Applying Nanoparticle Tracking Analysis to Characterize the Polydispersity of Aggregates Resulting from Tannin–Polysaccharide Interactions in Wine-Like Media
Abstract
1. Introduction
2. Results and Discussion
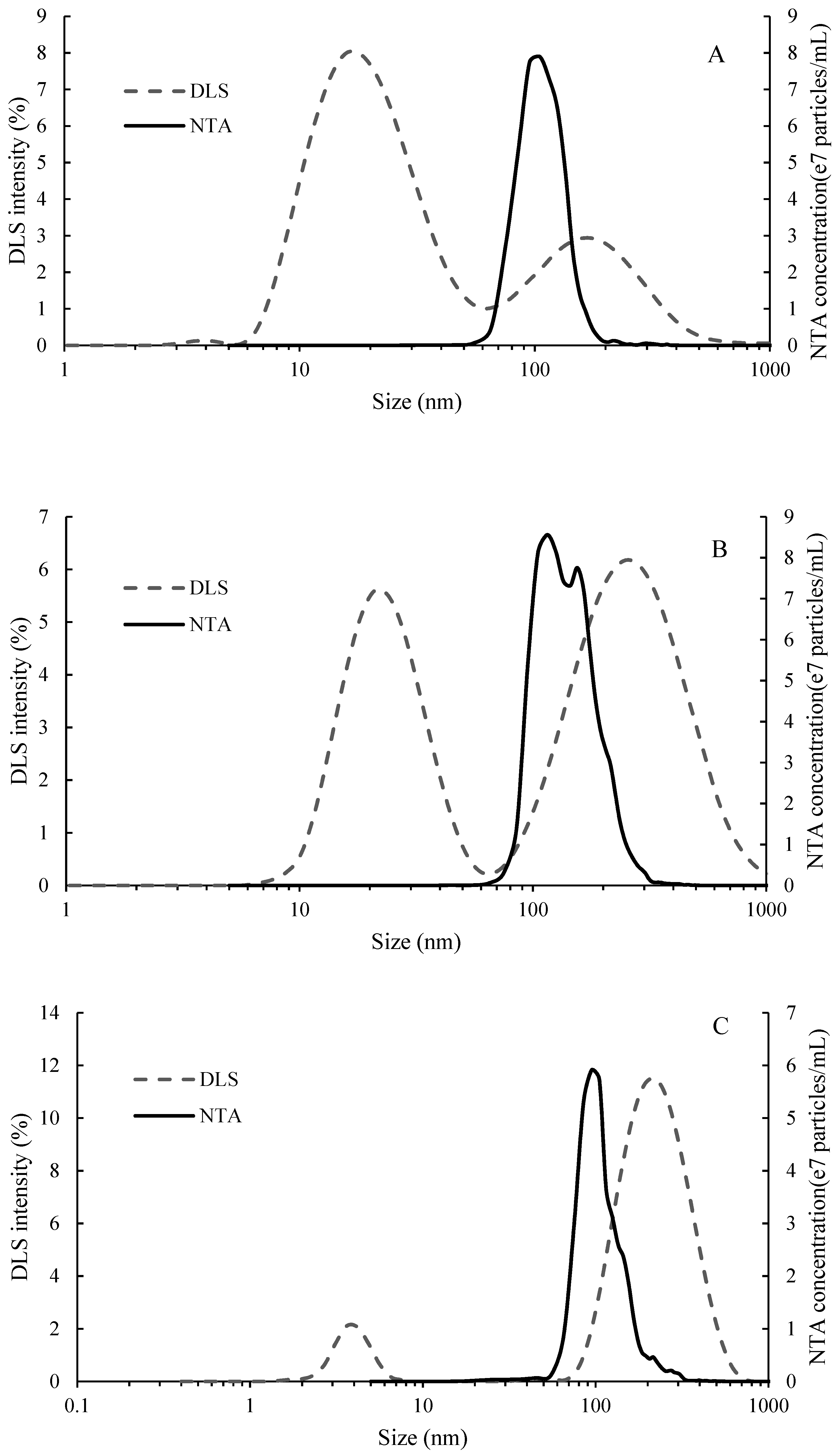
2.1. Molecular Weight and Particle Size Distribution of Seed Tannin, Mannoprotein and Gum Arabic
2.2. Interactions between Polysaccharides and Tannins Characterized by UV-Visible Spectrometry
2.3. Binding Experiment Characterized by NTA
3. Materials and Methods
3.1. Preparation of Polysaccharide and Tannin Materials
3.2. Macromolecule Characterization
3.2.1. Size Exclusion Chromatography Analysis
3.2.2. DLS Analysis
3.2.3. NTA Analysis
3.2.4. System Qualification for NTA and DLS Instruments
3.2.5. Particle Size of Tannin and Polysaccharide Determined by DLS and NTA
3.3. Characterization of Interactions between Polysaccharides and Tannins
3.3.1. UV-visible Spectroscopy Analysis
3.3.2. NTA and DLS Analyses
3.3.3. Tannin Solubility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, P.A.; McRae, J.M.; Bindon, K.A. Impact of winemaking practices on the concentration and composition of tannins in red wine. Aust. J. Grape Wine Res. 2015, 21, 601–614. [Google Scholar] [CrossRef]
- Poncet-Legrand, C.; Cartalade, D.; Putaux, J.L.; Cheynier, V.; Vernhet, A. Flavan-3-ol aggregation in model ethanolic solutions: Incidence of polyphenol structure, concentration, ethanol content, and ionic strength. Langmuir 2003, 19, 10563–10572. [Google Scholar] [CrossRef]
- Riou, V.; Vernhet, A.; Doco, T.; Moutounet, M. Aggregation of grape seed tannins in model wine—Effect of wine polysaccharides. Food Hydrocoll. 2002, 16, 17–23. [Google Scholar] [CrossRef]
- McRae, J.M.; Mierczynska-Vasilev, A.; Soden, A.; Barker, A.M.; Day, M.P.; Smith, P.A. Effect of commercial-scale filtration on sensory and colloidal properties of red wines over 18 months bottle aging. Am. J. Enol. Vitic. 2017, 68, 263–274. [Google Scholar] [CrossRef]
- Waters, E.J.; Peng, Z.; Pocock, K.F.; Jones, G.P.; Clarke, P.; Williams, P.J. Solid-State 13C-NMR investigation into insoluble deposits adhering to the inner glass surface of bottled red wine. J. Agric. Food Chem. 1994, 42, 1761–1766. [Google Scholar] [CrossRef]
- Watrelot, A.A.; Le Bourvellec, C.; Imberty, A.; Renard, C.M.G.C. Neutral sugar side chains of pectins limit interactions with procyanidins. Carbohydr. Polym. 2014, 99, 527–536. [Google Scholar] [CrossRef]
