica Acta 1986, 185, 1–17. [Google Scholar] [CrossRef]
- Savitzky, A.; Golay, M.J.E. Smoothing and Differentiation of Data by Simplified Least Squares Procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Afseth, N.K.; Kohler, A. Extended multiplicative signal correction in vibrational spectroscopy, a tutorial. Chemom. Intell. Lab. Syst. 2012, 117, 92–99. [Google Scholar] [CrossRef]
- Rinnan, Å.; van den Berg, F.; Engelsen, S.B. Review of the most common pre-processing techniques for near-infrared spectra. TrAC Trends Anal. Chem. 2009, 28, 1201–1222. [Google Scholar] [CrossRef]
- Martens, H.; Stark, E. Extended multiplicative signal correction and spectral interference subtraction: New preprocessing methods for near infrared spectroscopy. J. Pharm. Biomed. Anal. 1991, 9, 625–635. [Google Scholar] [CrossRef]
- Fearn, T. Assessing calibrations: SEP, RPD, RER and R2. NIR News 2002, 6, 12–13. [Google Scholar] [CrossRef]
- Williams, P.C.; Sobering, D.C. Comparison of Commercial near Infrared Transmittance and Reflectance Instruments for Analysis of Whole Grains and Seeds. J. Near Infrared Spectrosc. 1993, 1, 25–32. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |




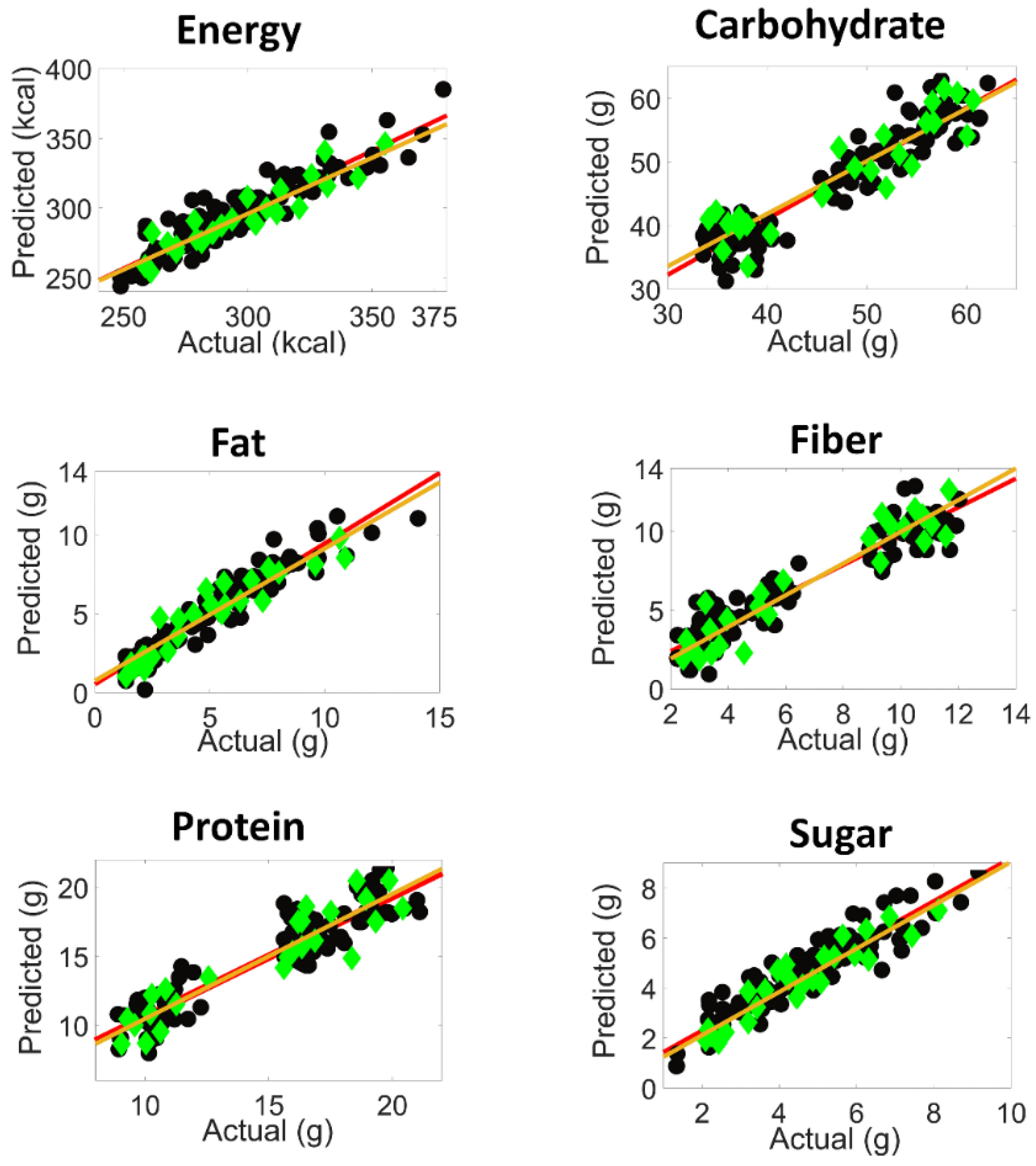
 ), prediction fit (
), prediction fit ( ), calibration samples (
), calibration samples ( ) and predicted test set samples (
) and predicted test set samples ( )).
