Antispasmodic Activity of Prenylated Phenolic Compounds from the Root Bark of Morus nigra
Abstract
1. Introduction
2. Results and Discussion
2.1. Isolation and Structure Elucidation of Compounds 1–7
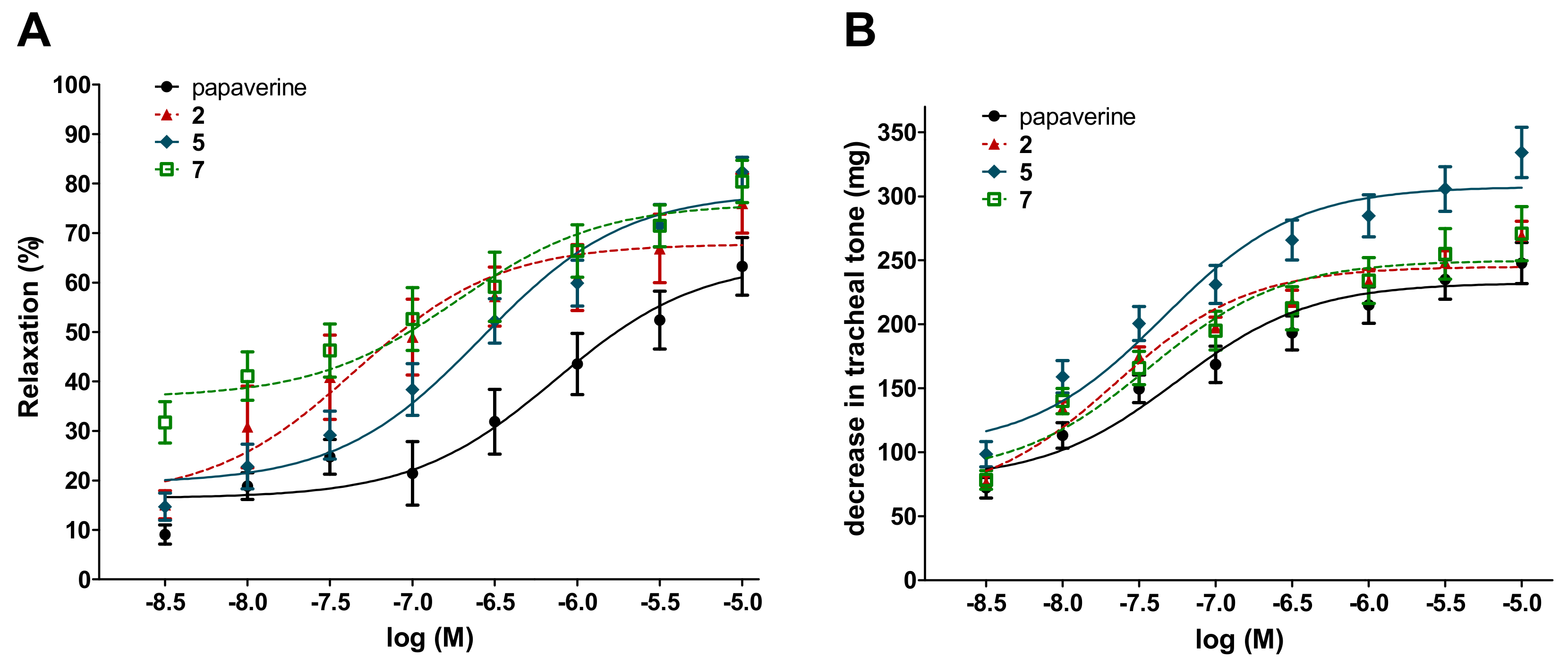
2.2. Antispasmodic Activity of Compounds 1–7
3. Materials and Methods
3.1. General
3.2. Plant Material, Extraction and Pre-Purification
3.3. Chromatographic Separation of Compounds 1–7
3.4. Structure Elucidation of the Isolated Compounds
3.5. Housing and Handling of the Animals
3.6. Isolated Organ Bath Studies
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Randall, O.L. Spasmolytic Activity of Synthetic Drugs. J. Am. Pharm. Assoc. (Pract. Pharm. Ed.) 1948, 9, 572–574. [Google Scholar]
- Doeing, D.C.; Solway, J. Airway smooth muscle in the pathophysiology and treatment of asthma. J. Appl. Physiol. 2013, 114, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Talley, N.J.; Spiegel, B.M.R.; Foxx-Orenstein, A.E.; Schiller, L.; Quigley, E.M.M.; Moayyedi, P. Effect of fibre, antispasmodics, and peppermint oil in the treatment of irritable bowel syndrome: Systematic review and meta-analysis. BMJ 2008, 337, a2313. [Google Scholar]
- Clyde, B.L.; Firlik, A.D.; Kaufmann, A.M.; Spearman, M.P.; Yonas, H. Paradoxical aggravation of vasospasm with papaverine infusion following aneurysmal subarachnoid hemorrhage. Case report. J. Neurosurg. 1996, 84, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Haney, S.; Hancox, R.J. Recovery from bronchoconstriction and bronchodilator tolerance. Clin. Rev. Allergy Immunol. 2006, 31, 181–196. [Google Scholar] [CrossRef]
- Bown, D. Encyclopedia of Herbs and Their Uses; Dorling Kindersley: London, UK, 1995. [Google Scholar]
- Rawat, P.; Singh, P.K.; Kumar, V. Evidence based traditional anti-diarrheal medicinal plants and their phytocompounds. Biomed. Pharmacother. 2017, 96, 1453–1464. [Google Scholar] [CrossRef]
- Huang, H.P.; Ou, T.T.; Wang, C.J. Mulberry (sang shen zi) and its bioactive compounds, the chemoprevention effects and molecular mechanisms in vitro and in vivo. J. Tradit. Complement. Med. 2013, 3, 7–15. [Google Scholar] [CrossRef]
- Ercisli, S.; Tosun, M.; Duralija, B.; Voca, S.; Sengul, M.; Turan, M. Phytochemical Content of Some Black (Morus nigra L.) and Purple (Morus rubra L.) Mulberry Genotypes. Food Technol. Biotechnol. 2010, 48, 5. [Google Scholar]
- Zoofishan, Z.; Hohmann, J.; Hunyadi, A. Phenolic antioxidants of Morus nigra roots, and antitumor potential of morusin. Phytochem. Rev. 2018, 17, 1031–1045. [Google Scholar] [CrossRef]
- Duke, J.A.; Bogenschutz-Godwin, M.J.; deCelliar, J.; Duke, P.A.K. Handbook of Medicinal Herbs, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Mohiuddin, E.; Usmanghani, K.; Akram, M.; Asif, H.M.; Akhtar, N.; Shah, P.A.; Uzair, M. Morus nigra-L.A. J. Med. Plants Res. 2011, 5, 3. [Google Scholar]