- Mekoue Nguela, J.; Poncet-Legrand, C.; Sieczkowski, N.; Vernhet, A. Interactions of grape tannins and wine polyphenols with a yeast protein extract, mannoproteins and β-glucan. Food Chem. 2016, 210, 671–682. [Google Scholar] [CrossRef]
- Poncet-Legrand, C.; Doco, T.; Williams, P.; Vernhet, A. Inhibition of grape seed tannin aggregation by wine mannoproteins: Effect of polysaccharide molecular weight. Am. J. Enol. Vitic. 2007, 58, 87–91. [Google Scholar]
- Guadalupe, Z.; Ayestarán, B.; Williams, P.; Doco, T. Determination of must and wine polysaccharides by gas chromatography-mass spectrometry (GC-MS) and size-exclusion chromatography (SEC). In Polysaccharides: Bioactivity and Biotechnology; Ramawat, K., Mérillon, J.M., Eds.; Springer International Publishing: Basel, Switzerland, 2015; pp. 1265–1297. [Google Scholar]
- Carvalho, E.; Mateus, N.; Plet, B.; Pianet, I.; Dufourc, E.; De Freitas, V. Influence of wine pectic polysaccharides on the interactions between condensed tannins and salivary proteins. J. Agric. Food Chem. 2006, 54, 8936–8944. [Google Scholar] [CrossRef]
- Mateus, N.; Carvalho, E.; Luís, C.; De Freitas, V. Influence of the tannin structure on the disruption effect of carbohydrates on protein-tannin aggregates. Anal. Chim. Acta 2004, 513, 135–140. [Google Scholar] [CrossRef]
- Rinaldi, A.; Gambuti, A.; Moio, L. Precipitation of salivary proteins after the interaction with wine: the effect of ethanol, pH, fructose, and mannoproteins. J. Food Sci. 2012, 77, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Watrelot, A.A.; Schulz, D.L.; Kennedy, J.A. Wine polysaccharides influence tannin-protein interactions. Food Hydrocoll. 2017, 63, 571–579. [Google Scholar] [CrossRef]
- Alcalde-Eon, C.; García-Estévez, I.; Puente, V.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Color stabilization of red wines. A chemical and colloidal approach. J. Agric. Food Chem. 2014, 62, 6984–6994. [Google Scholar] [CrossRef] [PubMed]
- Maury, C.; Sarni-Manchado, P.; Poinsaut, P.; Cheynier, V.; Moutounet, M. Influence of polysaccharides and glycerol on proanthocyanidin precipitation by protein fining agents. Food Hydrocoll. 2016, 60, 598–605. [Google Scholar] [CrossRef]
- Australian Government Standard 4.5.1—Wine Production Requirements (Australia Only), Version F2017C01001. Australia New Zealand Food Standards Code: 2014. Available online: https://www.legislation.gov.au/Details/F2017C01001 (accessed on 24 April 2019).
