Essential Oil of Algerian Eryngium campestre: Chemical Variability and Evaluation of Biological Activities
Abstract
:1. Introduction
2. Results
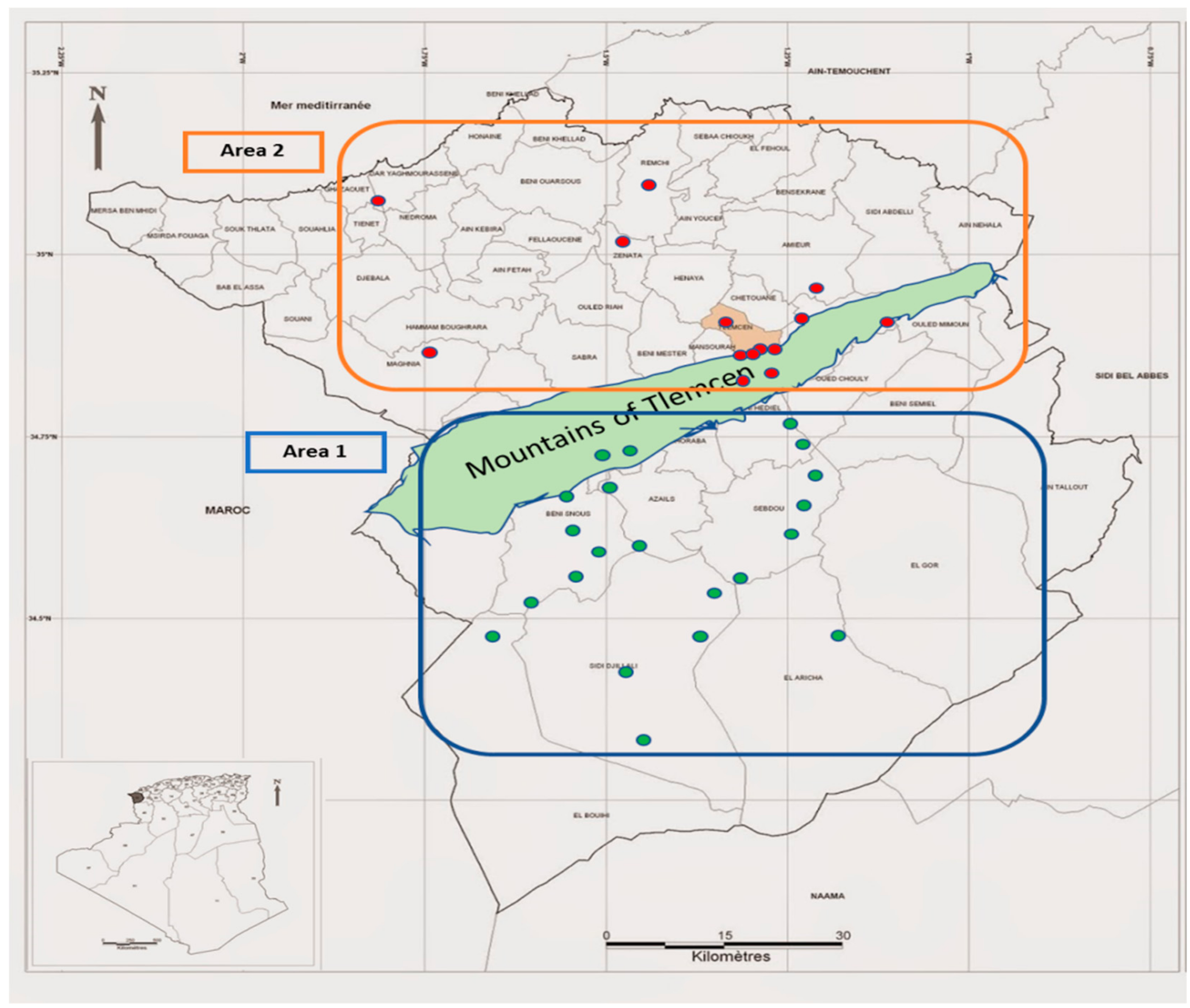
2.1. Harvest Locations
2.2. Chemical Composition of E. campestre Essential Oils
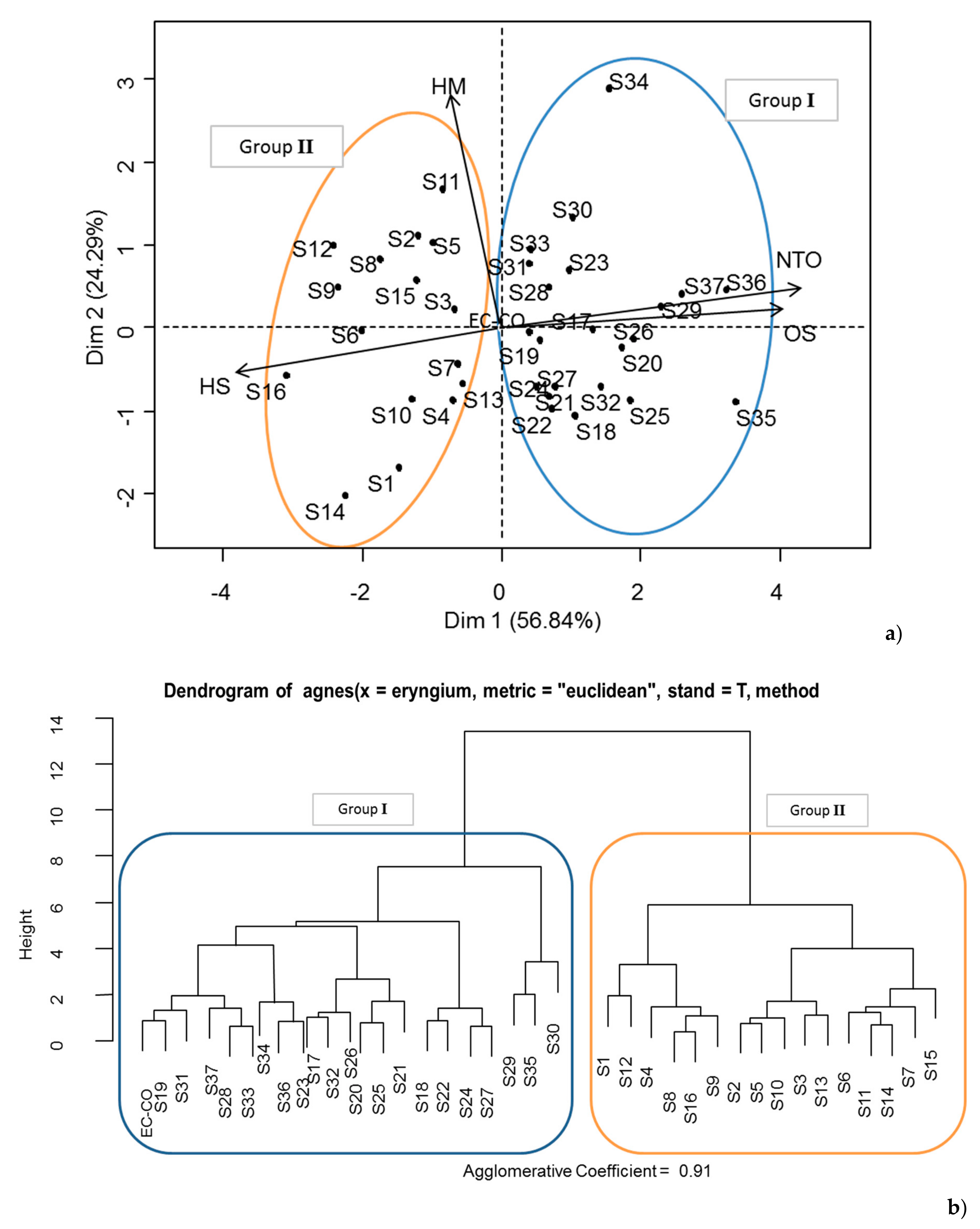
2.3. Chemical Varaibility of Algerian E. campestre Essential Oils
2.4. Growth Inhibition of Bacteria by Essential Oil
2.5. Cytotoxity and Antiprotozoal Activities of E. campestre Essential Oil
3. Materials and Methods
3.1. Plant Material
3.2. Essential-Oil Isolation
3.3. Oil Isolation
3.4. GC-FID Analysis
3.5. GC-MS Analysis
3.6. Compound Identification and Quantification
3.7. Statistical Analysis
3.8. Microbial Strains
3.9. Preparation of Inoculum
3.10. Sensitivity Tests—Disc Diffusion Method
3.11. MICs
3.12. Cytotoxity and Antiprotozoal Activities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Calvino, C.I.; Martinez, S.G.; Downie, S.R. The evolutionary history of Eryngium (Apiaceae, Saniculoideae): Rapid radiations, long distance dispersals, and hybridizations. Mol. Phylogenet. Evol. 2008, 46, 1129–1150. [Google Scholar] [CrossRef] [PubMed]
- Jaghabir, M. Hypoglycemic effects of Eryngium creticum. Arch. Pharm. Res. 1991, 14, 295–297. [Google Scholar] [CrossRef]
- Kartal, M.; Mitaine-Offer, A.-C.; Abu-Asaker, M.; Miyamoto, T.; Calis, I.; Wagner, H.; Lacaille-Dubois, M.-A. Two New Triterpene Saponins from Eryngium campestre. Chem. Amp. Pharm. Bull. 2008, 53, 1318–1320. [Google Scholar] [CrossRef] [PubMed]
- Baytop, T. Therapy with medicinal plants in Turkey (Past and Present), 2nd ed.; Nobel Tip Basimevi.
- Coste, H. Flore Descriptive et Illustrée de la France, de la Corse et des Contrées Limitrophes II; Librairie Scientifique et Technique Albert Blanchart: Le Val-Saint-Germain, France, 1980. [Google Scholar]