- Ziaei, S.A.; Heidari, M.R.; Amin, G.R.; Kochmeshki, A.; Heidari, M. Inhibitory Effects of Germinal Angiotensin Converting Enzyme by Medicinal Plants Used in Iranian Traditional Medicine as Antihypertensive. J. Kerman Univ. Med. Sci. 2009, 16, 134–143. [Google Scholar]
- The Ayurvedic Pharmacopoeia of India; Government Of India Ministry Of Health & Family Welfare Department Of Ayurveda, yoga & Naturopathy, Unani, Siddha and Homoeopathy (Ayush): New Delhi, India, 2008; p. 423.
- Khare, C.P. Indian Medicinal Plants, An Illustrated Dictionary; Springer-Verlag: Heidelberg, Germany, 2010; Volume 1, p. 426. [Google Scholar]
- Ahmad, J.; Farooqui, A.H.; Siddiqui, T.O. Morus nigra. Hamdard Med. 1985, 15, 3. [Google Scholar]
- Panda, H. Handbook on Ayurvedic Medicines with Formulae, Processes & Their Uses, 2nd ed.; National Institute of Industrial Research project consultency services: New Delhi, India, 2013; Volume 2, p. 136. [Google Scholar]
- Tag, H.M. Hepatoprotective effect of mulberry (Morus nigra) leaves extract against methotrexate induced hepatotoxicity in male albino rat. BMC Complement. Altern. Med. 2015, 15, 252. [Google Scholar] [CrossRef] [PubMed]
- Shibata, Y.; Kume, N.; Arai, H.; Hayashida, K.; Inui-Hayashida, A.; Minami, M.; Mukai, E.; Toyohara, M.; Harauma, A.; Murayama, T.; et al. Mulberry leaf aqueous fractions inhibit TNF-alpha-induced nuclear factor kappaB (NF-kappaB) activation and lectin-like oxidized LDL receptor-1 (LOX-1) expression in vascular endothelial cells. Atherosclerosis 2007, 193, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.V.; Bieski, I.G.C.; Balogun, S.O.; Martins, D.T.O. Ethnobotanical study of medicinal plants used by Ribeirinhos in the North Araguaia microregion, Mato Grosso, Brazil. J. Ethnopharmacol. 2017, 205, 69–102. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Mawla, A.M.; Mohamed, K.M.; Mostafa, A.M. Induction of biologically active flavonoids in cell cultures of morus nigra and testing their hypoglycemic efficacy. Sci. Pharm. 2011, 79, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Hunyadi, A.; Gergely, A.; Simon, A.; Tóth, G.; Veress, G.; Báthori, M. Preparative-Scale Chromatography of Ecdysteroids of Serratula wolffii Andrae. J. Chromatogr. Sci. 2007, 45, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Fukai, T.; Yamada, S.; Katayanagi, M. Phenolic Constituents of the Cultivated Mulberry Tree (Morus alba L.). Chem. Pharm. Bull. 1976, 24, 2898–2900. [Google Scholar] [CrossRef]
- Nomura, T.; Fukai, T. Constituents of the cultivated mulberry tree. Planta Med. 1981, 42, 79–88. [Google Scholar] [CrossRef]
- Lee, H.J.; Lyu da, H.; Koo, U.; Lee, S.J.; Hong, S.S.; Kim, K.; Kim, K.H.; Lee, D.; Mar, W. Inhibitory effect of 2-arylbenzofurans from the Mori Cortex Radicis (Moraceae) on oxygen glucose deprivation (OGD)-induced cell death of SH-SY5Y cells. Arch. Pharm. Res. 2011, 34, 1373–1380. [Google Scholar] [CrossRef]
- Alves, L.F.; Chimiak, A.; Milewska, M.J.; Nomura, T. Phenolic compounds of the mulberry tree and related plants. In Fortschritte der Chemie organischer Naturstoffe. Progress in the chemistry of organic natural products; Herz, W., Grisebach, H., Kirby, G.W., Tamm, C., Eds.; Springer-Verlag: New York, NY, USA, 1988; Volume 53, p. 93. [Google Scholar]
