Pharmacokinetics and Tissue Distribution of a Novel Bis-Chelated Gold(I) Diphosphine Compound, Bis(2,3-bis(tert-butylmethylphosphino)Quinoxaline)Aurate(I), in Rats
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of LC-MS/MS Conditions
2.2. Method Validation
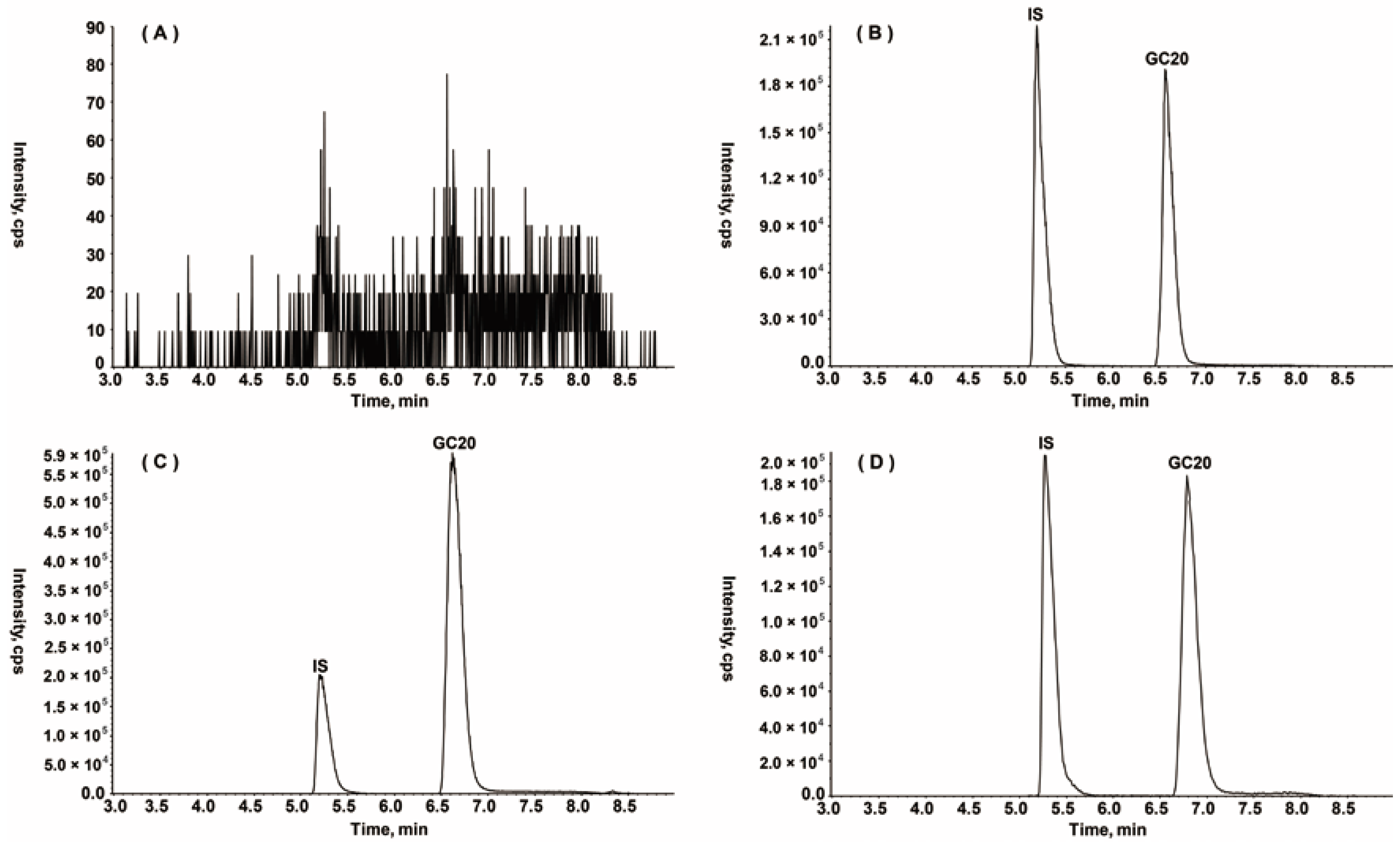
2.2.1. Specificity
2.2.2. Linearity and Sensitivity
2.2.3. Precision and Accuracy
2.2.4. Recovery and Matrix Effect
2.2.5. Stability Studies
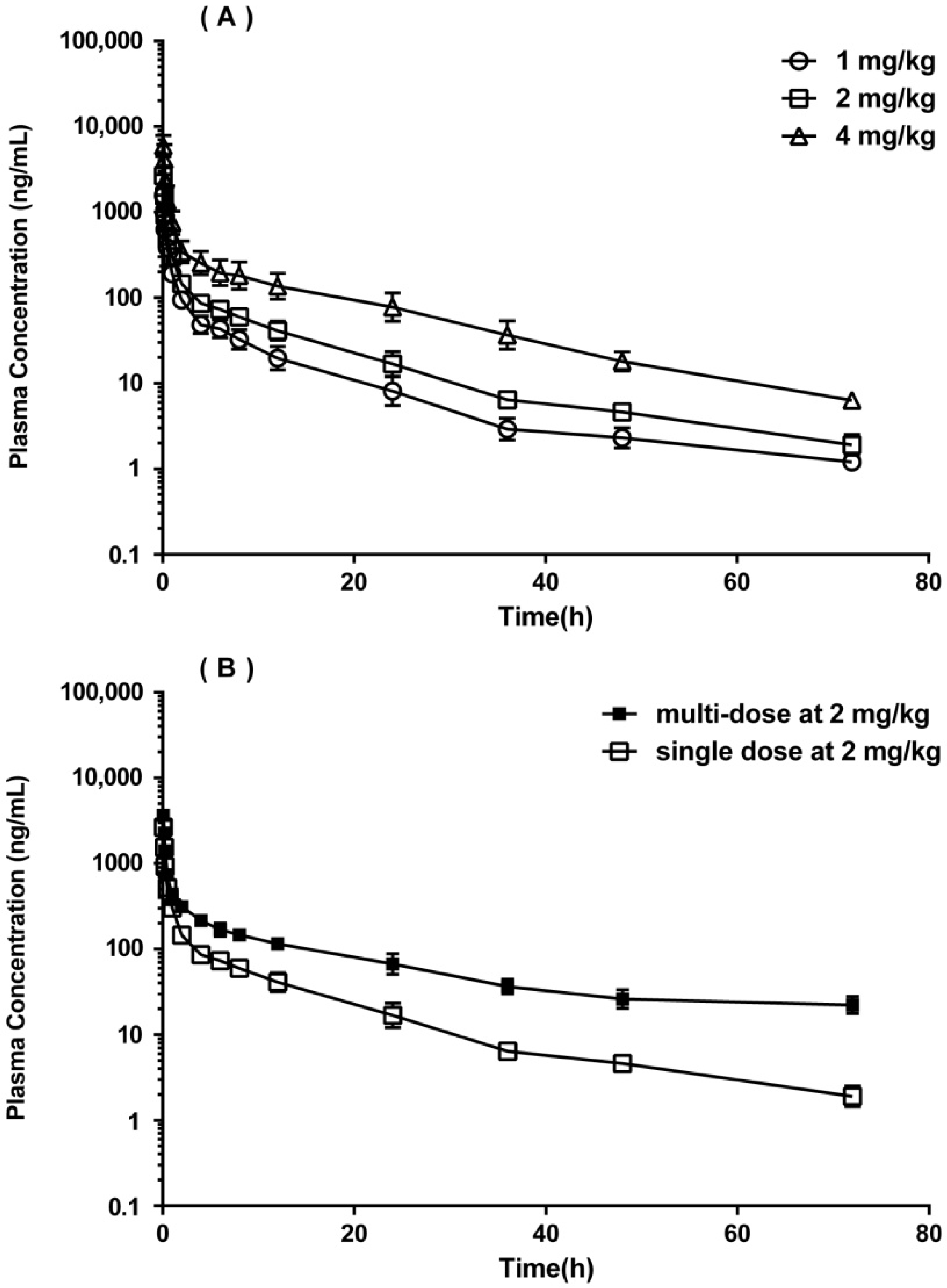
2.3. Pharmacokinetic Study in SD Rats
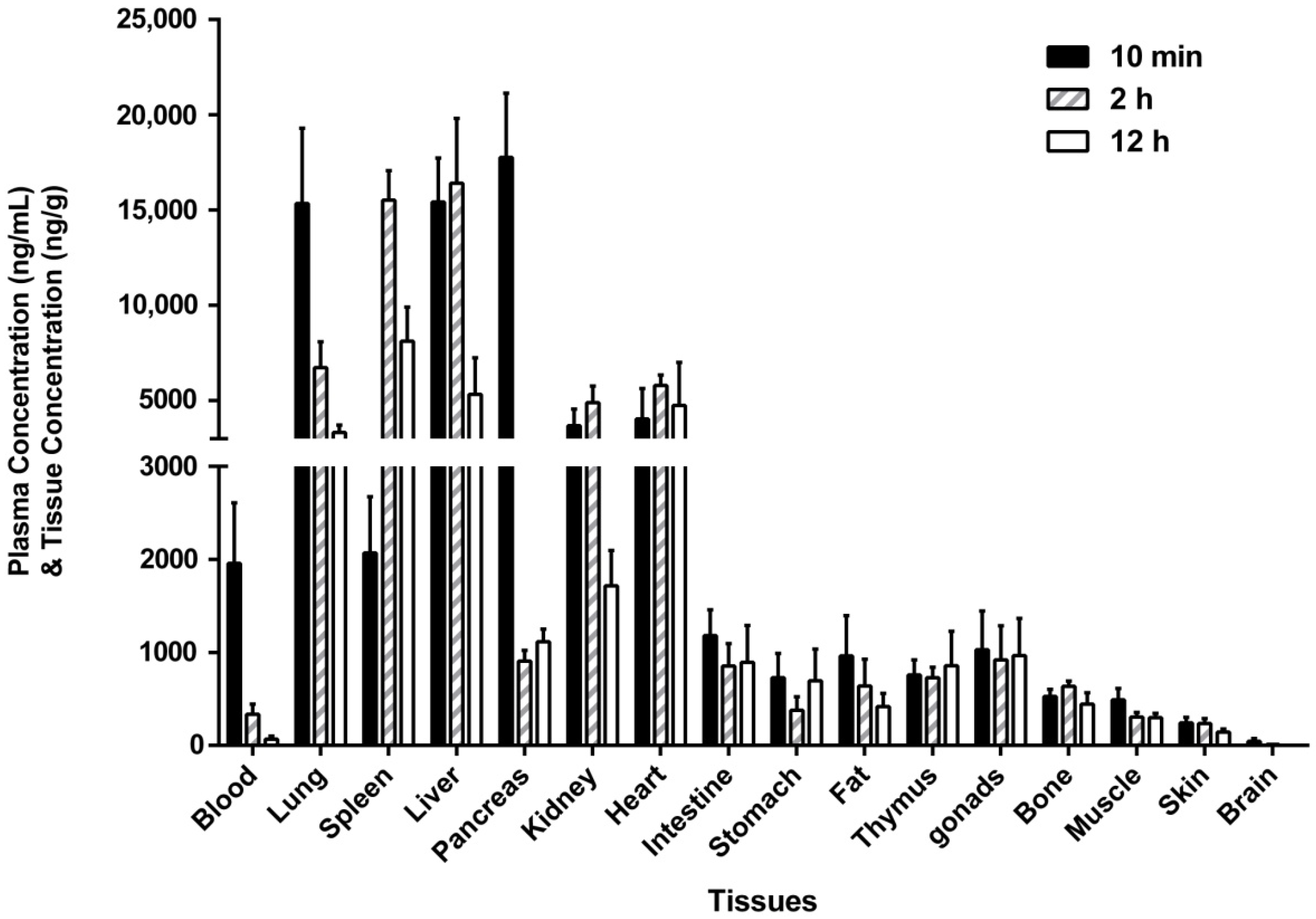
2.4. Tissue Distribution
3. Material and Methods
3.1. Chemicals and Reagents
3.2. Chromatographic and Mass Spectrometer Conditions
3.3. Preparation of Standard Solutions and Calibration Samples
3.4. Sample Preparation
3.5. Analysis Method Validation
3.6. Pharmacokinetic Study in Rats
3.7. Tissue Distribution in Rats
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Basenengquist, K. Intraperitoneal Cisplatin and Paclitaxel in Ovarian Cancer. Obstet. Gynecol. 2006, 107, 948–949. [Google Scholar]
- Mok, T.S.; Wu, Y.L.; Thongprasert, S.; Yang, C.H.; Chu, D.T.; Saijo, N.; Sunpaweravong, P.; Han, B.; Margono, B.; Ichinose, Y. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 2009, 361, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Fukuchi, M.; Manda, R.; Nakajima, M.; Miyazaki, T.; Sohda, M.; Masuda, N.; Fukai, Y.; Tsukada, K.; Kuwano, H. Efficacy and toxicity of nedaplatin and 5-FU with radiation treatment for advanced esophageal carcinomas. Anticancer Res. 2003, 23, 3493–3498. [Google Scholar] [PubMed]
