Homo- and Copolymerization of Ethylene with Norbornene Catalyzed by Vanadium(III) Phosphine Complexes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Polymerization of Ethylene
2.2. Copolymerization of Ethylene with Norbornene
3. Materials and Methods
3.1. General Procedures and Materials
3.2. General (co)polymerization Procedure
3.3. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Burt, J.; Levason, W.; Reid, G. Coordination chemistry of the main group elements with phosphine, arsine and stibine ligands. Coord. Chem. Rev. 2009, 260, 65–115. [Google Scholar] [CrossRef]
- Cheng, C.; Hartwig, J.F. Rhodium-catalyzed intermolecular C-H silylation of arenes with high steric regiocontrol. Science 2014, 343, 853–857. [Google Scholar] [CrossRef]
- Scheuermann, M.L.; Johnson, E.J.; Chirik, P.J. Alkene Isomerization–Hydroboration Promoted by Phosphine-Ligated Cobalt Catalysts. Org. Lett. 2015, 17, 2716–2719. [Google Scholar] [CrossRef] [PubMed]
- Murai, M.; Takeshima, H.; Morita, H.; Kuninobu, Y.; Takai, K. Acceleration Effects of Phosphine Ligands on the Rhodium-Catalyzed Dehydrogenative Silylation and Germylation of Unactivated C(sp3)–H Bonds. J. Org. Chem. 2015, 80, 5407–5414. [Google Scholar] [CrossRef]
- Ricci, G.; Sommazzi, A.; Masi, F.; Ricci, M.; Boglia, A.; Leone, G. Well Defined Transition Metal Complexes with Phosphorus and Nitrogen Ligands for 1,3-Dienes Polymerization. Coord. Chem. Rev. 2010, 254, 661–676. [Google Scholar] [CrossRef]
- Nomura, K.; Zhang, S. Design of Vanadium Complex Catalysts for Precise Olefin Polymerization. Chem. Rev. 2011, 111, 2342–2362. [Google Scholar] [CrossRef] [PubMed]
- Gambarotta, S. Vanadium-based Ziegler–Natta: challenges, promises, problems. Coord. Chem. Rev. 2003, 237, 229–243. [Google Scholar] [CrossRef]
- Wu, J.Q.; Li, Y.S. Well-defined vanadium complexes as the catalysts for olefin polymerization. Coord. Chem. Rev. 2011, 255, 2303–2314. [Google Scholar] [CrossRef]
- Hagen, H.; Boersma, J.; van Koten, G. Homogeneous vanadium-based catalysts for the Ziegler–Natta polymerization of α-olefins. Chem. Soc. Rev. 2002, 31, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Redshaw, C. Vanadium procatalysts bearing chelating aryloxides: Structure–activity trends in ethylene polymerisation. Dalton Trans. 2010, 39, 5595–5604. [Google Scholar] [CrossRef] [PubMed]