- Fukai, T.; Hano, Y.; Hirakura, K.; Nomura, T.; Uzawa, J.U.N.; Fukushima, K. Structures of two natural hypotensive diels alder type adducts mulberrofurans f and g from the cultivated mulberry tree morus lhou. Chem. Pharm. Bull. 1985, 33, 3195–3204. [Google Scholar] [CrossRef] [PubMed]
- Kapche, G.D.W.F.; Fozing, C.D.; Donfack, J.H.; Fotso, G.W.; Amadou, D.; Tchana, A.N.; Bezabih, M.; Moundipa, P.F.; Ngadjui, B.T.; Abegaz, B.M. Prenylated arylbenzofuran derivatives from Morus mesozygia with antioxidant activity. Phytochemical 2009, 70, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Hirakura, K.; Fujimoto, Y.; Fukai, T.; Nomura, T. Two Phenolic glycosides from the root bark of the cultivated mulberry tree (Morus lhou). J. Nat. Product. 1986, 49, 218–224. [Google Scholar] [CrossRef]
- Wang, Y.-N.; Liu, M.-F.; Hou, W.-Z.; Xu, R.-M.; Gao, J.; Lu, A.-Q.; Xie, M.-P.; Li, L.; Zhang, J.-J.; Peng, Y.; et al. Bioactive benzofuran derivatives from cortex Mori radicis, and their neuroprotective and analgesic activities mediated by mGluR₁. Molecules 2017, 22, 236. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.K.; Cao, Y.G.; Ke, Y.Y.; Zhang, Y.L.; Li, F.; Gong, J.H.; Zhao, X.; Kuang, H.X.; Feng, W.S. Phenolic constituents from the root bark of Morus alba L. and their cardioprotective activity in vitro. Phytochemistry 2017, 135, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, F.; Monacelli, B.; Messana, I. Comparison between in vivo and in vitro metabolite production of Morus nigra. Planta Med. 1999, 65, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.P.; Cheng, K.W.; Zhu, Q.; Wang, X.C.; Lin, Z.X.; Wang, M. Tyrosinase inhibitory constituents from the roots of Morus nigra: A structure-activity relationship study. J. Agric. Food Chem. 2010, 58, 5368–5373. [Google Scholar] [CrossRef]
- Dong, X.; Qi, L.; Jiang, C.; Chen, J.; Wei, E.; Hu, Y. Synthesis, biological evaluation of prenylflavonoids as vasorelaxant and neuroprotective agents. Bio. Med. Chem. Lett. 2009, 19, 3196–3198. [Google Scholar] [CrossRef]
- Kim, C.Y.; Kim, H.J.; Kim, K.M.; Oak, M.H. Vasorelaxant prenylated flavonoids from the roots of Sophora flavescens. Biosci. Biotechnol. Biochem. 2013, 77, 395–397. [Google Scholar] [CrossRef]
- Hejazian, S.H.; Bagheri, S.M.; Dashti-R, M.H. Relaxant effect of Humulus lupulus extracts on isotonic rat’s ileum contractions. Avicenna J. Phytomed. 2014, 4, 53–58. [Google Scholar]
- Peery, A.F.; Crockett, S.D.; Barritt, A.S.; Dellon, E.S.; Eluri, S.; Gangarosa, L.M.; Jensen, E.T.; Lund, J.L.; Pasricha, S.; Runge, T.; et al. Burden of gastrointestinal, liver, and pancreatic diseases in the United States. Gastroenterology 2015, 149, 1731–1741.e1733. [Google Scholar] [CrossRef] [PubMed]
