Novel Targeted Nano-Parthenolide Molecule against NF-kB in Acute Myeloid Leukemia
Abstract
:1. Introduction
2. Results
2.1. Patients’ Assessment for NF-κB Expression
2.2. Flowcytometry Study
2.3. Nanoparticle Characterization
2.4. Cell Vitality Assay
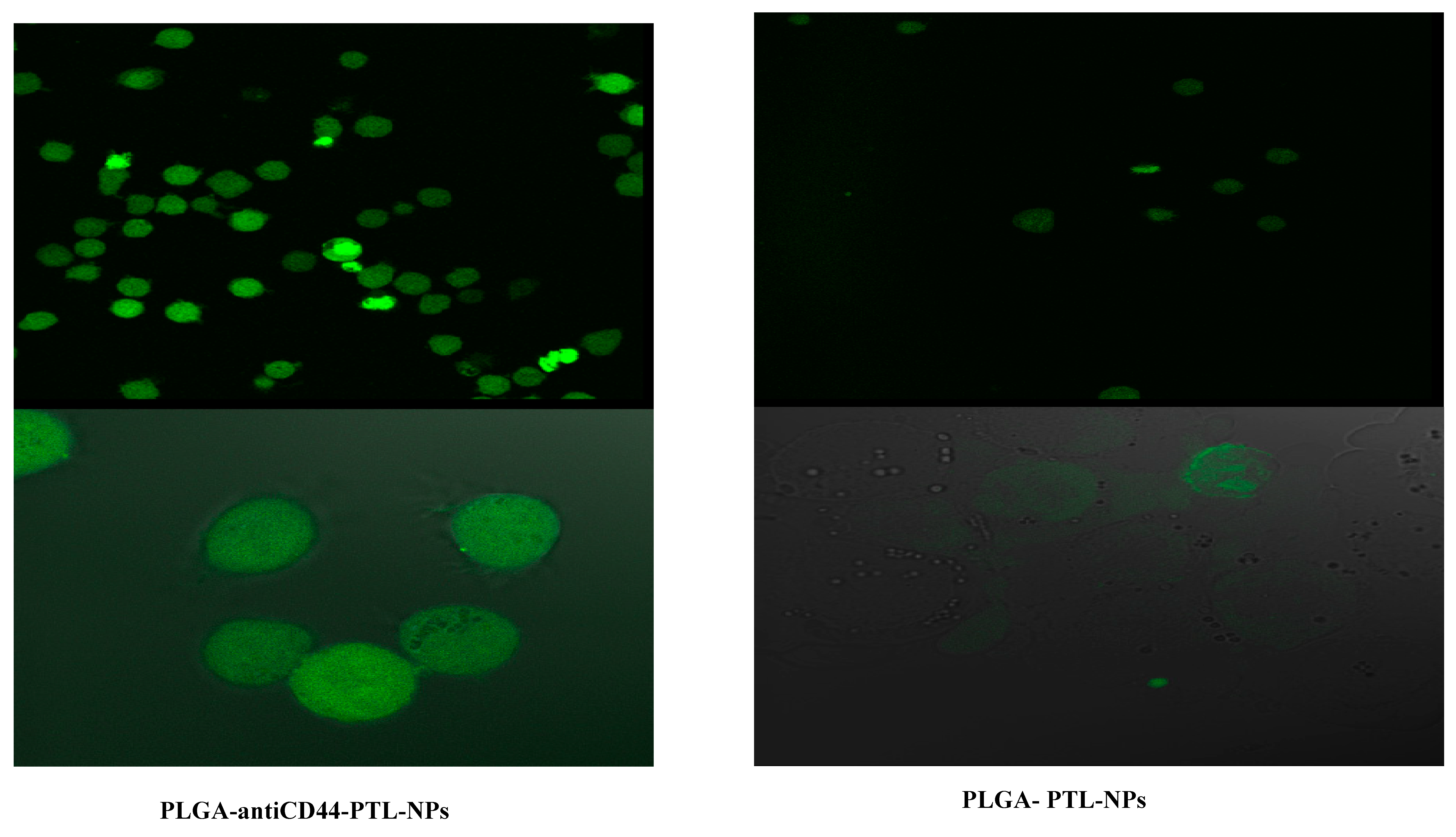
2.5. Assessment of Cellular Uptake of PLGA-antiCD44-PTL-NPs
3. Methods
3.1. Patient and Control Samples
3.2. Isolation of Mononuclear Cells (MNCs) from Bone Marrow Samples
3.3. RNA Extraction and Quantitative RT-PCR
3.4. Cell Culture Experiments
3.4.1. Cell Lines
3.4.2. Cell Vitality and Morphology
3.4.3. Flow Cytometry Analysis
3.5. Protein Analysis (Western Blot)
3.6. Synthesis and Characterization of Nanoparticles
3.6.1. Dynamic Laser Light Scattering (DLS)
3.6.2. Determination of PTL Amount in Nanoparticles
3.6.3. Fluorescence Intensity
3.7. Cell Vitality Assay (MTT Assay)
3.8. Statistical Analysis
4. Discussion
4.1. NF-κB and Acute Myeloid Leukemia
4.2. NF-κB Targeting
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- American Cancer Society. Cancer Facts and Figures 2017. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf (accessed on 19 October 2018).