- Christman, D.L.; Keim, G.I. Reactivities of Nonconjugated Dienes Used in Preparation of Terpolymers in Homogeneous Systems. Macromolecules 1968, 1, 358–363. [Google Scholar] [CrossRef]
- Zambelli, A.; Pasquon, I.; Signorini, R.; Natta, G. Polymerization of propylene to syndiotactic polymer. III. Behaviour of the catalyst system VCl4–Al(C2H5)2Cl in the presence of Lewis bases. Makromol. Chem. 1968, 112, 60–182. [Google Scholar]
- Junghanns, E.; Gumboldt, O.; Bier, G. Polymerization of ethylene and propylene to amorphous copolymers with catalysts of vanadium oxychloride and alkyl aluminum halides. Makromol. Chem. 1962, 58, 18–42. [Google Scholar] [CrossRef]
- Murphy, V.J.; Turner, H. Low-Coordinate (Arylimido) vanadium(V) Alkyls: Synthesis and Reactivity of V(NAr)(CH2Ph)3 (Ar = C6H3-2,6-iPr2). Organometallics 1997, 16, 2495–2497. [Google Scholar] [CrossRef]
- Doi, Y.; Suzuki, S.; Soga, K. Living coordination polymerization of propene with a highly active vanadium-based catalyst. Macromolecules 1986, 19, 2896–2900. [Google Scholar] [CrossRef]
- Zanchin, G.; Vendier, L.; Pierro, I.; Bertini, F.; Ricci, G.; Lorber, C.; Leone, G. Homo- and co-polymerization of ethylene with cyclic olefins catalyzed by phosphine adducts of (imido)vanadium(IV) complexes. Organometallics 2018, 37, 3181–3195. [Google Scholar] [CrossRef]
- Zanchin, G.; Pierro, I.; Parisini, E.; Martì-Rujas, J.; Ricci, G.; Leone, G. Synthesis, structure and behavior of vanadium(III) diphosphine complexes in the homo- and co-polymerization of ethylene with norbornene: The ligand donor strength and bite angle make the difference. J. Organomet. Chem. 2018, 861, 142–150. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, W.C.; Shang, D.D.; Wu, Y.X. Synthesis of ultra-high-molecular-weight ethylene-propylene copolymer via quasi-living copolymerization with N-heterocyclic carbene ligated vanadium complexes. J. Polym. Sci. A: Polym. Chem. 2019, 57, 553–561. [Google Scholar] [CrossRef]
- Bialek, M.; Bisz, E. Dichlorovanadium(IV) diamine-bis(phenolate) complexes for ethylene (co)polymerization and 1-olefin isospecific polymerization. J. Catal. 2018, 362, 65–73. [Google Scholar] [CrossRef]