- Quézel, P.; Santa, S. Nouvelle Flore de l′Algérie et des Régions Désertiques Méridionales; Éditions du Centre national de la Recherche scientifique: Paris, France, 1962.
- Kartnig, T.; Wolf, J. Flavonoids from the aboveground parts of Eryngium campestre. Planta Med. 1993, 59, 285–286. [Google Scholar] [CrossRef] [PubMed]
- Thiem, B.; Goślin′ska, O.; Kikowska, M.; Budzianowski, J. Antimicrobial activity of three Eryngium, L. species (Apiaceae). Herba. Pol. 2010, 56, 52–58. [Google Scholar]
- Hawas, U.W.; El-Kassem, T.; Lamia, A.; Awad, M.; Hanem, A.A.; Taie, H. Anti-Alzheimer, antioxidant activities and flavonol glycosides of Eryngium campestre L. Curr. Chem. Biol. 2013, 7, 188–195. [Google Scholar] [CrossRef]
- Erdelmeier, C.A.J.; Sticher, O. A cyclohexenone and a cyclohexadienone glycoside from Eryngium campestre. Phytochemistry 1986, 25, 741–743. [Google Scholar] [CrossRef]
- Erdelmeier, C.A.J.; Sticher, O. Coumarin Derivatives from Eryngium campestre L. Planta Med. 1985, 51, 407–409. [Google Scholar] [CrossRef]
- Kartal, M.; Mitaine-Offer, A.-C.; Paululat, T.; Abu-Asaker, M.; Wagner, H.; Mirjolet, J.-F.; Guilbaud, N.; Lacaille-Dubois, M.-A. Triterpene Saponins from Eryngium campestre. J. Nat. Prod. 2006, 69, 1105–1108. [Google Scholar] [CrossRef]
- Fernandes, E.S.d.S. Identication of active compounds in extracts of Eryngium species from the Iberia. Ph.D. Thesis, Faculty of Pharmacy of University of Coimbra, Coimbra, Portugal, 2013. [Google Scholar]
- Cianfaglione, K.; Cianfaglione, K.; Blomme, E.E.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Dall′Acqua, S.; Maggi, F. Cytotoxic essential oils from Eryngium campestre and Eryngium amethystinum (Apiaceae) growing in central Italy. Chem. Biodivers. 2017, 14, e1700096. [Google Scholar] [CrossRef]
- Camarda, L.; Di Stefano, V.; Merikech, M. Essential oil of inflorescences of Eryngium campestre L. (Apiaceae). Riv. Ital. EPPOS 2004, 38, 25–28. [Google Scholar]