- Darwish, N.H.E.; Sudha, T.; Godugu, K.; Elbaz, O.; Abdelghaffar, H.A.; Hassan, E.E.A.; Mousa, S.A. Acute myeloid leukemia stem cell markers in prognosis and targeted therapy: Potential impact of BMI-1, TIM-3 and CLL-1. Oncotarget 2016, 7, 57811–57820. [Google Scholar] [CrossRef]
- Trendowski, M. The inherent metastasis of leukaemia and its exploitation by sonodynamic therapy. Crit. Rev. Oncol. Hematol. 2015, 94, 149–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takao, J.; Yudate, T.; Das, A.; Shikano, S.; Bonkobara, M.; Ariizumi, K.; Cruz, P.D. Expression of NF-kB in epidermis and the relationship between NF-kB activation and inhibition of keratinocyte growth. Br. J. Dermatol. 2003, 148, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.-Y.W.; Wu, P.-Y.; Wu, T.-J.; Hou, H.-A.; Chou, W.-C.; Teng, C.-L.J.; Lin, C.-R.; Chen, J.-M.M.; Lin, T.-Y.; Su, H.-C. Aurora A and NF-κB survival pathway drive chemoresistance in acute myeloid leukemia via the TRAF-interacting protein TIFA. Cancer Res. 2017, 77, 494–508. [Google Scholar] [CrossRef]
- Godwin, P.; Baird, A.M.; Heavey, S.; Barr, M.P.; O’Byrne, K.J.; Gately, K. Targeting nuclear factor-kappa B to overcome resistance to chemotherapy. Front. Oncol. 2013, 3, 120. [Google Scholar] [CrossRef]
- Mehta, S.V.; Shukla, S.N.; Vora, H.H. Overexpression of Bcl2 protein predicts chemoresistance in acute myeloid leukemia: Its correlation with FLT3. Neoplasma 2013, 60, 666–675. [Google Scholar] [CrossRef]
- Zhou, J.; Ching, Y.Q.; Chng, W.J. Aberrant nuclear factor-kappa B activity in acute myeloid leukemia: From molecular pathogenesis to therapeutic target. Oncotarget 2015, 6, 5490–5500. [Google Scholar] [CrossRef] [PubMed]
- Griessinger, E.; Frelin, C.; Cuburu, N.; Imbert, V.; Dageville, C.; Hummelsberger, M.; Sirvent, N.; Dreano, M.; Peyron, J.F. Preclinical targeting of NF-kB and FLT3 pathways in AML cells. Leukemia 2008, 22, 1466–1469. [Google Scholar] [CrossRef]
- Braun, T.; Carvalho, G.; Fabre, C.; Grosjean, J.; Fenaux, P.; Kroemer, G. Targeting NF-kB in hematologic malignancies. Cell Death Differ. 2006, 13, 748–758. [Google Scholar] [CrossRef]
- Li, C.; Li, F.; Zhao, K.; Yao, J.; Cheng, Y.; Zhao, L.; Li, Z.; Lu, N.; Guo, Q. LFG-500 inhibits the invasion of cancer cells via down-regulation of PI3K/AKT/NF-κB signaling pathway. PLoS ONE 2014, 9, e91332. [Google Scholar] [CrossRef]
- Hall, I.H.; Lee, K.H.; Starnes, C.O.; Sumida, Y.; Wu, R.Y.; Waddell, T.G.; Cochran, J.W.; Gerhart, K.G. Anti-inflammatory activity of sesquiterpene lactones and related compounds. J. Pharm. Sci. 1979, 68, 537–542. [Google Scholar] [CrossRef]
- Diamanti, P.; Cox, C.V.; Moppett, J.P.; Blair, A. Parthenolide eliminates leukemia-initiating cell populations and improves survival in xenografts of childhood acute lymphoblastic leukemia. Blood 2013, 121, 1384–1393. [Google Scholar] [CrossRef] [Green Version]
- Guzman, M.L.; Rossi, R.M.; Neelakantan, S.; Li, X.; Corbett, C.A.; Hassane, D.C.; Becker, M.W.; Bennett, J.M.; Sullivan, E.; Lachowicz, J.L.; et al. An orally bioavailable parthenolide analog selectively eradicates acute myelogenous leukemia stem and progenitor cells. Blood 2007, 110, 4427–4435. [Google Scholar] [CrossRef]
- Sun, D.; Zhou, J.K.; Zhao, L.; Zheng, Z.Y.; Li, J.; Pu, W.; Liu, S.; Liu, X.S.; Liu, S.J.; Zheng, Y.; et al. Novel curcumin liposome modified with hyaluronan targeting CD44 plays an anti-leukemic role in acute myeloid leukemia in vitro and in vivo. ACS Appl. Mater. Interfaces 2017, 9, 16857–16868. [Google Scholar] [CrossRef] [PubMed]