- Lamonte, R.R.; Mc Nally, D. Cyclic olefin copolymers. Adv. Mater. Process. 2001, 3, 33–36. [Google Scholar]
- Nunes, S.P.; Ohlsson, P.D.; Ordeig, O.; Kutter, P.J. Cyclic olefin polymers: Emerging materials for lab-on-a-chip applications. Microfluid. Nanofluid. 2010, 9, 145–161. [Google Scholar] [CrossRef]
- Kaminsky, W.; Bark, A.; Arndt, M. New polymers by homogenous zirconocene/aluminoxane catalysts. Macromol. Symp. 1991, 47, 83–93. [Google Scholar] [CrossRef]
- Li, X.; Hou, Z. Organometallic catalysts for copolymerization of cyclic olefins. Coord. Chem. Rev. 2008, 252, 1842–1869. [Google Scholar] [CrossRef]
- Leone, G.; Pierro, I.; Zanchin, G.; Forni, A.; Bertini, F.; Rapallo, A.; Ricci, G. Vanadium(III)–catalyzed copolymerization of ethylene with norbornene: Microstructure at tetrad level and reactivity ratios. J. Mol. Catal. A: Chem. 2016, 424, 220–231. [Google Scholar] [CrossRef]
- Bansemer, R.L.; Huffman, J.C.; Caulton, K.G. Synthesis and characterization of VCl3(PMePh2)2. Inorg. Chem. 1985, 24, 3003–3006. [Google Scholar] [CrossRef]
- Holt, D.G.L.; Larkworthy, L.F.; Povey, D.C.; Smith, G.W.; Leigh, G.J. Synthesis and structural studies of complexes of vanadium(II) and vanadium(III) halides with tertiary phosphines. Inorg. Chim. Acta 1993, 207, 11–19. [Google Scholar] [CrossRef]
- Bultitude, J.; Larkworthy, L.F.; Povey, D.C.; Smith, G.W.; Dilworth, J.R. The preparation and crystal and molecular structures of trichlorobis(methyldiphenylphosphine)vanadium(III) and its acetonitrile adduct. J. Chem. Soc. Dalton Trans. 1986, 2253–2258. [Google Scholar] [CrossRef]
- Nieman, J.; Teuben, J.H.; Huffman, J.C.; Caulton, K.G. Preparation and characterization of mono-cyclopentadienylvanadium dihalide bis-phosphine complexes -Crystal-structure of (η-5-C5H5)VCl2(PMe3)2. J. Org. Chem. 1983, 255, 193–204. [Google Scholar] [CrossRef]
- Wang, W.; Nomura, K. Notable effects of aluminum alkyls and solvents for highly efficient ethylene (co)polymerizations catalyzed by (arylimido)-(aryloxo)vanadium complexes. Adv. Synth. Catal. 2006, 348, 743–750. [Google Scholar] [CrossRef]