- Pala-Paul, J.; Usano-Alemany, J.; Soria, A.C.; Perez-Alonso, M.J.; Brophy, J.J. Essential oil composition of Eryngium campestre L. growing in different soil types. A preliminary study. Nat. Prod. Commun. 2008, 3, 1121–1126. [Google Scholar] [CrossRef]
- Abd-Elmonem, A.R.; Shehab, N.G. Study of the volatile oil of Eryngium campestre L. growing in Egypt. Bull. Fac. Pharm. Cairo Univ. 2006, 44, 53–57. [Google Scholar]
- Nebija, F.; Stefkov, G.; Karapandzova, M.; Stafilov, T.; Panovska, T.K.; Kulevanova, S. Chemical characterization and antioxidant activity of Eryngium campestre L., Apiaceae from Kosovo. Makedon. Farm. Bilt. 2009, 55, 22–32. [Google Scholar] [CrossRef]
- Küpeli, E.; Kartal, M.; Aslan, S.; Yesilada, E. Comparative evaluation of the anti-inflammatory and antinociceptive activity of Turkish Eryngium species. J. Ethnopharmacol. 2006, 107, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Usta, C.; Yildirim, A.B.; Turker, A.U. Antibacterial and antitumour activities of some plants grown in Turkey. Biotechnol. Biotechnol. Equip. 2014, 28, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Celik, A.; Aydınlık, N.; Arslan, I. Phytochemical constituents and inhibitory activity towards methicillin-resistant Staphylococcus aureus strains of Eryngium species (Apiaceae). Chem. Biodivers. 2011, 8, 454–459. [Google Scholar] [CrossRef]
- Medbouhi, A.; Tintaru, A.; Beaufay, C.; Naubron, J.-V.; Djabou, N.; Costa, J.; Quetin-Leclercq, J.; Muselli, A. Structural Elucidation and Cytotoxicity of a New 17-Membered Ring Lactone from Algerian Eryngium campestre. Molecules 2018, 23, 3250. [Google Scholar] [CrossRef]
- Ghennou, S. Contribution à une étude de stipa tenacissima L dans le Sud-ouest de la région de Tlemcen. Phytodynamique des écosystèmes motorrals menacés. Ph.D. Thesis, Université de Tlemcen, Tlemcen, Algeria, 2014. [Google Scholar]
