Osmanicin, a Polyketide Alkaloid Isolated from Streptomyces osmaniensis CA-244599 Inhibits Elastase in Human Fibroblasts
Abstract
:1. Introduction
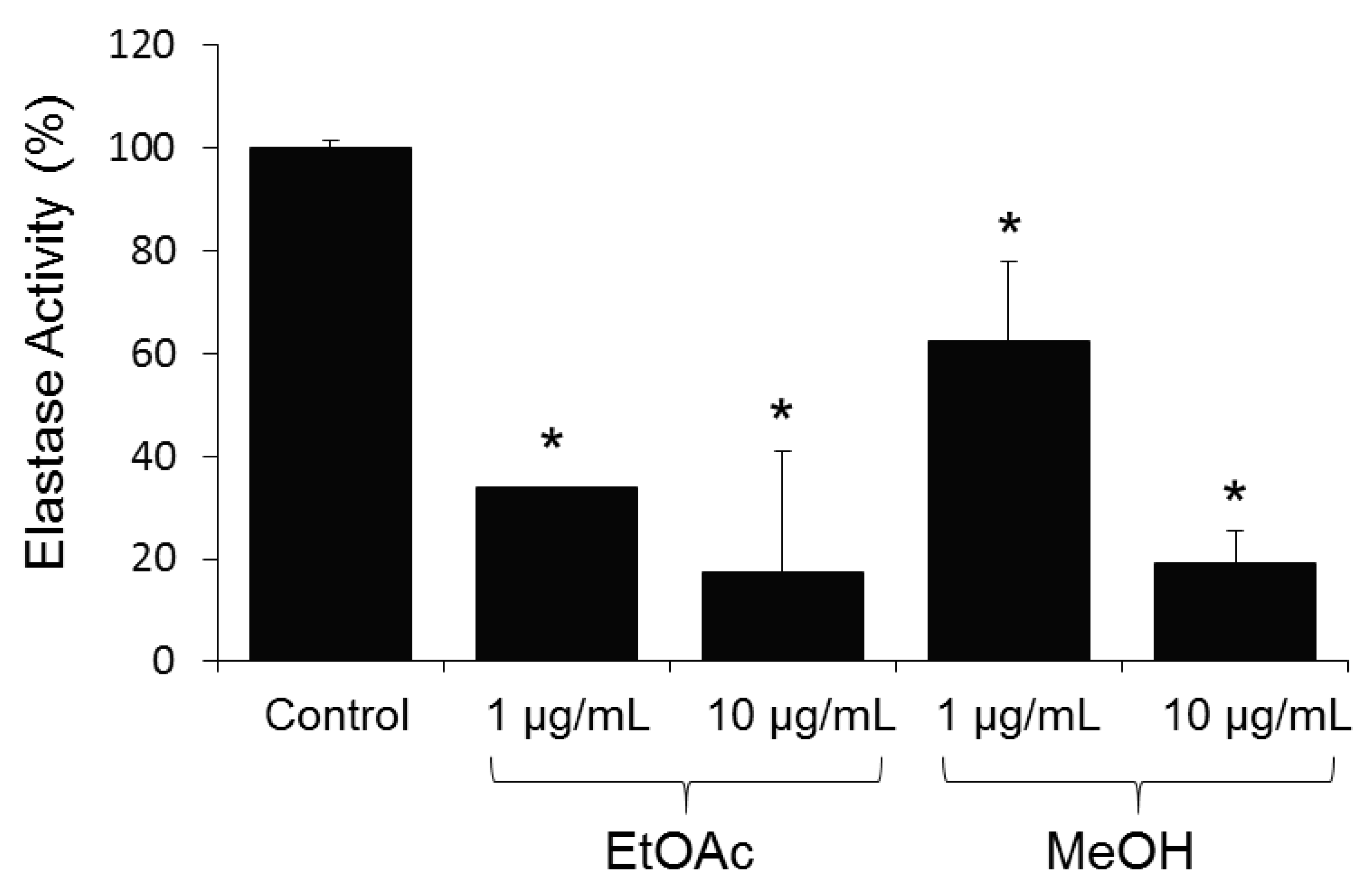
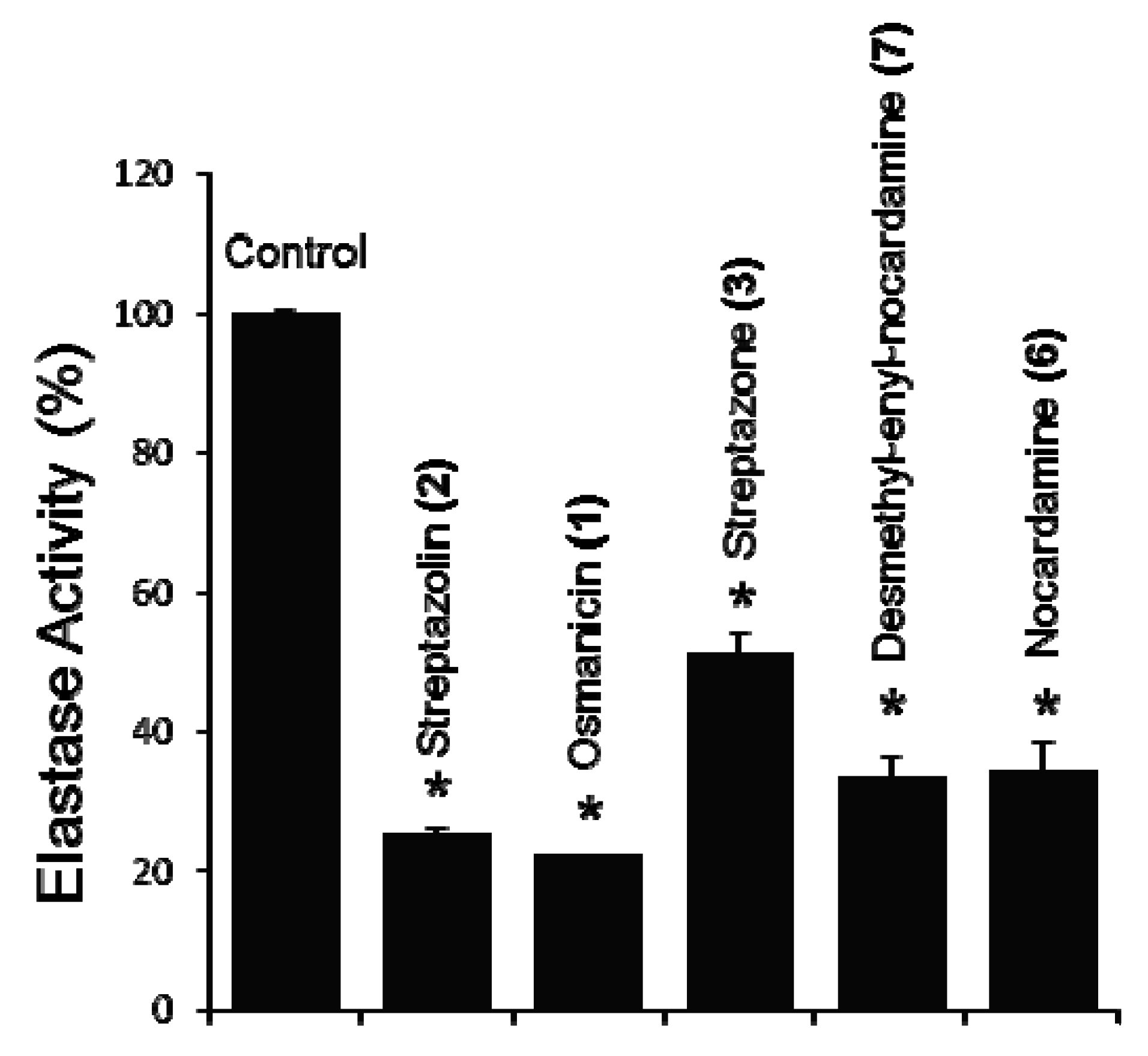
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Bacterial Strain
3.3. 16. S rDNA Sequence and Phylogenetic Analysis
3.4. Fermentation, Isolation and Structure Elucidation of Compounds
3.5. Cell Based Elastase Bioassay
3.6. Determination of Osmanicin IC50 Inhibitory Concentration against Elastase
3.7. Cell Survival Assay
3.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hwang, K.-S.; Kima, H.U.; Charusanti, P.; Palsson, B.Ø.; Lee, S.Y. Systems biology and biotechnology of Streptomyces species for the production of secondary metabolites. Biotechnol. Adv. 2014, 32, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.M.; Dhakal, D.; Sohng, J.K. An Insight into the “-Omics” Based Engineering of Streptomycetes for Secondary Metabolite Overproduction. BioMed. Res. Int. 2013, 2013, 968518. [Google Scholar] [CrossRef] [PubMed]
- Le Goff, G.; Martin, M.-T.; Iorga, B.I.; Adelin, I.; Servy, C.; Cortial, S.; Ouazzani, J. Isolation and Characterization of Unusual Hydrazides from Streptomyces sp. Impact of the Cultivation Support and Extraction Procedure. J. Nat. Prod. 2013, 76, 142–149. [Google Scholar] [PubMed]
- Markus Nett, M.; Ikeda, H.; Moore, B.S. Genomic basis for natural product biosynthetic diversity in the actinomycetes. Nat. Prod. Rep. 2009, 26, 1362–1384. [Google Scholar] [CrossRef] [PubMed]
- Georgousaki, K.; Katsinas, N.; Tsafantakis, N.; Gumeni, S.; Oves-Costales, D.; González, I.; Almeida, C.; Genilloud, O.; Trougakos, I.N.; Fokialakis, N. Actinobacteria of global biodiversity as a source of bioactive metabolites for the discovery and development of novel cosmeuceutical agents. PMIO 2017, 4, S1–S202. [Google Scholar]
- Imokawa, G.; Ishida, K. Biological mechanisms underlying the ultraviolet radiation-induced formation of skin wrinkling and sagging I: Reduced skin elasticity, highly associated with enhanced dermal elastase activity, triggers wrinkling and sagging. Int. J. Mol. Sci. 2015, 16, 7753–7775. [Google Scholar] [CrossRef] [PubMed]