- Wang, J.B.; Lu, L.P.; Liu, J.Y.; Li, Y.S. [ONN]-type amine pyridine (s) phenolate-based oxovanadium (v) catalysts for ethylene homo-and copolymerization. Dalton Trans. 2014, 43, 12926–12934. [Google Scholar] [CrossRef]
- Wu, J.Q.; Mu, J.S.; Zhang, S.W.; Li, Y.S. Vanadium(V) complexes containing tetradentate amine trihydroxy ligands as catalysts for copolymerization of cyclic olefins. J. Polym. Sci. Part A Pol. Chem. 2010, 48, 1122–1132. [Google Scholar] [CrossRef]
- Lorber, C.; Wolff, F.; Choukroun, R.; Vendier, L. Ethylene Homo- and Copolymerization Activity of a Series of [ONNO]-Type Amine Bis(phenolate) Based Vanadium(II–V) Catalysts. Eur. J. Inorg. Chem. 2005, 2850–2859. [Google Scholar] [CrossRef]
- Tang, L.M.; Wu, J.Q.; Duan, Y.Q.; Pan, L.; Li, Y.G.; Li, Y.S. Ethylene polymerizations, and the copolymerizations of ethylene with hexene or norbornene with highly active mono(β-enaminoketonato) vanadium(III) catalysts. J. Polym. Sci. Part A Pol. Chem. 2008, 46, 2038–2048. [Google Scholar] [CrossRef]
- Wu, J.Q.; Pan, L.; Pan, L.; Li, Y.G.; Liu, S.R.; Li, Y.S. Synthesis, structural characterization, and olefin polymerization behavior of Vanadium(III) complexes bearing tridentate Schiff base ligands. Organometallics 2009, 28, 1817–1825. [Google Scholar] [CrossRef]
- Tolman, A.C. Steric effects of phosphorus ligands in organometallic chemistry and homogeneous catalysis. Chem. Rev. 1977, 77, 313–348. [Google Scholar] [CrossRef]
- Fernandez, A.; Reyes, C.; Wilson, M.R.; Woska, D.C.; Prock, A.; Giering, W.P. Examination of Drago’s EB and CB parameters for phosphines through the Quantitative Analysis of Ligand Effects (QALE). Organometallics 1997, 16, 342–348. [Google Scholar] [CrossRef]
- Woska, D.C.; Prock, A.; Giering, W.P. Determination of the stereoelectronic parameters of PF3, PCl3, PH3, and P(CH2CH2CN)(3). The quantitative analysis of ligand effects (QALE). Organometallics 2000, 19, 4629–4638. [Google Scholar] [CrossRef]
- Joerg, S.; Drago, R.S.; Sales, J. Reactivity of phosphorus donors. Organometallics 1998, 17, 589–599. [Google Scholar] [CrossRef]
- Bent, H.A. An Appraisal of Valence-bond Structures and Hybridization in Compounds of the First-row elements. Chem. Rev. 1961, 61, 275–311. [Google Scholar] [CrossRef]