- Bharali, D.J.; Sudha, T.; Cui, H.; Mian, B.M.; Mousa, S.A. Anti-CD24 nano-targeted delivery of docetaxel for the treatment of prostate cancer. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 263–273. [Google Scholar] [CrossRef]
- Srinivasan, M.; Bharali, D.J.; Sudha, T.; Khedr, M.; Guest, I.; Sell, S.; Glinsky, G.V.; Mousa, S.A. Downregulation of Bmi1 in breast cancer stem cells suppresses tumor growth and proliferation. Oncotarget 2017, 8, 38731–38742. [Google Scholar] [CrossRef] [Green Version]
- Benicio, M.T.L.; Scheucher, P.S.; Garcia, A.B.; Falcao, R.P.; Rego, E.M. Characterization of leukemic stem cells in AML cell lines using ALDH staining. Blood 2013, 122, 5409. [Google Scholar]
- Shen, J.P.; Yang, H.; Ni, W.M.; Qian, W.B. Cytotoxicity of homoharringtonine on leukemic stem-like cells in AML cell line KG-1. J. Zhejiang Univ. Med. Sci. 2012, 41, 485–490. [Google Scholar]
- Larizza, L.; Magnani, I.; Beghini, A. The kasumi-1 cell line: At (8; 21)-kit mutant model for acute myeloid leukemia. Leuk. Lymphoma 2005, 46, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Mrózek, K. Cytogenetic, molecular genetic, and clinical characteristics of acute myeloid leukemia with a complex karyotype. Semin. Oncol. 2008, 35, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Odero, M.D.; Zeleznik-Le, N.J.; Chinwalla, V.; Rowley, J.D. Cytogenetic and molecular analysis of the acute monocytic leukemia cell line THP-1 with an MLL-AF9 translocation. Genes Chromosomes Cancer 2000, 29, 333–338. [Google Scholar] [CrossRef]
- Vial, J.P.; Lacombe, F. Immunophenotyping of acute leukemia: Utility of CD45 for blast cell identification. Methods Cell Biol. 2001, 64, 343–358. [Google Scholar] [PubMed]
- Sudha, T.; Bharali, D.J.; Yalcin, M.; Darwish, N.H.; Debreli Coskun, M.; Keating, K.A.; Lin, H.Y.; Davis, P.J.; Mousa, S.A. Targeted delivery of paclitaxel and doxorubicin to cancer xenografts via the nanoparticle of nano-diamino-tetrac. Int. J. Nanomed. 2017, 12, 1305–1315. [Google Scholar] [CrossRef]
- Sudha, T.; Bharali, D.J.; Yalcin, M.; Darwish, N.H.; Coskun, M.D.; Keating, K.A.; Lin, H.Y.; Davis, P.J.; Mousa, S.A. Targeted delivery of cisplatin to tumor xenografts via the nanoparticle component of nano-diamino-tetrac. Nanomedicine 2017, 12, 195–205. [Google Scholar] [CrossRef] [Green Version]
- Hariri, W.; Sudha, T.; Bharali, D.J.; Cui, H.; Mousa, S.A. Nano-targeted delivery of toremifene, an estrogen receptor-a blocker in prostate cancer. Pharm. Res. 2015, 32, 2764–2774. [Google Scholar] [CrossRef]
- Sudha, T.; Bharali, D.J.; Sell, S.; Darwish, N.H.E.; Davis, P.J.; Mousa, S.A. Nanoparticulate tetrac inhibits growth and vascularity of glioblastoma xenografts. Horm. Cancer 2017, 8, 157–165. [Google Scholar] [CrossRef]
- Ghosh, S.S.; Kao, P.M.; McCue, A.W.; Chappelle, H.L. Use of maleimide-thiol coupling chemistry for efficient syntheses of oligonucleotide-enzyme conjugate hybridization probes. Bioconj. Chem. 1990, 1, 71–76. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Ghosh, S. The NF-κB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef]
- Hoesel, B.; Schmid, J.A. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef]
- Imbert, V.; Peyron, J.F. NF-kB in hematological malignancies. Biomedicines 2017, 5, 27. [Google Scholar] [CrossRef]