- Bindon, K.A.; Carew, A.L.; Mierczynska-Vasilev, A.; Kassara, S.; Kerslake, F.; Smith, P.A. Characterization of macromolecular complexes in red wine: Composition, molecular mass distribution and particle size. Food Chem. 2016, 199, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Brandão, E.; Silva, M.S.; García-Estévez, I.; Williams, P.; Mateus, N.; Doco, T.; de Freitas, V.; Soares, S. The role of wine polysaccharides on salivary protein-tannin interaction: A molecular approach. Carbohydr. Polym. 2017, 177, 77–85. [Google Scholar] [CrossRef]
- Carn, F.; Guyot, S.; Baron, A.; Pérez, J.; Buhler, E.; Zanchi, D. Structural properties of colloidal complexes between condensed tannins and polysaccharide hyaluronan. Biomacromolecules 2012, 13, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Mamet, T.; Ge, Z.Z.; Zhang, Y.; Li, C.M. Interactions between highly galloylated persimmon tannins and pectins. Int. J. Biol. Macromol. 2018, 106, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.I.; Gonçalves, R.M.; Fernandes, I.; Mateus, N.; de Freitas, V. Mechanistic approach by which polysaccharides inhibit α-amylase/procyanidin aggregation. J. Agric. Food Chem. 2009, 57, 4352–4358. [Google Scholar] [CrossRef]
- Watrelot, A.A.; Le Bourvellec, C.; Imberty, A.; Renard, C.M.G.C. Interactions between pectic compounds and procyanidins are influenced by methylation degree and chain length. Biomacromolecules 2013, 14, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Filipe, V.; Hawe, A.; Jiskoot, W. Critical evaluation of nanoparticle tracking analysis (NTA) by NanoSight for the measurement of nanoparticles and protein aggregates. Pharm. Res. 2010, 27, 796–810. [Google Scholar] [CrossRef]
- Jarzębski, M.; Bellich, B.; Białopiotrowicz, T.; Śliwa, T.; Kościński, J.; Cesàro, A. Particle tracking analysis in food and hydrocolloids investigations. Food Hydrocoll. 2017, 68, 90–101. [Google Scholar] [CrossRef]
- Bindon, K.A.; Smith, P.A.; Holt, H.; Kennedy, J.A. Interaction between grape-derived proanthocyanidins and cell wall material. 2. implications for vinification. J. Agric. Food Chem. 2010, 58, 10736–10746. [Google Scholar] [CrossRef]
- Technical Note: Size Quality Report for the Zetasizer Nano. Available online: http://www.malvern.com/en/support/resource-center/technical-notes/TN101104SizeQualityReportZetasizerNano.aspx (accessed on 3 April 2017).
- ISO 19430:2016 Particle Size Analysis—Particle Tracking Analysis (PTA) Method. 2016. Available online: https://www.iso.org/standard/64890.html (accessed on 24 April 2019).
