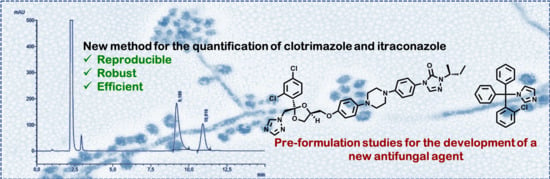
Development of a Method for the Quantification of Clotrimazole and Itraconazole and Study of Their Stability in a New Microemulsion for the Treatment of Sporotrichosis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Study of the Compatibility between Clotrimazole and Itraconazole
2.2. Determination of the Concentration of Clotrimazole and Itraconazole in Microemulsions Using HPLC Analyses
2.3. Study of the Stability of a Novel Microemulsion Containing Clotrimazole and Itraconazole
3. Experimental Methods
3.1. Materials for Analytical Method Development
3.2. Compatibility Study of Clotrimazole and Itraconazole
3.2.1. Preparation of Clotrimazole/Itraconazole Binary Mixtures
3.2.2. X-ray Powder Diffraction (PXRD)
3.2.3. Fourier Transform Infrared Spectroscopy (FTIR)
3.2.4. Thermal Analyses
3.3. Instruments and Chromatographic Conditions
3.4. Standard Stock Solutions and Calibration Standards
3.5. Sample Preparation
3.6. Method Validation Protocol
3.6.1. Linearity Range
3.6.2. Selectivity
3.6.3. Sensitivity
3.6.4. Precision and Accuracy
3.6.5. Stability
3.6.6. Robustness
3.7. Application of the Method
3.7.1. Microemulsion Preparation
3.7.2. Stability Study
3.8. Characterization of the Synthetized Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chakrabarti, A.; Bonifaz, A.; Gutierrez-Galhardo, M.C.; Mochizuki, T.; Li, S. Global epidemiology of sporotrichosis. Med. Mycol. 2015, 53, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Gremião, I.D.; Miranda, L.H.; Reis, E.G.; Rodrigues, A.M.; Pereira, S.A. Zoonotic epidemic of sporotrichosis: Cat to human transmission. PLoS Pathog. 2017, 13, e1006077. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.M.; de Hoog, G.S.; de Camargo, Z.P. Sporothrix species causing outbreaks in animals and humans driven by animal-animal transmission. PLoS Pathog. 2016, 12, e1005638. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.B.L.; Schubach, T.P.; Coll, J.O.; Gremião, I.D.; Wanke, B.; Schubach, A. Esporotricose: A evolução e os desafios de uma epidemia. Rev. Panam. Salud Publica 2010, 27, 455–460. [Google Scholar] [PubMed]
- Gremião, I.D.; Menezes, R.C.; Schubach, T.M.; Figueiredo, A.B.; Cavalcanti, M.C.; Pereira, S.A. Feline sporotrichosis: Epidemiological and clinical aspects. Med. Mycol. 2015, 53, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, B.; Campos, P.E. Sporotrichosis: A forgotten disease in the drug research. Expert Rev. Anti-Infect. Ther. 2004, 2, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Kadavakollu, S.; Stailey, C.; Kunapareddy, C.S.; White, S. Clotrimazole as a cancer drug: A short review. Med. Chem. 2014, 4, 722–724. [Google Scholar] [CrossRef]
- Gagini, T.; Borba-Santos, L.P.; Rodrigues, A.M.; Camargo, Z.P.; Rozental, S. Clotrimazole is highly effective in vitro against feline Sporothrix brasiliensis isolates. J. Med. Microbiol. 2011, 66, 1573–1580. [Google Scholar] [CrossRef]
- Pai, V.; Ganavalli, A.; Kikkeri, N.N. Antifungal resistance in dermatology. Indian J. Dermatol. 2018, 63, 361–368. [Google Scholar] [CrossRef]
- Carvalho, A.L.M.; da Silva, J.A.; Lira, A.A.M.; Conceição, T.M.F.; Nunes, R.S.; Junior, R.L.C.A.; Sarmento, V.H.V.; Leal, L.B.; Santana, D.P. Evaluation of microemulsion and lamellar liquid crystalline systems for transdermal zidovudine delivery. J. Pharm. Sci. 2016, 105, 1–6. [Google Scholar] [CrossRef]
- Padula, C.; Telò, I.; Ianni, A.D.; Pescina, S.; Nicoli, S.; Santi, P. Microemulsion containing triamcinolone acetonide for buccal administration. Eur. J. Pharm. Sci. 2018, 115, 233–239. [Google Scholar] [CrossRef]