- Billem, A. Contribution à l′étude histologique du Chamaerops humilis L.: Approche comparative des peuplements des Monts de Traras et des Monts de Tlemcen. Ph.D. Thesis, Université Es-senia Oran, Oran, Algeria, 2012. [Google Scholar]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Picaud, S.; Olsson, M.E.; Brodelius, M.; Brodelius, P.E. Cloning, expression, purification and characterization of recombinant (+)-germacrene D synthase from Zingiber officinale. Arch. Biochem. Biophys. 2006, 452, 17–28. [Google Scholar] [CrossRef]
- Şahin, F.; Güllüce, M.; Daferera, D.; Sökmen, A.; Sökmen, M.; Polissiou, M.; Agar, G.; Özer, H. Biological activities of the essential oils and methanol extract of Origanum vulgare ssp. vulgare in the Eastern Anatolia region of Turkey. Food Control. 2004, 15, 549–557. [Google Scholar] [CrossRef]
- Dorman, H.J.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Kpadonou, B.G.; Kpoviessi, S.D.; Yayi, L.E.; Gbaguidi, F.; Frédérich, M.; Moudachirou, M.; Quetin-Leclercq, J.; Accrombessi, G.C.; Bero, J. In vitro antitrypanosomal and antiplasmodial activities of crude extracts and essential oils of Ocimum gratissimum Linn from Benin and influence of vegetative stage. J. Ethnopharmacol. 2014, 155, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Council of Europe. European Pharmacopoeia; Council of Europe: Strasbourg, France, 1997. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- König, W.A.; Joulain, D.; Hochmuth, D.H. Terpenoids and Related Constituents of Essential Oils, Library of Mass Finder 2.1; Institute of Organic Chemistry, University of Hamburg: Hamburg, Germany, 2011. [Google Scholar]
- NIST. PC Version 1.7 of the NIST/EPA/NIH Mass Spectral Library; National Institute of Standards and Technology (NIST): Gaithersburg, MD, USA, 1999.