- Al Assaf, S.; Phillips, G.O.; Williams, P.A. Studies on acacia exudate gums. Part I: the molecular weight of Acacia senegal gum exudate. Food Hydrocoll. 2005, 19, 647–660. [Google Scholar] [CrossRef]
- Li, X.; Fang, Y.; Al-Assaf, S.; Phillips, G.O.; Nishinari, K.; Zhang, H. Rheological study of gum arabic solutions: Interpretation based on molecular self-association. Food Hydrocoll. 2009, 23, 2394–2402. [Google Scholar] [CrossRef]
- Lankhorst, P.P.; Voogt, B.; Tuinier, R.; Lefol, B.; Pellerin, P.; Virone, C. Prevention of tartrate crystallization in wine by hydrocolloids: The mechanism studied by Dynamic light scattering. J. Agric. Food Chem. 2017, 65, 8923–8929. [Google Scholar] [CrossRef] [PubMed]
- Zanchi, D.; Vernhet, A.; Poncet-Legrand, C.; Cartalade, D.; Tribet, C.; Schweins, R.; Cabane, B. Colloidal Dispersions of tannins in water−ethanol solutions. Langmuir 2007, 23, 9949–9959. [Google Scholar] [CrossRef] [PubMed]
- Zanchi, D.; Konarev, P.V.; Tribet, C.; Baron, A.; Svergun, D.I.; Guyot, S. Rigidity, conformation, and solvation of native and oxidized tannin macromolecules in water-ethanol solution. J. Chem. Phys. 2009, 130, 245103. [Google Scholar] [CrossRef]
- Poncet-Legrand, C.; Cabane, B.; Bautista-Ortín, A.-B.; Carrillo, S.; Fulcrand, H.; Pérez, J.; Vernhet, A. Tannin oxidation: Intra- versus intermolecular reactions. Biomacromolecules 2010, 11, 2376–2386. [Google Scholar] [CrossRef] [PubMed]
- Cheah, K.Y.; Howarth, G.S.; Bindon, K.A.; Kennedy, J.A.; Bastian, S.E.P. Low molecular weight procyanidins from grape seeds enhance the impact of 5-Fluorouracil chemotherapy on Caco-2 human colon cancer cells. PLoS ONE 2014. [Google Scholar] [CrossRef] [PubMed]
- Hixson, J.L.; Bindon, K.A.; Smith, P.A. Evaluation of Direct Phloroglucinolysis and colorimetric depolymerization assays and their applicability for determining condensed tannins in grape marc. J. Agric. Food Chem. 2015, 63, 9954–9962. [Google Scholar] [CrossRef] [PubMed]
- Le Bourvellec, C.; Renard, C.M.G.C. Interactions between polyphenols and macromolecules: quantification methods and mechanisms. Crit. Rev. Food Sci. Nutr. 2012, 52, 213–248. [Google Scholar] [CrossRef] [PubMed]
- Guadalupe, Z.; Ayestarán, B. Effect of commercial mannoprotein addition on polysaccharide, polyphenolic, and color composition in red wines. J. Agric. Food Chem. 2008, 56, 9022–9029. [Google Scholar] [CrossRef]
- Rowe, J.D.; Harbertson, J.F.; Osborne, J.P.; Freitag, M.; Lim, J.; Bakalinsky, A.T. Systematic identification of yeast proteins extracted into model wine during aging on the yeast lees. J. Agric. Food Chem. 2010, 58, 2337–2346. [Google Scholar] [CrossRef] [PubMed]
- Quijada-Morín, N.; Williams, P.; Rivas-Gonzalo, J.C.; Doco, T.; Escribano-Bailón, M.T. Polyphenolic, polysaccharide and oligosaccharide composition of Tempranillo red wines and their relationship with the perceived astringency. Food Chem. 2014, 154, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wilkinson, K.L.; Bindon, K.A. Compositional variability in commercial tannin and mannoprotein products. Am. J. Enol. Vitic. 2018, 69, 176–181. [Google Scholar] [CrossRef]