- Rittie, L.; Fisher, G.J. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 2002, 1, 705–720. [Google Scholar] [CrossRef]
- Robert, L.; Molinari, J.; Ravelojaona, V.; Andrès, E.; Robert, A.M. Age- and passage-dependent upregulation of fibroblast elastase-type endopeptidase activity. Role of advanced glycation endproducts, inhibition by fucose- and rhamnose-rich oligosaccharides. Arch. Gerontol. Geriatr. 2010, 50, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Le Goff, G.; Martin, M.-T.; Servy, C.; Cortial, S.; Lopes, P.; Bialecki, A.; Smadja, J.; Ouazzani, J. Isolation and Characterization of α,β-Unsaturated γ-Lactono-Hydrazides from Streptomyces sp. J. Nat. Prod. 2012, 75, 915–919. [Google Scholar] [CrossRef]
- Adelin, E.; Servy, S.; Martin, M.-T.; Arcile, G.; Iorga, B.I.; Retailleau, P.; Bonfill, M.; Ouazzani, J. Bicyclic and tetracyclic diterpenes from a Trichoderma symbiont of Taxus baccata. Phytochemistry 2014, 97, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Djinni, I.; Djoudi, W.; Souagui, S.; Rabia, F.; Rahmouni, S.; Mancini, I.; Kecha, M. Streptomyces thermoviolaceus SRC3 strain as a novel source of the antibiotic adjuvant streptazolin: A statistical approach toward the optimized production. J. Microbial. Meth. 2018, 148, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Puder, C.; Krastel, P.; Zeeck, A. Streptazones A, B1, B2, C, and D: New piperidine alkaloids from streptomycetes. J. Nat. Prod. 2000, 63, 1258–1260. [Google Scholar] [CrossRef] [PubMed]
- Tarazona, G.; Schleissner, C.; Rodríguez, P.; Pérez, M.; Cañedo, L.M.; Cuevas, C. Streptenols F–I Isolated from the Marine-Derived Streptomyces misionensis BAT-10-03-023. J. Nat. Prod. 2017, 80, 1034–1038. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Shin, H.J.; Jang, K.H.; Kim, T.S.; Oh, K.B.; Shin, J. Cyclic peptides of the nocardamine class from a marine-derived bacterium of the genus Streptomyces. J. Nat. Prod. 2005, 68, 623–625. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.Y.S.; Graziani, E.; Waters, B.; Pan, W.; Li, X.; McDermott, J.; Davies, J. Novel natural products from soil DNA libraries in a Streptomycete host. Org. Lett. 2000, 2, 2401–2404. [Google Scholar] [CrossRef]