- Rashida, M.A.; Naza, T.; Abbasa, M.; Nazirb, S.; Younasa, N.; Majeeda, S.; Qureshic, N.; Akhtard, M.N. Chloramphenicol loaded microemulsions: Development, characterization and stability. Colloid Interface Sci. Commun. 2019, 28, 41–48. [Google Scholar] [CrossRef]
- Seok, S.H.; Lee, S.-A.; Park, E.-S. Formulation of a microemulsion-based hydrogel containing celecoxib. J. Drug Deliv. Sci. Technol. 2018, 43, 409–414. [Google Scholar] [CrossRef]
- Kumar, S.K.; Dhancinamoorthi, D.; Sarvanan, R.; Gopal, U.K.; Shanmugam, V. Microemulsions as a carrier for novel drug delivery: A review. Int. J. Pharm. Sci. Rev. Res. 2011, 10, 37–45. [Google Scholar]
- Hu, X.-B.; Kang, R.-R.; Tang, T.-T.; Li, Y.-J.; Wu, J.-Y.; Wang, J.-M.; Liu, X.-Y.; Xiang, D.-X. Topical delivery of 3,5,4′-trimethoxy-trans-stilbene-loaded microemulsion-based hydrogel for the treatment of osteoarthritis in a rabbit model. Drug Deliv. Transl. Res. 2019, 9, 357–365. [Google Scholar] [CrossRef]
- Hájková, R.; Sklenárová, H.; Matysová, L.; Svecová, P.; Solich, P. Development and validation of HPLC method for determination of clotrimazole and its two degradation products in spray formulation. Talanta 2007, 73, 483–489. [Google Scholar] [CrossRef]
- Abdel-Moety, E.M.; Khattab, F.I.; Kelani, K.M.; AbouAl-Alamein, A.M. Chromatographic determination of clotrimazole, ketoconazole and fluconazole in pharmaceutical formulations. Farmaco 2002, 57, 931–938. [Google Scholar] [CrossRef]
- Bharate, S.S.; Bharate, S.B.; Bajaj, A.N. Interactions and incompatibilities of pharmaceutical excipientes with active pharmaceutical ingredients: A comprehensive review. J. Excipients and Food Chem. 2010, 1, 3–26. [Google Scholar] [CrossRef]
- Ceschel, G.C.; Badiello, R.; Ronchi, C.; Maffei, P. Degradation of components in drug formulations: A comparison between HPLC and DSC methods. J. Pharm. Biomed. Anal. 2003, 32, 1067–1072. [Google Scholar] [CrossRef]
- Deshmukha, P.R.; Gaikwadb, V.L.; Tamanea, P.K.; Mahadikc, K.R.; Purohit, R.N. Development of stability-indicating HPLC method and accelerated stability studies for osmotic and pulsatile tablet formulations of Clopidogrel Bisulfate. J. Pharm. Biomed. Anal. 2019, 165, 346–356. [Google Scholar] [CrossRef]
- Lee, T.-K.; Ryoo, S.-J.; Lee, Y.-S. A new method for the preparation of 2-chlorotrityl resin and its application to solid-phase peptide synthesis. Tetrahedron Lett. 2007, 48, 389–391. [Google Scholar] [CrossRef]
- Nandi, I.; Bari, M.; Joshi, H. Study of isopropyl myristate micremulsion systems containing cyclodextrins to improve the solubility of two model hydrophobic drugs. AAPS PharmaSciTech. 2003, 4, 1–9. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |












| Standard Solutions | Parameters of the Method | Validation Results |
|---|---|---|
| Clotrimazole | Linearity | Calibration range (μg mL−1): 5–200 y = 233647.7939x − 312039.9299 (R2 = 0.9988) |
| LOD | 0.84 μg mL−1 | |
| LOQ | 2.54 μg mL−1 | |
| Slope | 233647.7939 ± 976.8015153 | |
| Interception | −312039.9299 ± 59416.57811 | |
| Itraconazole | Linearity | Calibration range (μg mL−1): 5–160 y = 89946.6896x − 79996.5373 (R2 = 0.9999) |
| LOD | 0.86 μg mL−1 | |
| LOQ | 2.60 μg mL−1 | |
| Slope | 89946.6896 ± 780.1420761 | |
| Interception | −79996.53731 ± 23351.48986 |
| Concentration (µg/mL) | Clotrimazole | Itraconazole | ||||
|---|---|---|---|---|---|---|