- Park, M.H.; Hong, J.T. Roles of NF-κB in cancer and inflammatory diseases and their therapeutic approaches. Cells 2016, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- de Graffenried, L.A.; Chandrasekar, B.; Friedrichs, W.E.; Donzis, E.; Silva, J.; Hidalgo, M.; Freeman, J.W.; Weiss, G.R. NF-kB inhibition markedly enhances sensitivity of resistant breast cancer tumor cells to tamoxifen. Ann. Oncol. 2004, 15, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Crowell, J.A.; Steele, V.E.; Sigman, C.C.; Fay, J.R. Is inducible nitric oxide synthase a target for chemoprevention? Mol. Cancer Ther. 2003, 2, 815–823. [Google Scholar]
- Pautz, A.; Art, J.; Hahn, S.; Nowag, S.; Voss, C.; Kleinert, H. Regulation of the expression of inducible nitric oxide synthase. In Nitric Oxide; Academic Press: Cambridge, MA, USA, 2010; Volume 23, pp. 75–93. [Google Scholar]
- Brandao, M.M.; Soares, E.; Salles, T.S.; Saad, S.T. Expression of inducible nitric oxide synthase is increased in acute myeloid leukaemia. Acta Haematol. 2001, 106, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Dolcet, X.; Llobet, D.; Pallares, J.; Matias-Guiu, X. NF-kB in development and progression of human cancer. Virchows Arch. 2005, 446, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Rushworth, S.A.; Zaitseva, L.; Langa, S.; Bowles, K.M.; MacEwan, D.J. Flip regulation of HO-1 and TNF signalling in human acute myeloid leukemia provides a unique secondary anti-apoptotic mechanism. Oncotarget 2010, 1, 359–366. [Google Scholar]
- Gyrd-Hansen, M.; Meier, P. IAPs: From caspase inhibitors to modulators of NF-kB, inflammation and cancer. Nat. Rev. Cancer 2010, 10, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Abdel-Mageed, A.B.; Mondal, D.; Kandil, E. The nuclear factor kappa-B signaling pathway as a therapeutic target against thyroid cancers. Thyroid 2013, 23, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Carbone, C.; Melisi, D. NF-kB as a target for pancreatic cancer therapy. Expert Opin. Ther. Targets 2012, 16, S1–S10. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.; Kumar, R. Crosstalk between NFkB and glucocorticoid signaling: A potential target of breast cancer therapy. Cancer Lett. 2012, 322, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Jain, G.; Cronauer, M.V.; Schrader, M.; Moller, P.; Marienfeld, R.B. NF-kB signaling in prostate cancer: A promising therapeutic target? World J. Urol. 2012, 30, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Hjortso, M.D.; Andersen, M.H. The expression, function and targeting of haem oxygenase-1 in cancer. Curr. Cancer Drug Targets 2014, 14, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Jeney, V.; Balla, J.; Yachie, A.; Varga, Z.; Vercellotti, G.M.; Eaton, J.W.; Balla, G. Pro-oxidant and cytotoxic effects of circulating heme. Blood 2002, 100, 879–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rushworth, S.A.; MacEwan, D.J. HO-1 underlies resistance of AML cells to TNF-induced apoptosis. Blood 2008, 111, 3793–3801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heasman, S.A.; Zaitseva, L.; Bowles, K.M.; Rushworth, S.A.; Macewan, D.J. Protection of acute myeloid leukaemia cells from apoptosis induced by front-line chemotherapeutics is mediated by haem oxygenase-1. Oncotarget 2011, 2, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.L.; Neering, S.J.; Upchurch, D.; Grimes, B.; Howard, D.S.; Rizzieri, D.A.; Luger, S.M.; Jordan, C.T. Nuclear factor-κB is constitutively activated in primitive human acute myelogenous leukemia cells. Blood 2001, 98, 2301–2307. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, B.; Weber, M.; Quirling, M.; Fischer, C.; Page, S.; Adam, M.; Von Schilling, C.; Waterhouse, C.; Schmid, C.; Neumeier, D. Increased IkB kinase activity is associated with activated NF-kB in acute myeloid blasts. Leukemia 2002, 16, 2062. [Google Scholar] [CrossRef] [PubMed]