- Drautz, H.; Zähner, H. Isolation and structure of streptazolin. Helv. Chim. Acta 1981, 64, 1752–1765. [Google Scholar] [CrossRef]
- Karrer, A.; Dobler, M. Stoffwechselprodukte von mikroorganismen 217. Mitteilung röntgenstrukturanalyse von O-acetyldihydrostreptazolin. Helv. Chim. Acta 1982, 65, 1432–1435. [Google Scholar] [CrossRef]
- Awodi, U.R.; Ronan, J.L.; Masschelein, J.; De los Santos, E.L.C.; Challis, G. Thioester reduction and aldehyde transaminationare universal steps in actinobacterial polyketidealkaloid biosynthesis. Chem. Sci. 2017, 8, 411–415. [Google Scholar] [CrossRef]
- Byrne, M.J.; Lees, N.R.; Han, L.-C.; Van der Kamp, M.W.; Mulholland, A.J.; Stach, J.E.M.; Willis, C.L.; Paul, R.; Race, P.R. The catalytic mechanism of a natural Diels-Alderase revealed in molecular detail. J. Am. Chem. Soc. 2016, 138, 6095–6098. [Google Scholar] [CrossRef]
- Klas, K.; Tsukamoto, S.; Sherman, D.H.; Williams, R.M. Natural Diels-Alderases: Elusive and Irresistible. J. Org. Chem. 2015, 80, 11672–11685. [Google Scholar] [CrossRef]
- Watanabe, K.; Mie, T.; Ichihara, A.; Oikawa, H.; Honma, M. An Enzyme-Catalyzed [4+2] Cycloaddition is a Key Step in the Biosynthesis of Spinosyn A. J. Biol. Chem. 2000, 275, 38393–38401. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, N.; Kemter, K.; Richter, G.; Cushman, M.; Bacher, A. Domain structure of riboflavin synthase. Eur. J. Biochem. 2001, 268, 4315–4323. [Google Scholar] [CrossRef] [PubMed]
- Eiben, C.B.; Siegel, J.B.; Bale, J.B.; Cooper, S.; Khatib, F.; Shen, B.W.; Players, F.; Stoddard, B.L.; Popovic, Z.; Baker, D. Increased Diels-Alderase activity through backbone remodeling guided by Foldit players. Nat. Biotechnol. 2012, 30, 190–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preiswerka, N.; Becka, T.; Schulza, J.D.; Milovníka, P.; Mayera, C.; Siegel, J.B.; Baker, D.; Hilvert, D. Impact of scaffold rigidity on the design and evolution of an artificial Diels-Alderase. Proc. Natl. Acad. Sci. USA 2014, 111, 8013–8018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leman-Loubière, C.; Le Goff, G.; Retailleau, P.; Debitus, C.; Ouazzani, J. Sporothriolide-Related Compounds from the Fungus Hypoxylon monticulosum CLL-205 Isolated from a Sphaerocladina Sponge from the Tahiti Coast. J. Nat. Prod. 2017, 80, 2850–2854. [Google Scholar] [CrossRef] [PubMed]