- Scollary, G.R.; Pásti, G.; Kállay, M.; Blackman, J.; Clark, A.C. Astringency response of red wines: Potential role of molecular assembly. Trends Food Sci. Technol. 2012, 27, 25–36. [Google Scholar] [CrossRef]
- McRae, J.M.; Ziora, Z.M.; Kassara, S.; Cooper, M.A.; Smith, P.A. Ethanol concentration influences the mechanisms of wine tannin interactions with poly(l-proline) in model wine. J. Agric. Food Chem. 2015, 63, 4345–4352. [Google Scholar] [CrossRef]
- Fontoin, H.; Saucier, C.; Teissedre, P.L.; Glories, Y. Effect of pH, ethanol and acidity on astringency and bitterness of grape seed tannin oligomers in model wine solution. Food Qual. Preference 2008, 19, 286–291. [Google Scholar] [CrossRef]
- Vidal, S.; Courcoux, P.; Francis, L.; Kwiatkowski, M.; Gawel, R.; Williams, P.; Waters, E.; Cheynier, V. Use of an experimental design approach for evaluation of key wine components on mouth-feel perception. Food Qual. Preference 2004, 15, 209–217. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Jones, G.P. Analysis of proanthocyanidin cleavage products following acid-catalysis in the presence of excess phloroglucinol. J. Agric. Food Chem. 2001, 49, 1740–1746. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.A.; Taylor, A.W. Analysis of proanthocyanidins by high-performance gel permeation chromatography. J. Chromatogr. A 2003, 995, 99–107. [Google Scholar] [CrossRef]
- Bindon, K.A.; Kennedy, J.A. Ripening-induced changes in grape skin proanthocyanidins modify their interaction with cell walls. J. Agric. Food Chem. 2011, 59, 2696–2707. [Google Scholar] [CrossRef]
- ISO 22412:2008 Particle size analysis—Dynamic light scattering (DLS). International Organization of Standardization. 2008. Available online: https://www.iso.org/standard/40942.html (accessed on 24 April 2019).
- Mercurio, M.D.; Dambergs, R.G.; Herderich, M.J.; Smith, P.A. High throughput analysis of red wine and grape phenolics—Adaptation and validation of methyl cellulose precipitable tannin assay and modified Somers color assay to a rapid 96 well plate format. J. Agric. Food Chem. 2007, 55, 4651–4657. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Sample of the purified seed tannin is available from the authors. Commercial mannoprotein and gum arabic products are available from the producing companies (details are provided in the Materials and Methods section). |






| Material | Mean Mr (kg/mol) | PdI | Mr10 (kg/mol) | Mr90 (kg/mol) |
|---|---|---|---|---|
| Mannoprotein (MP) | 33.6 ± 1.9 | 2.25 ± 0.07 | 9.6 ± 0.3 | 97.9 ± 8.8 |
| Gum arabic (AG) | 132.5 ± 1.0 | 1.75 ± 0.01 | 47.6 ± 0.4 | 321.6 ± 1.4 |
| Seed tannin (ST) | 1.92 ± 0.02 | 2.14 ± 0.01 | 0.5 ± 0.01 | 6.0 ± 0.09 |