- Li, G.; Chen, X.; Wu, X.; Xie, J.; Liang, Y.; Zhao, X.; Chen, W.; Fu, L. Effect of Dicycloplatin, a Novel Platinum Chemotherapeutical Drug, on Inhibiting Cell Growth and Inducing Cell Apoptosis. PLoS ONE 2012, 7, e48994. [Google Scholar] [CrossRef] [PubMed]
- André, T.; Boni, C.; Mounedjiboudiaf, L.; Navarro, M.; Tabernero, J.; Hickish, T.; Topham, C.; Zaninelli, M.; Clingan, P.; Bridgewater, J. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N. Engl. J. Med. 2004, 350, 2343–2351. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.Y.; Xu, Y.P.; Jin, W.; Lou, L.G. Antitumor activity of lobaplatin alone or in combination with antitubulin agents in non-small-cell lung cancer. Anti-Cancer Drug 2012, 23, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Giaccone, G. Clinical Perspectives on Platinum Resistance. Drugs 2000, 59, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Kelland, L.R. Preclinical perspectives on platinum resistance. Drugs 2000, 59, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bruijnincx, P.C.; Sadler, P.J. New trends for metal complexes with anticancer activity. Curr. Opin. Chem. Biol. 2008, 12, 197–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nobili, S.; Mini, E.; Landini, I.; Gabbiani, C.; Casini, A.; Messori, L. Gold compounds as anticancer agents: Chemistry, cellular pharmacology, and preclinical studies. Med. Res. Rev. 2010, 30, 550–580. [Google Scholar] [CrossRef] [PubMed]
- Gandin, V.; Fernandes, A.P.; Rigobello, M.P.; Dani, B.; Sorrentino, F.; Tisato, F.; Björnstedt, M.; Bindoli, A.; Sturaro, A.; Rella, R. Cancer cell death induced by phosphine gold(I) compounds targeting thioredoxin reductase. Biochem. Pharmacol. 2010, 79, 90–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernersprice, S.J.; Filipovska, A. Gold compounds as therapeutic agents for human diseases. Metallomics 2011, 3, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.C.; Rodriguez, L. Phosphine-gold(I) compounds as anticancer agents: General description and mechanisms of action. Anticancer Agents Med. Chem. 2011, 11, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Craig, S.; Gao, L.; Lee, I.; Gray, T.; Berdis, A.J. Gold-containing indoles as anticancer agents that potentiate the cytotoxic effects of ionizing radiation. J. Med. Chem. 2012, 55, 2437–2451. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, M.; Cao, R.; Zhang, W.; Yin, M.; Xiao, X.; Liu, Q.; Huang, N. A soluble bis-chelated gold(I) diphosphine compound with strong anticancer activity and low toxicity. J. Med. Chem. 2013, 56, 1455–1466. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services Food and Drug Administration. Guidance for Industry, Bioanalytical Method Validation. Federal Register 2001, 66, 206–207. [Google Scholar]