- Andreani, S.; Barboni, T.; Desjobert, J.M.; Paolini, J.; Costa, J.; Muselli, A. Essential oil composition and chemical variability of Xanthium italicum Moretti from Corsica. Flavour. Fragr. J. 2012, 27, 227–236. [Google Scholar] [CrossRef]
- Breneton, R. Chemometrics and the Analytical Process. Data Handl. Sci. Technol. 2003, 2, 5–11. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-First Informational Supplement; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2011.
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Irith Wiegand, K.H. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Hoet, S.; Stévigny, C.; Block, S.; Opperdoes, F.; Colson, P.; Baldeyrou, B.; Lansiaux, A.; Bailly, C.; Quetin-Leclercq, J. Alkaloids from Cassytha filiformis and related aporphines: Antitrypanosomal activity, cytotoxicity, and interaction with DNA and topoisomerases. Planta Med. 2004, 70, 407–413. [Google Scholar]
- Le, T.B.; Beaufay, C.; Nghiem, D.T.; Mingeot-Leclercq, M.-P.; Quetin-Leclercq, J. In Vitro Anti-Leishmanial Activity of Essential Oils Extracted from Vietnamese Plants. Molecules 2017, 22, 1071. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds S1–S37 are available from the authors. |
| Origins | Plant Organs | Main Components | % | Ref. |
|---|---|---|---|---|
| Portugal | Aerial parts | (E)-β-Farnesene β-Bisabolene | 31.31 7.8 | [13] |
| Germacrene D Germacrene A β-Elemene | 18.4 9.2 19.7 | |||
| β-Bisabolene Germacrene D α-Cadinol | 15.6 12.3 8.2 | |||
| Italy | Aerial parts | Germacrene D Allo-Aromadendrene Spathulenol Ledol | 13.8 7.7 7.0 5.7 | [14] |
| Italy | Inflorescences | Germacrene D Apiol α-Himachalene | 49.5 19.5 15.1 | [15] |
| Spain | Inflorescences | Germacrene D β-Curcumene Myrcene (E)-β-Farnesene | 30.3–40.3 0.7–22.2 3–21.7 0.1–19 | [16] |
| Egypt | Aerial parts | γ-Cadinen-15-al Spathulenol Octanoic acid α-Curcumene | 23.3 10.7 9.8 8.6 | [17] |
| N° | Sample Location of Harvest | GPS | Alt | Voucher Codes | Area | |
|---|---|---|---|---|---|---|
| Latitude | Longitude | |||||
| S1 | Lalla setti | 34°51′52.42″ N | 1°18′18.09″ O | 996 | EC01 | Area 1: high steppe plains/Limestone acumulation soils/Low organic matter/Law water resources |
| S2 | 34°51′52.68″ N | 1°18′20.72″ O | 996 | EC02 | ||
| S3 | 34°51′52.35″ N | 1°18′20.72″ O | 997 | EC03 | ||
| S4 | 34°51′38.24″ N | 1°19′4.80″ O | 1037 | EC04 | ||
| S5 | Bouhanak | 34°52′38.75″ N | 1°21′33.88″ O | 774 | EC28 | |
| S6 | Mafrouch | 34°50′55.59″ N | 1°17′53.21″ O | 1148 | EC29 | |
| S7 | 34°49′45.37″ N | 1°18′44.75″ O | 1133 | EC30 | ||
| S8 | Zenata | 34°56′51.27″ N | 1°26′58.89″ O | 300 | EC33 | |