)).
 ), prediction fit (
), prediction fit ( ), calibration samples (
), calibration samples ( ) and predicted test set samples (
) and predicted test set samples ( )).
)).
| Sample | Energy (kcal) | Carbohydrate (g) | Fat (g) | Fiber (g) | Protein (g) | Sugar (g) |
|---|---|---|---|---|---|---|
| Pasta | ||||||
| 1 | 374.0 | 75.0 | 1.8 | 3.0 | 13.5 | 3.0 |
| 2 | 347.0 | 69.0 | 2.1 | 4.0 | 12.0 | 6.0 |
| 3 | 360.0 | 61.0 | 2.8 | 6.5 | 21.0 | 3.4 |
| 4 | 335.0 | 47.4 | 2.9 | 12.0 | 25.0 | 1.8 |
| 5 | 348.0 | 45.1 | 7.3 | 14.0 | 21.0 | 2.9 |
| Sauce | ||||||
| 1 | 97.0 | 6.8 | 7.7 | 1.8 | 3.4 | 5.0 |
| 2 | 136.0 | 8.6 | 11.3 | 2.0 | 3.0 | 6.5 |
| 3 | 74.0 | 6.6 | 4.7 | 2.1 | 1.5 | 4.8 |
| 4 | 91.0 | 6.0 | 7.4 | 1.4 | 1.5 | 5.4 |
| 5 | 33.0 | 4.8 | 0.9 | 1.0 | 1.0 | 3.9 |
| Parameter | Energy | Carbohydrate | Fat | Fiber | Protein | Sugar |
|---|---|---|---|---|---|---|
| # LVs | 8 | 8 | 7 | 8 | 8 | 8 |
| RMSEC | 11.15 a | 2.97 b | 0.83 b | 1.10 b | 1.36 b | 0.65 b |
| RMSECV | 13.10 a | 3.43 b | 0.94 b | 1.27 b | 1.56 b | 0.74 b |
| RMESEP | 10.64 a | 3.59 b | 0.95 b | 1.11 b | 1.39 b | 0.61 b |
| Content Range | 248.67–378.54 a | 33.55–62.13 b | 1.34–14.06 b | 2.23–12.03 b | 8.89–21.67 b | 1.34–9.15 b |
| R2 Cal | 0.85 | 0.89 | 0.91 | 0.89 | 0.87 | 0.86 |
| R2 CV | 0.80 | 0.85 | 0.88 | 0.85 | 0.83 | 0.82 |
| R2 Pred | 0.86 | 0.85 | 0.89 | 0.90 | 0.86 | 0.88 |
| RPD | 2.02 | 2.54 | 2.77 | 2.45 | 2.26 | 2.19 |
| Slope CV | 0.85 | 0.89 | 0.91 | 0.89 | 0.87 | 0.86 |
| Offset CV | 43.12 a | 5.21 b | 0.46 b | 0.73 b | 1.92 b | 0.62 b |
| Slope Pred | 0.80 | 0.83 | 0.84 | 0.99 | 0.91 | 0.87 |
| Offset Pred | 55.0 a | 8.81 b | 0.75 b | 0.37 b | 1.40 b | 0.38 b |
| Energy (kcal) | Carbohydrate (g) | Fat (g) | ||||||
|---|---|---|---|---|---|---|---|---|
| Actual | Predicted | Relative Error (%) | Actual | Predicted | Relative Error (%) | Actual | Predicted | Relative Error (%) |
| 299.8 | 307.9 | 2.7 | 60.1 | 54.1 | 9.9 | 1.4 | 1.0 | 25.3 |
| 355.2 | 346.3 | 2.5 | 59.1 | 61.1 | 3.4 | 6.8 | 7.1 | 4.4 |
| 281.4 | 275.7 | 2.0 | 60.7 | 59.8 | 1.3 | 4.8 | 6.5 | 35.1 |
| 331.9 | 315.8 | 4.9 | 57.8 | 61.3 | 6.2 | 1.6 | 1.8 | 16.7 |