Sample Availability: Samples of the GC20 and IS (Ketoconazole) are available from the authors. |

| Matrix | y = a + bx * | ||
|---|---|---|---|
| a | b | r | |
| Plasma | 3.92 × 10−4 | 2.34 × 10−4 | 0.9998 |
| Heart | −1.04 × 10−4 | 2.23 × 10−3 | 0.9998 |
| Liver | 4.72 × 10−4 | 2.56 × 10−3 | 0.9997 |
| Spleen | 8.98 × 10−4 | 2.39 × 10−3 | 0.9998 |
| Lung | −7.26 × 10−4 | 2.37 × 10−3 | 0.9995 |
| Kidney | 5.69 × 10−4 | 2.56 × 10−3 | 0.9996 |
| Stomach | 4.04 × 10−4 | 2.40 × 10−3 | 0.9999 |
| Intestine | −9.33 × 10−5 | 2.55 × 10−3 | 0.9998 |
| Pancreas | 6.58 × 10−3 | 2.60 × 10−3 | 0.9998 |
| Thymus | 4.19 × 10−4 | 2.49 × 10−3 | 0.9998 |
| Gonds | 5.32 × 10−4 | 2.07 × 10−3 | 0.9999 |
| Skin | 1.06 × 10−3 | 3.12 × 10−3 | 0.9999 |
| Muscle | 8.77 × 10−4 | 3.61 × 10−3 | 0.9999 |
| Fat | 6.61 × 10−4 | 1.94 × 10−3 | 0.9997 |
| Brain | 6.85 × 10−4 | 2.14 × 10−3 | 0.9997 |
| Bone | 4.00 × 10−4 | 2.13 × 10−3 | 0.9997 |
| Spiked Concentration (ng/mL) | Intra-day | Inter-day | ||||
|---|---|---|---|---|---|---|
| Measured Concentration (ng/mL) | Precision (RSD%) | Accuracy (RE%) | Measured Concentration (ng/mL) | Precision (RSD%) | Accuracy (RE%) | |
| 2 | 2.0 ± 0.1 | 2.9 | 0.5 | 2.0 ± 0.0 | 2.2 | 0.2 |
| 50 | 50.8 ± 2.7 | 5.3 | 1.6 | 52.4 ± 0.8 | 1.5 | 4.8 |