- Kuenzig, M.E.; Bishay, K.; Leigh, R.; Kaplan, G.G.; Benchimol, E.I.; Crowdscreen SR Review Team. Co-occurrence of asthma and the inflammatory bowel diseases: A systematic review and meta-analysis. Clin. Transl. Gastroenterol. 2018, 9, 188. [Google Scholar] [CrossRef] [PubMed]
- Duddeck, H.; Dietrich, W.; ToÓth, G.B. Structure Elucidation by Modern NMR: A Workbook, 3rd ed.; Steinkopff Verlag-Springer: Darmstadt, Germany, 1998; p. 211. [Google Scholar]
- Pretsch, E.; Tóth, G.; Munk, M.E.; Badertscher, M. Computer-Aided Structure Elucidation: Spectra Interpretation and Structure Generation; Wiley-VCH: Weinheim, Germany, 2002; p. 279. [Google Scholar]
Sample Availability: Samples of the compounds 1, and 3–7 are available from the authors. |


| Compound | Maximal Inhibition of Ileal Contraction (%) | Tracheal Tone Reduction (mg) |
|---|---|---|
| papaverine a | 47.9 ± 10.5 | 134.4 ± 41.4 |
| MeOH extract | −33.2 ± 27.9 *** | −22.0 ± 11.9 *** |
| EtOAc fraction | 24.4 ± 16.2 ** | −5.0 ± 11.9 *** |
| n-Hexane fraction | no action | no action |
| Water fraction | −9.1 ± 10.8 *** | −32.5 ± 10.3 *** |
| morusin (1) | no action | 90.7 ± 20.4 * |
| kuwanon U (2) a | 39.6 ± 4.5 | 145.5 ± 16.7 |
| kuwanon E (3) | 26.6 ± 9.1 * | no action |
| moracin P (4) | no action | 42.4 ± 7.7 ** |
| moracin O (5) a | 47.2 ± 11.6 | 123.6 ± 36.1 |
| albanol A (6) | 20.5 ± 9.1 ** | 194.7 ± 42.3 * |
| albanol B (7) a | 35.5 ± 8.4 | 100.6 ± 43.6 * |
| Compound | Ileal Contractions | Tracheal Tone | ||
|---|---|---|---|---|
| EC50 ± SEM (µM) | Emax ± SEM (%) | EC50 ± SEM (µM) | Emax (mg ± SEM) | |
| kuwanon U (2) | 0.13 ± 0.04 | 70.5 ± 6.1 | 0.033 ± 0.05 | 247.8 ± 9.9 |
| moracin O (5) | 1.1 ± 0.43 | 85.3 ± 4.4* | 0.062 ± 0.01 | 309.5 ± 17.7 * |
| albanol B (7) | 1.3 ± 0.98 | 83.2 ± 3.9 | 0.100 ± 0.05 | 254.9 ± 19.3 |
| papaverine | 0.44 ± 0.15 | 63.6 ± 6.3 | 0.074 ± 0.03 | 233.7 ± 15.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zoofishan, Z.; Kúsz, N.; Csorba, A.; Tóth, G.; Hajagos-Tóth, J.; Kothencz, A.; Gáspár, R.; Hunyadi, A. Antispasmodic Activity of Prenylated Phenolic Compounds from the Root Bark of Morus nigra. Molecules 2019, 24, 2497. https://doi.org/10.3390/molecules24132497
Zoofishan Z, Kúsz N, Csorba A, Tóth G, Hajagos-Tóth J, Kothencz A, Gáspár R, Hunyadi A. Antispasmodic Activity of Prenylated Phenolic Compounds from the Root Bark of Morus nigra. Molecules. 2019; 24(13):2497. https://doi.org/10.3390/molecules24132497
Chicago/Turabian StyleZoofishan, Zoofishan, Norbert Kúsz, Attila Csorba, Gábor Tóth, Judit Hajagos-Tóth, Anna Kothencz, Róbert Gáspár, and Attila Hunyadi. 2019. "Antispasmodic Activity of Prenylated Phenolic Compounds from the Root Bark of Morus nigra" Molecules 24, no. 13: 2497. https://doi.org/10.3390/molecules24132497
APA StyleZoofishan, Z., Kúsz, N., Csorba, A., Tóth, G., Hajagos-Tóth, J., Kothencz, A., Gáspár, R., & Hunyadi, A. (2019). Antispasmodic Activity of Prenylated Phenolic Compounds from the Root Bark of Morus nigra. Molecules, 24(13), 2497. https://doi.org/10.3390/molecules24132497