| 285.7 | 282.0 | 1.3 | 56.0 | 56.2 | 0.3 | 5.7 | 5.5 | 2.8 |
| 260.8 | 254.4 | 2.5 | 51.7 | 54.0 | 4.5 | 3.2 | 2.6 | 17.9 |
| 279.3 | 277.7 | 0.6 | 56.6 | 59.3 | 4.7 | 7.3 | 5.8 | 20.5 |
| 330.9 | 340.5 | 2.9 | 54.5 | 50.0 | 8.3 | 1.7 | 1.6 | 9.2 |
| 289.4 | 287.4 | 0.7 | 56.7 | 56.4 | 0.5 | 3.6 | 4.7 | 27.9 |
| 258.6 | 258.4 | 0.1 | 53.3 | 51.6 | 3.2 | 5.1 | 5.6 | 10.0 |
| 271.2 | 272.2 | 0.4 | 45.5 | 44.2 | 3.0 | 4.3 | 5.0 | 15.2 |
| 307.5 | 299.7 | 2.5 | 47.2 | 52.0 | 10.3 | 2.1 | 2.3 | 8.4 |
| 344.2 | 321.6 | 6.6 | 48.8 | 49.0 | 0.4 | 2.2 | 1.5 | 32.8 |
| 325.6 | 323.5 | 0.7 | 50.4 | 48.3 | 4.2 | 3.6 | 3.6 | 1.0 |
| 303.1 | 288.7 | 4.8 | 51.9 | 45.7 | 12.0 | 10.6 | 9.8 | 8.1 |
| 320.6 | 300.2 | 6.4 | 45.7 | 44.7 | 2.3 | 5.6 | 7.0 | 23.7 |
| 267.7 | 274.8 | 2.7 | 50.1 | 45.6 | 8.9 | 2.2 | 2.2 | 1.8 |
| 293.6 | 291.8 | 0.6 | 35.5 | 36.2 | 2.0 | 7.6 | 7.8 | 3.8 |
| 302.3 | 290.5 | 3.9 | 37.2 | 39.4 | 5.8 | 2.5 | 2.3 | 9.4 |
| 278.5 | 290.8 | 4.4 | 40.3 | 39.1 | 3.1 | 2.8 | 4.7 | 67.4 |
| 271.2 | 268.7 | 0.9 | 37.9 | 40.2 | 6.1 | 6.3 | 5.8 | 8.4 |
| 311.6 | 296.4 | 4.9 | 36.1 | 39.9 | 10.6 | 8.0 | 7.6 | 5.2 |
| 313.0 | 313.3 | 0.1 | 38.4 | 45.4 | 18.1 | 5.5 | 5.0 | 8.9 |
| 261.4 | 282.3 | 8.0 | 37.2 | 40.7 | 9.4 | 9.6 | 8.1 | 15.5 |
| 286.0 | 282.5 | 1.2 | 34.1 | 41.3 | 21.1 | 10.9 | 8.5 | 21.6 |
| Average Relative Error (%) | 2.7 | Average Relative Error (%) | 6.4 | Average Relative Error (%) | 16.1 | |||
| Fiber (g) | Protein (g) | Sugar (g) | ||||||
|---|---|---|---|---|---|---|---|---|
| Actual | Predicted | Relative Error (%) | Actual | Predicted | Relative Error (%) | Actual | Predicted | Relative Error (%) |
| 3.2 | 5.5 | 70.2 | 12.6 | 13.5 | 7.1 | 3.2 | 2.6 | 17.9 |
| 3.4 | 3.8 | 12.3 | 10.0 | 8.7 | 13.4 | 3.4 | 3.2 | 5.6 |
| 2.6 | 3.1 | 22.2 | 10.6 | 9.5 | 10.3 | 6.3 | 5.1 | 18.6 |
| 2.4 | 1.9 | 20.7 | 11.3 | 11.5 | 2.3 | 2.2 | 2.1 | 5.6 |
| 2.8 | 2.4 | 14.8 | 10.8 | 12.6 | 16.4 | 8.1 | 7.1 | 12.4 |
| 4.0 | 4.4 | 12.0 | 10.3 | 12.2 | 18.6 | 4.5 | 3.6 | 18.6 |
| 3.7 | 2.7 | 26.2 | 9.3 | 10.5 | 12.5 | 5.6 | 6.1 | 8.1 |
| 3.0 | 1.9 | 36.7 | 9.6 | 10.0 | 4.4 | 6.9 | 6.9 | 0.1 |