- Yoshida, Y.; Mohri, J.I.; Ishii, S.I.; Mitani, M.; Saito, J.; Matsui, S.; Makio, H.; Nakano, T.; Tanaka, H.; Onda, M.; et al. Living Copolymerization of Ethylene with Norbornene Catalyzed by Bis(Pyrrolide−Imine) Titanium Complexes with MAO. J. Am. Chem. Soc. 2004, 126, 12023–12032. [Google Scholar] [CrossRef] [PubMed]
- Popeney, C.S.; Guan, Z. Effect of Ligand Electronics on the Stability and Chain Transfer Rates of Substituted Pd(II) α-Diimine Catalysts. Macromolecules 2010, 43, 4091–4097. [Google Scholar] [CrossRef]
- Leone, G.; Mauri, M.; Losio, S.; Bertini, F.; Ricci, G.; Porri, L. Copolymerization of ethylene with α-olefins and cyclic olefins catalyzed by a Ti(IV) diisopropoxy complex bearing a tridentate [O−,S,O−]-type bis(phenolato) ligand. Polim. Chem. 2014, 5, 3412–3423. [Google Scholar] [CrossRef]
- Simanke, A.G.; Alamo, R.G.; Galland, G.B.; Mauler, R. Wide-Angle X-ray Scattering of Random Metallocene−Ethylene Copolymers with Different Types and Concentration of Comonomer. Macromolecules 2001, 34, 6959–6971. [Google Scholar] [CrossRef]
- Arndt, M.; Beulich, I. C1-symmetric metallocenes for olefin polymerisation, 1. Catalytic performance of [Me2C(3-tertBuCp)(Flu)]ZrCl2 in ethene/norbornene copolymerisation. Macromol. Chem. Phys. 1998, 199, 1221–1232. [Google Scholar] [CrossRef]
- Hasan, T.; Ikeda, T.; Shiono, T. Ethene−Norbornene Copolymer with High Norbornene Content Produced by ansa-Fluorenylamidodimethyltitanium Complex Using a Suitable Activator. Macromolecules 2004, 37, 8503–8509. [Google Scholar] [CrossRef]
- Kiesewetter, J.; Arikan, B.; Kaminsky, W. Copolymerization of ethene with norbornene using palladium(II) α-diimine catalysts: Influence of feed composition, polymerization temperature, and ligand structure on copolymer properties and microstructure. Polymer 2006, 47, 3302–3314. [Google Scholar] [CrossRef]
- Leone, G.; Zanchin, G.; Pierro, I.; Sommazzi, A.; Forni, A.; Ricci, G. Synthesis, structure and 1,3-Butadiene polymerization behavior of Vanadium(III) phosphine complexes. Catalysts 2017, 7, 369. [Google Scholar] [CrossRef]
Sample Availability: Not available. |

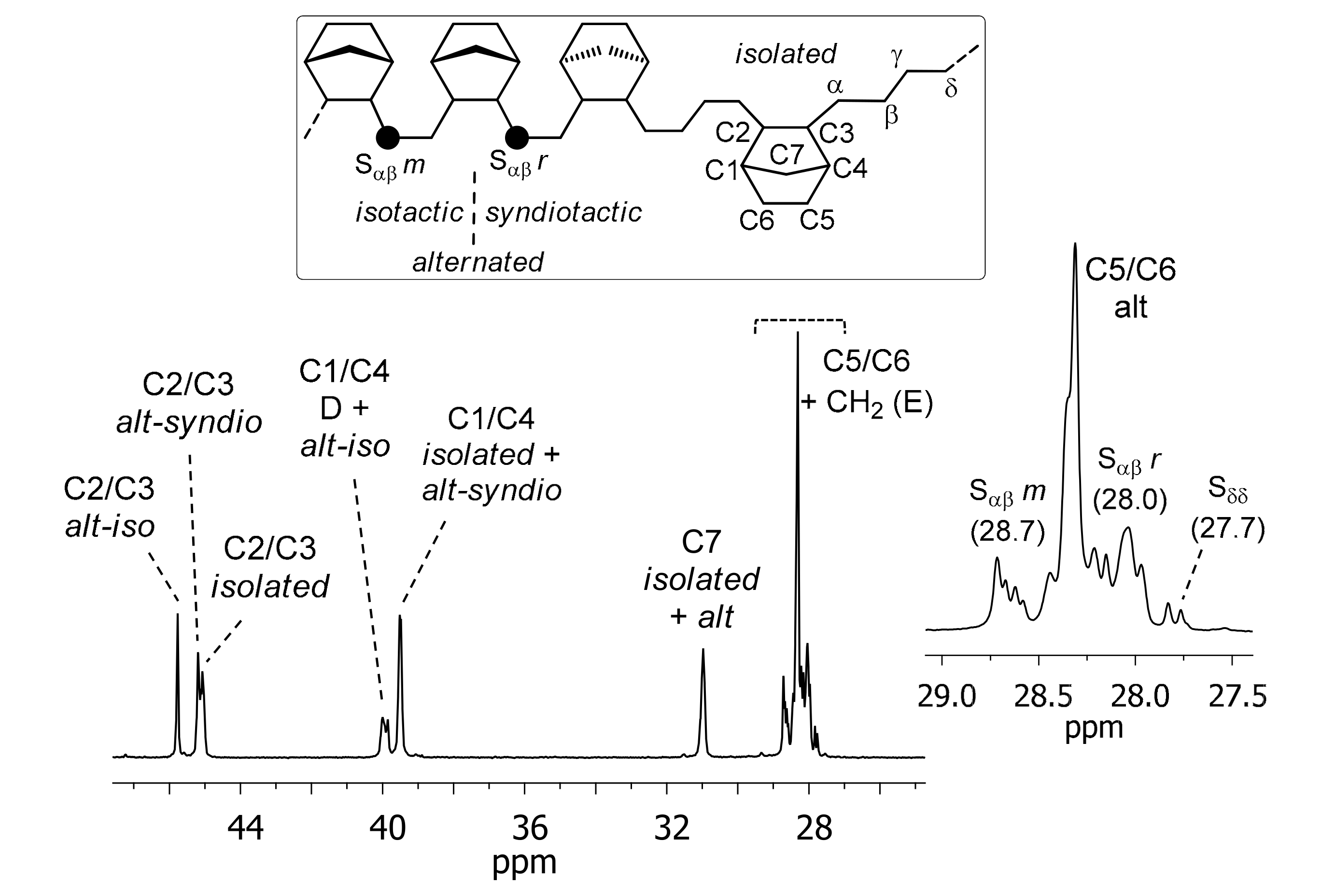
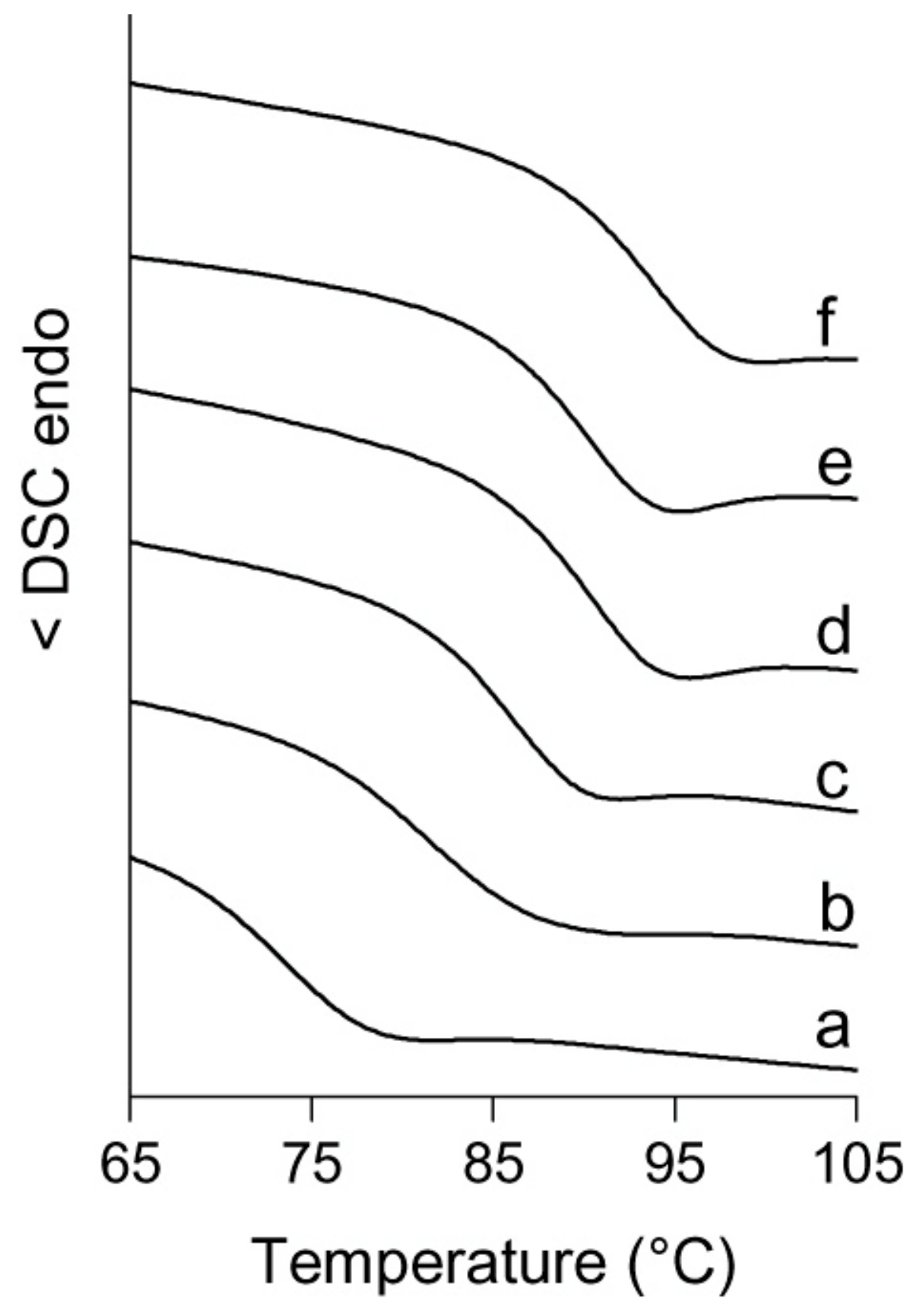