- Seite, S.; Zucchi, H.; Septier, D.; Igondjo-Tchen, S.; Senni, K.; Godeau, G. Elastin changes during chronological and photo-ageing: The important role of lysozyme. J. Eur. Acad. Dermatol. Venereol. 2006, 20, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Innis, M.A.; Gelfand, D.H.; Sninsky, J.J.; White, T.J. PCR Protocols: A Guide to Methods and application. Trends Genet. 1990, 6, 229. [Google Scholar]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef] [Green Version]
- Lane, D.J. 16S/23S rRNA sequencing. In Nucleic Acid Techniques in Bacterial Systematics; Stackebrandt, E., Goodfellow, M., Eds.; John Wiley and Sons: New York, NY, USA, 1991; pp. 115–175. [Google Scholar]
- Yoon, S.H.; Ha, S.M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA and whole genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic. Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Jukes, T.H.; Cantor, C.R. Evolution of Protein Molecules. In Mammalian Protein Metabolism; Munro, H.N., Ed.; Academic Press: New York, NY, USA, 1969; pp. 21–132. [Google Scholar]
- Stratford, F.L.; Chondrogianni, N.; Trougakos, I.P.; Gonos, E.S.; Rivett, A.J. Proteasome response to interferon-gamma is altered in senescent human fibroblasts. FEBS Lett. 2006, 580, 3989–3994. [Google Scholar] [CrossRef] [PubMed]
- Sklirou, A.D.; Gaboriaud-Kolar, N.; Papassideri, I.; Skaltsounis, A.L.; Trougakos, I.P. 6-bromo-indirubin-3’-oxime (6BIO), a Glycogen synthase kinase-3β inhibitor, activates cytoprotective cellular modules and suppresses cellular senescence-mediated biomolecular damage in human fibroblasts. Sci. Rep. 2017, 7, 11713. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of osmanicin are available from the authors. |






| Osmanicin (1) a | |||
|---|---|---|---|
| No. | δC, type | δH, mult. (J in Hz) | HMBC |
| 2 | 43.4, CH2 | 3.62, bt (7.4) | C-3, 4, 19 |
| 3 | 36.2, CH2 | 2.39, (dt (8.4, 3.9) | C-2, 4, 5 |
| 4 | 185.7, C | ||