| 3.4 | 2.2 | 35.0 | 9.1 | 8.6 | 4.7 | 7.4 | 6.1 | 18.2 |
| 4.6 | 2.3 | 49.7 | 10.2 | 10.8 | 6.0 | 5.4 | 5.3 | 3.0 |
| 5.9 | 6.9 | 16.4 | 15.8 | 14.9 | 5.4 | 6.3 | 6.3 | 1.5 |
| 5.1 | 6.1 | 17.9 | 18.4 | 14.9 | 19.1 | 3.6 | 3.9 | 7.9 |
| 5.0 | 5.2 | 3.9 | 16.9 | 16.1 | 4.4 | 4.6 | 4.1 | 10.9 |
| 5.4 | 4.7 | 12.9 | 16.0 | 15.3 | 4.2 | 2.6 | 2.2 | 14.3 |
| 10.1 | 10.3 | 1.4 | 15.6 | 14.2 | 9.3 | 3.2 | 3.9 | 21.0 |
| 10.5 | 11.4 | 8.8 | 19.3 | 17.5 | 9.4 | 4.9 | 4.3 | 11.2 |
| 9.4 | 11.1 | 18.8 | 19.9 | 20.5 | 3.2 | 2.4 | 1.8 | 25.3 |
| 8.9 | 9.6 | 7.5 | 20.4 | 18.5 | 9.5 | 2.1 | 1.9 | 7.9 |
| 9.3 | 8.0 | 13.6 | 18.6 | 20.5 | 10.3 | 4.3 | 4.4 | 2.9 |
| 9.7 | 10.4 | 7.2 | 18.9 | 19.2 | 1.2 | 6.0 | 5.3 | 10.6 |
| 10.8 | 11.0 | 2.2 | 16.3 | 15.7 | 4.2 | 2.2 | 2.3 | 8.7 |
| 11.1 | 10.4 | 6.0 | 17.5 | 18.2 | 4.0 | 4.0 | 4.7 | 18.8 |
| 11.7 | 12.6 | 8.1 | 16.2 | 17.5 | 8.1 | 4.2 | 4.9 | 18.0 |
| 10.8 | 9.4 | 13.4 | 16.3 | 17.5 | 7.4 | 5.2 | 5.3 | 1.8 |
| 11.6 | 9.7 | 16.2 | 16.5 | 18.6 | 12.7 | 5.1 | 4.2 | 16.8 |
| Average Relative Error (%) | 18.2 | Average Relative Error (%) | 8.3 | Average Relative Error (%) | 11.4 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neves, M.D.G.; Poppi, R.J.; Siesler, H.W. Rapid Determination of Nutritional Parameters of Pasta/Sauce Blends by Handheld Near-Infrared Spectroscopy. Molecules 2019, 24, 2029. https://doi.org/10.3390/molecules24112029
Neves MDG, Poppi RJ, Siesler HW. Rapid Determination of Nutritional Parameters of Pasta/Sauce Blends by Handheld Near-Infrared Spectroscopy. Molecules. 2019; 24(11):2029. https://doi.org/10.3390/molecules24112029
Chicago/Turabian StyleNeves, Marina D. G., Ronei J. Poppi, and Heinz W. Siesler. 2019. "Rapid Determination of Nutritional Parameters of Pasta/Sauce Blends by Handheld Near-Infrared Spectroscopy" Molecules 24, no. 11: 2029. https://doi.org/10.3390/molecules24112029
APA StyleNeves, M. D. G., Poppi, R. J., & Siesler, H. W. (2019). Rapid Determination of Nutritional Parameters of Pasta/Sauce Blends by Handheld Near-Infrared Spectroscopy. Molecules, 24(11), 2029. https://doi.org/10.3390/molecules24112029