| Entry | V-cat | Phosphine | Yield (mg) | Activity 4 (×10 2) | Mw5 (×10 3) | Mw/Mn5 | ||
|---|---|---|---|---|---|---|---|---|
| (type) | υCO 2 (cm−1) | θ3 (°) | ||||||
| 1 | 1a | PMe2Ph | 2065.3 | 122 | 195 | 390 | 65.5 | 6.8 |
| 2 | 1b | PMePh2 | 2067.0 | 136 | 281 | 562 | 44.6 | 3.9 |
| 3 | 1c | PPh3 | 2068.9 | 145 | 302 | 604 | 35.3 | 4.2 |
| 4 | 1d | PCy3 | 2056.4 | 170 | 221 | 442 | 56.9 | 6.3 |
| 5 | 1e | PtBu3 | 2056.1 | 182 | 295 | 590 | 84.2 | 7.9 |
| 6 | VCl3(THF)3 | 361 | 722 | 34.7 | 5.0 | |||
| Entry | V-cat | Phosphine | Yield (mg) | Activity 4 (×10 2) | NB 5 (mol%) | Mw6 (×10 3) | Mw/Mn6 | Tg7 (°C) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| (type) | υCO 2 (cm−1) | θ3 (°) | ||||||||
| 7 | 1a | PMe2Ph | 2065.3 | 122 | 160 | 320 | 38.0 | 90.3 | 4.2 | 90 |
| 8 | 1b | PMePh2 | 2067.0 | 136 | 241 | 482 | 37.8 | 61.4 | 3.8 | 90 |
| 9 | 1c | PPh3 | 2068.9 | 145 | 367 | 734 | 38.7 | 42.6 | 4.2 | 81 |
| 10 | 1d | PCy3 | 2056.4 | 170 | 226 | 452 | 39.3 | 86.4 | 5.9 | 93 |
| 11 | 1e | PtBu3 | 2056.1 | 182 | 247 | 494 | 38.8 | 130.0 | 7.0 | 85 |
| 12 | VCl3(THF)3 | 395 | 790 | 41.2 | 43.6 | 4.4 | 73 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanchin, G.; Gavezzoli, A.; Bertini, F.; Ricci, G.; Leone, G. Homo- and Copolymerization of Ethylene with Norbornene Catalyzed by Vanadium(III) Phosphine Complexes. Molecules 2019, 24, 2088. https://doi.org/10.3390/molecules24112088
Zanchin G, Gavezzoli A, Bertini F, Ricci G, Leone G. Homo- and Copolymerization of Ethylene with Norbornene Catalyzed by Vanadium(III) Phosphine Complexes. Molecules. 2019; 24(11):2088. https://doi.org/10.3390/molecules24112088
Chicago/Turabian StyleZanchin, Giorgia, Alessia Gavezzoli, Fabio Bertini, Giovanni Ricci, and Giuseppe Leone. 2019. "Homo- and Copolymerization of Ethylene with Norbornene Catalyzed by Vanadium(III) Phosphine Complexes" Molecules 24, no. 11: 2088. https://doi.org/10.3390/molecules24112088
APA StyleZanchin, G., Gavezzoli, A., Bertini, F., Ricci, G., & Leone, G. (2019). Homo- and Copolymerization of Ethylene with Norbornene Catalyzed by Vanadium(III) Phosphine Complexes. Molecules, 24(11), 2088. https://doi.org/10.3390/molecules24112088