| 5 | 112.4, C | ||
| 6 | 61.0, C | ||
| 7 | 38.9, CH | 2.81, b quint (7.6) | C-6, 21, 22 |
| 8 | 33.2, CH | 2.60, m | C-6, 23 |
| 9 | 141.3, C | ||
| 10 | 80.0, CH | 4.68, m | C-9, 11 |
| 11 | 83.5, CH | 4.69, m | C-10, 12, 13, 18 |
| 12 | 64.5, CH | 4.53, b d (1.4) | C-18 |
| 13 | 138.7, C | ||
| 14 | 38.8, CH | 3.36, m | C-13, 16 |
| 15 | 28.6, CH2 | 1.02, m 1.41, d quint (13.3, 2.9) | |
| 16 | 43.6, CH2 | 3.11, td (13.3, 3.2) | C-18 |
| 3.58, dd (5.1, 2.3) | |||
| 18 | 160.0, C | ||
| 19 | 169.2, C | ||
| 20 | 127.4, CH | 6.33, d (5.8) | C-5, 6, 19, 21 |
| 21 | 154.6, CH | 6.78, d (5.8) | C-5, 6, 20 |
| 22 | 12.8, CH3 | 1.05, d (7.4) | C-6, 7, 8 |
| 23 | 13.4, CH3 | 0.62, d (7.1) | C-7, 8, 9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samy, M.N.; Le Goff, G.; Lopes, P.; Georgousaki, K.; Gumeni, S.; Almeida, C.; González, I.; Genilloud, O.; Trougakos, I.; Fokialakis, N.; et al. Osmanicin, a Polyketide Alkaloid Isolated from Streptomyces osmaniensis CA-244599 Inhibits Elastase in Human Fibroblasts. Molecules 2019, 24, 2239. https://doi.org/10.3390/molecules24122239
Samy MN, Le Goff G, Lopes P, Georgousaki K, Gumeni S, Almeida C, González I, Genilloud O, Trougakos I, Fokialakis N, et al. Osmanicin, a Polyketide Alkaloid Isolated from Streptomyces osmaniensis CA-244599 Inhibits Elastase in Human Fibroblasts. Molecules. 2019; 24(12):2239. https://doi.org/10.3390/molecules24122239
Chicago/Turabian StyleSamy, Mamdouh N., Géraldine Le Goff, Philippe Lopes, Katerina Georgousaki, Sentiljana Gumeni, Celso Almeida, Ignacio González, Olga Genilloud, Ioannis Trougakos, Nikolas Fokialakis, and et al. 2019. "Osmanicin, a Polyketide Alkaloid Isolated from Streptomyces osmaniensis CA-244599 Inhibits Elastase in Human Fibroblasts" Molecules 24, no. 12: 2239. https://doi.org/10.3390/molecules24122239
APA StyleSamy, M. N., Le Goff, G., Lopes, P., Georgousaki, K., Gumeni, S., Almeida, C., González, I., Genilloud, O., Trougakos, I., Fokialakis, N., & Ouazzani, J. (2019). Osmanicin, a Polyketide Alkaloid Isolated from Streptomyces osmaniensis CA-244599 Inhibits Elastase in Human Fibroblasts. Molecules, 24(12), 2239. https://doi.org/10.3390/molecules24122239