| 1000 | 1020.9 ± 76.8 | 7.5 | 2.1 | 1074.3 ± 22.6 | 2.1 | 7.4 |
| Analyte | Spiked Concentration (ng/mL) | Recovery (%) | Matrix Effect (%) |
|---|---|---|---|
| GC20 | 2 | 99.3 ± 5.5 | 100.0 ± 4.8 |
| 50 | 97.2 ± 4.7 | 103.4 ± 2.5 | |
| 1000 | 95.5 ± 1.2 | 98.3 ± 1.3 |
| Storage Conditions | Spiked Concentration (ng/mL) | Measured Concentration (ng/mL) | Precision (RSD%) | Accuracy (RE%) |
|---|---|---|---|---|
| Room temperature for 12 h | 2 | 1.8 ± 0.1 | 2.9 | −9.2 |
| 50 | 51.0 ± 0.4 | 0.8 | 2.0 | |
| 1000 | 1020.7 ± 23.6 | 2.3 | 2.1 | |
| Frozen (−80 °C) for 7 days | 2 | 2.2 ± 0.2 | 1.8 | 8.2 |
| 50 | 52.0 ± 0.9 | 1.7 | 4.1 | |
| 1000 | 1030.5 ± 15.9 | 1.5 | 3.1 | |
| Frozen (−80 °C) for 60 days | 2 | 1.8 ± 0.1 | 2.8 | −8.9 |
| 50 | 47.3 ± 0.6 | 1.3 | −5.4 | |
| 1000 | 970.1 ± 27.3 | 2.8 | −3.0 | |
| Three freeze-thaw cycles | 2 | 2.2 ± 0.1 | 1.5 | 8.7 |
| 50 | 51.1 ± 0.3 | 0.7 | 2.3 | |
| 1000 | 1001.8 ± 19.0 | 1.9 | 0.2 | |
| Autosampler stability (48 h at 4 °C) | 2 | 1.8 ± 0.0 | 2.6 | −10.1 |
| 50 | 45.2 ± 0.1 | 0.3 | −9.5 | |
| 1000 | 901.3 ± 17.1 | 1.9 | −9.9 |
| Non-Compartmental Parameters | Single Dose | Multiple Dose | ||
|---|---|---|---|---|
| i.v. 1 mg/kg | i.v. 2 mg/kg | i.v. 4 mg/kg | i.v. 2 mg/kg | |
| C5min (ng/mL) | 1564.6 ± 387.5 | 2657.6 ± 632.7 | 5902.6 ± 1955.2 | 3645.8 ± 388.3 |
| AUC0–t (h*ng/mL) * | 1419.9 ± 345.5 | 2466.4 ± 298.3 | 7237.1 ± 2695.0 | 4551.2 ± 440.3 # |