| Average (µg/mL) | Accuracy (%) | Precision (%) | Average (µg/mL) | Accuracy (%) | Precision (%) | |
| 5 | 4.883 | 97.7 | 0.20 | 5.593 | 111.9 | 0.86 |
| 10 | 9.292 | 92.9 | 0.57 | 10.115 | 101.2 | 0.91 |
| 20 | 19.233 | 96.2 | 0.40 | 20.029 | 100.1 | 0.58 |
| 40 | 38.927 | 97.3 | 0.01 | 39.621 | 99.1 | 1.12 |
| 80 | 77.731 | 97.2 | 1.08 | 79.154 | 98.9 | 1.81 |
| 160 | 151.888 | 94.9 | 0.71 | 160.488 | 100.3 | 0.82 |
| 200 | 204.631 | 102.3 | 0.65 | - | - | - |
| Samples (μg mL−1) | Intra-Day Precision (Repeatability) | Inter-Day Precision (Intermediate Precision) | ||||
|---|---|---|---|---|---|---|
| Clotrimazole | Concentration Found (μg mL−1) | Accuracy (%) | Precision (%) | Concentration Found (μg mL−1) | Accuracy (%) | Precision (%) |
| 7 | 6.818 | 97.4 ±2.25 | 0.47 | 6.865 | 98.07 ± 1.17 | 2.35 |
| 15 | 14.510 | 96.7 ±1.13 | 1.18 | 14.075 | 93.83 ± 3.17 | 0.95 |
| 120 | 116.679 | 97.2 ±0.27 | 0.28 | 121.108 | 100.92 ± 4.28 | 0.28 |
| Itraconazole | Concentration Found (μg mL−1) | Accuracy (%) | Precision (%) | Concentration Found (μg mL−1) | Accuracy (%) | Precision (%) |
| 7 | 7.206 | 102.9 ± 1.33 | 1.48 | 7.305 | 104.35 ± 1.25 | 1.20 |
| 70 | 70.809 | 101.2 ± 1.15 | 1.16 | 70.374 | 100.53 ± 2.41 | 2.40 |
| 150 | 152.745 | 101.8 ± 0.85 | 0.84 | 160.98 | 100.61 ± 4.9 | 1.59 |
| Days | Accuracy (%) | Precision (%) | Accuracy (%) | Precision (%) |
|---|---|---|---|---|
| Clotrimazole | Itraconazole | |||
| 0 | 97.3 ± 0.94 (25 °C) | 1.18 | 101.4 ± 0.62 | 0.84 |
| 7 | 105.7 ± 0.89 (25 °C) | 0.85 | 98.6 ± 4.48 | 4.57 |
| 104.9 ± 0.07 (−5 °C) | 0.07 | 98.4 ± 7.79 | 7.96 | |
| 15 | 105.3 ± 1.51 (25 °C) | 1.45 | 101.3 ± 0.51 | 0.50 |
| 105.5 ± 0.39 (−5 °C) | 0.38 | 100.1 ± 3.71 | 3.74 | |
| 30 | 97.3 ± 3.15 (25 °C) | 0.62 | 91.3 ± 2.71 | 3.21 |
| 94.2 ± 0.34 (−5 °C) | 0.73 | 88.7 ± 1.63 | 3.04 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia Ferreira, P.; Guimarães de Souza Lima, C.; Noronha, L.L.; de Moraes, M.C.; Silva, F.d.C.d.; Lifsitch Viçosa, A.; Omena Futuro, D.; Francisco Ferreira, V. Development of a Method for the Quantification of Clotrimazole and Itraconazole and Study of Their Stability in a New Microemulsion for the Treatment of Sporotrichosis. Molecules 2019, 24, 2333. https://doi.org/10.3390/molecules24122333
Garcia Ferreira P, Guimarães de Souza Lima C, Noronha LL, de Moraes MC, Silva FdCd, Lifsitch Viçosa A, Omena Futuro D, Francisco Ferreira V. Development of a Method for the Quantification of Clotrimazole and Itraconazole and Study of Their Stability in a New Microemulsion for the Treatment of Sporotrichosis. Molecules. 2019; 24(12):2333. https://doi.org/10.3390/molecules24122333
Chicago/Turabian StyleGarcia Ferreira, Patricia, Carolina Guimarães de Souza Lima, Letícia Lorena Noronha, Marcela Cristina de Moraes, Fernando de Carvalho da Silva, Alessandra Lifsitch Viçosa, Débora Omena Futuro, and Vitor Francisco Ferreira. 2019. "Development of a Method for the Quantification of Clotrimazole and Itraconazole and Study of Their Stability in a New Microemulsion for the Treatment of Sporotrichosis" Molecules 24, no. 12: 2333. https://doi.org/10.3390/molecules24122333
APA StyleGarcia Ferreira, P., Guimarães de Souza Lima, C., Noronha, L. L., de Moraes, M. C., Silva, F. d. C. d., Lifsitch Viçosa, A., Omena Futuro, D., & Francisco Ferreira, V. (2019). Development of a Method for the Quantification of Clotrimazole and Itraconazole and Study of Their Stability in a New Microemulsion for the Treatment of Sporotrichosis. Molecules, 24(12), 2333. https://doi.org/10.3390/molecules24122333