| ST a (mg/mL) | Model Wine b | Treatment | Percentile and Mean Particle Size by NTA (nm) | Treatment | Percentile and Mean Particle Size by NTA (nm) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| D10 | D50 | D90 | Mean | D10 | D50 | D90 | Mean | ||||
| 12% | ST | 56.4 ± 0.8 | 79.1 ± 1.3 | 121.9 ± 2.5 | 91.4 ± 1.4 | ST | 56.4 ± 0.8 | 79.1 ± 1.3 | 121.9 ± 2.5 | 91.4 ± 1.4 | |
| MP | 62.8 ± 0.6 | 87.7 ± 0.9 | 158.3 ± 5.0 | 105.7 ± 1.0 | AG | 96.0 ± 0.9 | 143.5 ± 0.8 | 226.9 ± 2.9 | 160.1 ± 1.0 | ||
| 1.25 c | ST + MP | 80.2 ± 0.8 | 104.0 ± 0.9 | 152.5 ± 3.3 | 116.5 ± 1.0 | ST + AG | 85.8 ± 1.3 | 133.1 ± 1.2 | 214.1 ± 2.8 | 147.6 ± 1.4 | |
| 15% | ST | 48.3 ± 0.9 | 71.4 ± 1.9 | 124.0 ± 4.9 | 85.7 ± 1.8 | ST | 48.3 ± 0.9 | 71.4 ± 1.9 | 124.0 ± 4.9 | 85.7 ± 1.8 | |
| MP | 68.7 ± 1.0 | 101.1 ± 1.2 | 155.6 ± 1.3 | 113.2 ± 0.8 | AG | 98.2 ± 1.4 | 141.0 ± 2.1 | 223.1 ± 4.3 | 157.5 ± 1.7 | ||
| ST + MP | 73.3 ± 0.9 | 99.0 ± 0.8 | 160.1 ± 3.3 | 113.9 ± 1.0 | ST + AG | 97.3 ± 0.9 | 134.5 ± 1.0 | 209.9 ± 3.4 | 151.0 ± 0.9 | ||
| 12% | ST | 78.3 ± 1.2 | 99.6 ± 1.2 | 147.3 ± 4.7 | 112.5 ± 1.3 | ST | 78.3 ± 1.2 | 99.6 ± 1.2 | 147.3 ± 4.7 | 112.5 ± 1.3 | |
| MP | 71.5 ± 1.3 | 100.8 ± 2.1 | 160.9 ± 4.9 | 113.4 ± 2.0 | AG | 93.8 ± 0.7 | 132.6 ± 1.8 | 193.4 ± 4.1 | 144.2 ± 1.9 | ||
| 5 d | ST + MP | 134.5 ± 2.8 | 229.2 ± 3.5 | 316.2 ± 3.8 | 231.2 ± 3.1 | ST + AG | 77.3 ± 1.0 | 108.6 ± 1.5 | 160.9 ± 6.0 | 121.4 ± 2.0 | |
| 15% | ST | 90.5 ± 1.3 | 119.7± 1.7 | 170.4 ± 2.9 | 131.4 ± 1.5 | ST | 90.5 ± 1.3 | 119.7± 1.7 | 170.4 ± 2.9 | 131.4 ± 1.5 | |
| MP | 68.0 ± 1.0 | 100.2 ± 4.2 | 184.1 ± 7.7 | 120.7 ± 3.2 | AG | 107.2 ± 1.1 | 148.2 ± 2.2 | 237.9 ± 5.6 | 167.1 ± 2.2 | ||
| ST + MP | 117.9 ± 1.5 | 153.3 ± 2.5 | 249.5 ± 6.2 | 176.1 ± 2.5 | ST + AG | 105.1 ± 1.0 | 132.9 ± 1.0 | 192.8 ± 2.6 | 147.3 ± 1.3 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Wilkinson, K.L.; Mierczynska-Vasilev, A.; Bindon, K.A. Applying Nanoparticle Tracking Analysis to Characterize the Polydispersity of Aggregates Resulting from Tannin–Polysaccharide Interactions in Wine-Like Media. Molecules 2019, 24, 2100. https://doi.org/10.3390/molecules24112100
Li S, Wilkinson KL, Mierczynska-Vasilev A, Bindon KA. Applying Nanoparticle Tracking Analysis to Characterize the Polydispersity of Aggregates Resulting from Tannin–Polysaccharide Interactions in Wine-Like Media. Molecules. 2019; 24(11):2100. https://doi.org/10.3390/molecules24112100
Chicago/Turabian StyleLi, Sijing, Kerry L. Wilkinson, Agnieszka Mierczynska-Vasilev, and Keren A. Bindon. 2019. "Applying Nanoparticle Tracking Analysis to Characterize the Polydispersity of Aggregates Resulting from Tannin–Polysaccharide Interactions in Wine-Like Media" Molecules 24, no. 11: 2100. https://doi.org/10.3390/molecules24112100
APA StyleLi, S., Wilkinson, K. L., Mierczynska-Vasilev, A., & Bindon, K. A. (2019). Applying Nanoparticle Tracking Analysis to Characterize the Polydispersity of Aggregates Resulting from Tannin–Polysaccharide Interactions in Wine-Like Media. Molecules, 24(11), 2100. https://doi.org/10.3390/molecules24112100