| AUC0-∞ (h*ng/mL) | 1457.0 ± 354.5 | 2540.5 ± 308.6 | 7562.2 ± 2709.6 | 6936.7 ± 1005.4 |
| t1/2 (h) | 11.33 ± 0.57 | 11.24 ± 0.75 | 12.58 ± 1.80 | 25.1 ± 3.0 |
| V/F (L/kg) | 11.89 ± 3.35 | 13.00 ± 2.39 | 11.11 ± 5.28 | 10.6 ± 1.7 |
| CL/F (L/h/kg) | 0.72 ± 0.19 | 0.80 ± 0.10 | 0.60 ± 0.24 | 0.29 ± 0.04 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.; Qi, H.; Chang, Q.; Zhang, Y.; Liu, W.; Liu, M.; Liu, Q.; Wang, G.; Sun, J. Pharmacokinetics and Tissue Distribution of a Novel Bis-Chelated Gold(I) Diphosphine Compound, Bis(2,3-bis(tert-butylmethylphosphino)Quinoxaline)Aurate(I), in Rats. Molecules 2019, 24, 2082. https://doi.org/10.3390/molecules24112082
Peng Y, Qi H, Chang Q, Zhang Y, Liu W, Liu M, Liu Q, Wang G, Sun J. Pharmacokinetics and Tissue Distribution of a Novel Bis-Chelated Gold(I) Diphosphine Compound, Bis(2,3-bis(tert-butylmethylphosphino)Quinoxaline)Aurate(I), in Rats. Molecules. 2019; 24(11):2082. https://doi.org/10.3390/molecules24112082
Chicago/Turabian StylePeng, Ying, Huanhuan Qi, Qingqing Chang, Yu Zhang, Weiyi Liu, Minyu Liu, Quanhai Liu, Guangji Wang, and Jianguo Sun. 2019. "Pharmacokinetics and Tissue Distribution of a Novel Bis-Chelated Gold(I) Diphosphine Compound, Bis(2,3-bis(tert-butylmethylphosphino)Quinoxaline)Aurate(I), in Rats" Molecules 24, no. 11: 2082. https://doi.org/10.3390/molecules24112082