- Cilloni, D.; Martinelli, G.; Messa, F.; Baccarani, M.; Saglio, G. Nuclear factor κB as a target for new drug development in myeloid malignancies. Haematologica 2007, 92, 1224–1229. [Google Scholar] [CrossRef] [PubMed]
- Kordes, U.; Krappmann, D.; Heissmeyer, V.; Ludwig, W.; Scheidereit, C. Transcription factor NF-kB is constitutively activated in acute lymphoblastic leukemia cells. Leukemia 2000, 14, 399. [Google Scholar] [CrossRef] [PubMed]
- Ghantous, A.; Sinjab, A.; Herceg, Z.; Darwiche, N. Parthenolide: From plant shoots to cancer roots. Drug Discov. Today 2013, 18, 894–905. [Google Scholar] [CrossRef]
- Wuerzberger-Davis, S.M.; Chang, P.Y.; Berchtold, C.; Miyamoto, S. Enhanced G2-M arrest by nuclear factor-kB-dependent p21waf1/cip1 induction. Mol. Cancer Res. 2005, 3, 345–353. [Google Scholar] [CrossRef]
- Zhou, J.; Chng, W.J. Identification and targeting leukemia stem cells: The path to the cure for acute myeloid leukemia. World J. Stem Cells 2014, 6, 473–484. [Google Scholar] [CrossRef]
- Guzman, M.L.; Rossi, R.M.; Karnischky, L.; Li, X.; Peterson, D.R.; Howard, D.S.; Jordan, C.T. The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells. Blood 2005, 105, 4163–4169. [Google Scholar] [CrossRef]
- Steele, A.; Jones, D.; Ganeshaguru, K.; Duke, V.; Yogashangary, B.; North, J.; Lowdell, M.; Kottaridis, P.; Mehta, A.; Prentice, A. The sesquiterpene lactone parthenolide induces selective apoptosis of B-chronic lymphocytic leukemia cells in vitro. Leukemia 2006, 20, 1073. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |





| Cell Line | CD34 (%) | CD44 (%) | CD34/CD44 (%) |
|---|---|---|---|
| Kasumi-1 | 63 | 99 | 61 |
| KG-1a | 98 | 98 | 97 |
| THP-1 Lucia | 21 | 78 | 13 |
| Nanoparticle | Size (nm) | PDI | Zeta Potential (ζ) |
|---|---|---|---|
| PLGA-NPs (void) | 176 | 0.056 | −13.6 |
| PLGA-PTL-NPs | 147 | 0.096 | −15.2 |
| PLGA-antiCD44-PTL-NPs | 162 | 0.098 | −15.8 |
| Gene Symbol | Gene Description | Assay ID | Amplicon Length |
|---|---|---|---|
| RELA | Nuclear factor kappa B (NF-κB) | Hs00428211_m1 | 87 |
| HPRT1 | Human hypoxanthine phosphoribosyl transferase 1 (house-keeping gene) | Hs02800695_m1 | 82 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darwish, N.H.E.; Sudha, T.; Godugu, K.; Bharali, D.J.; Elbaz, O.; El-ghaffar, H.A.A.; Azmy, E.; Anber, N.; Mousa, S.A. Novel Targeted Nano-Parthenolide Molecule against NF-kB in Acute Myeloid Leukemia. Molecules 2019, 24, 2103. https://doi.org/10.3390/molecules24112103
Darwish NHE, Sudha T, Godugu K, Bharali DJ, Elbaz O, El-ghaffar HAA, Azmy E, Anber N, Mousa SA. Novel Targeted Nano-Parthenolide Molecule against NF-kB in Acute Myeloid Leukemia. Molecules. 2019; 24(11):2103. https://doi.org/10.3390/molecules24112103
Chicago/Turabian StyleDarwish, Noureldien H. E., Thangirala Sudha, Kavitha Godugu, Dhruba J. Bharali, Osama Elbaz, Hasan A. Abd El-ghaffar, Emad Azmy, Nahla Anber, and Shaker A. Mousa. 2019. "Novel Targeted Nano-Parthenolide Molecule against NF-kB in Acute Myeloid Leukemia" Molecules 24, no. 11: 2103. https://doi.org/10.3390/molecules24112103
APA StyleDarwish, N. H. E., Sudha, T., Godugu, K., Bharali, D. J., Elbaz, O., El-ghaffar, H. A. A., Azmy, E., Anber, N., & Mousa, S. A. (2019). Novel Targeted Nano-Parthenolide Molecule against NF-kB in Acute Myeloid Leukemia. Molecules, 24(11), 2103. https://doi.org/10.3390/molecules24112103