| S9 | Remchi | 35°03′00″ N | 1° 26′ 00″ O | 82 | EC34 | |
| S10 | Maghniya | 34° 51′ 42″ N | 1° 43′ 50″ O | 455 | EC36 | |
| S11 | Chlaida | 34°57′8.14″ N | 1°13′28.33″ O | 609 | EC37 | |
| S12 | Tirni | 34°49′1.90″ N | 1°19′36.26″ O | 1159 | EC05 | |
| S13 | 34°47′8.00″ N | 1°20′37.88″ O | 1213 | EC06 | ||
| S14 | Bni aad | 35° 2′32.70″ N | 1°40′9.43″ O | 228 | EC35 | |
| S15 | Ain lekbira | 35° 2′32.70″ N | 1°40′9.43″ O | 228 | EC31 | |
| S16 | Mdig | 34°58′6.66″ N | 1°15′48.65″ O | 497 | EC32 | |
| S17 | Sebdou | 34°40′55.15″ N | 1°18′47.37″ O | 1013 | EC07 | Area 2: Mountains of Traras and palins of Tlemcen/calcareous humiferous soils/Rich in organic matter/Rich in water resources |
| S18 | 34°39′43.52″ N | 1°19′21.42″ O | 920 | EC08 | ||
| S19 | 34°38′15.18″ N | 1°20′9.95″ O | 938 | EC09 | ||
| S20 | Abed | 34°28′42.76″ N | 1°40′5.82″ O | 1446 | EC18 | |
| S21 | Bni behdel | 34°41′10.07″ N | 1°30′16.82″ O | 780 | EC10 | |
| S22 | 34°42′38.22″ N | 1°27′12.97″ O | 776 | EC11 | ||
| S23 | Sidi bounoir | 34°42′26.39″ N | 1°19′9.80″ O | 1110 | EC12 | |
| S24 | 34°40′55.15″ N | 1°18′47.37″ O | 1030 | EC13 | ||
| S25 | Sebdou/Sid djillali | 34°36′0.53″ N | 1°23′35.57″ O | 997 | EC14 | |
| S26 | 34°34′26.89″ N | 1°26′6.66″ O | 1005 | EC15 | ||
| S27 | Abed/Lekhmis | 34°37′5.86″ N | 1°35′34.98″ O | 867 | EC20 | |
| S28 | 34°34′40.59″ N | 1°36′4.15″ O | 980 | EC21 | ||
| S29 | 34°33′28.61″ N | 1°37′21.11″ O | 1123 | EC22 | ||
| S30 | 34°32′22.19″ N | 1°38′34.81″ O | 1294 | EC23 | ||
| S31 | 34°31′18.90″ N | 1°40′55.59″ O | 1360 | EC24 | ||
| S32 | 34°29′19.73″ N | 1°40′55.63″ O | 1253 | EC25 | ||
| S33 | Boughadou | 34°30′49.71″ N | 1°30′20.98″ O | 1336 | EC19 | |
| S34 | Sid djilali | 34°26′11.75″ N | 1°34′31.83″ O | 1245 | EC16 | |
| S35 | 34°23′27.94″ N | 1°36′23.71″ O | 1163 | EC17 | ||
| S36 | Laouadj | 34°29′3.67″ N | 1°16′18.01″ O | 1009 | EC27 | |
| S37 | Lekhmis | 34°38′20.61″ N | 1°33′45.40″ O | 840 | EC26 | |
| No a | Components | lRIab | RIa c | RIp d | EC-CO e | Seasonal Variation f | Plant Organs g | Identification h | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 04 May | 24 May | 15 June | 05 July | Roots | Stems | Leaves | Flowers | |||||||
| 1 | β-Pinene | 970 | 972 | 1110 | 0.1 | 0.1 | 0.1 | 0.1 | 0.3 | tr | tr | tr | tr | RI, MS |
| 2 | Myrcene | 979 | 982 | 1153 | 2.7 | 0.3 | 2.4 | 4.6 | 8.4 | 3.2 | 1.9 | 1.4 | 4.7 | RI, MS |
| 3 | p-Cymene | 1011 | 1013 | 1258 | tr | tr | tr | tr | tr | tr | tr | tr | tr | RI, MS |
| 4 | Limonene | 1020 | 1022 | 1199 | 0.1 | 0.1 | 0.1 | 0.1 | tr | tr | tr | tr | 0.2 | RI, MS |
| 5 | (Z)-β-Ocimene | 1024 | 1026 | 1230 | 0.1 | 0.1 | tr | tr | tr | 0.1 | tr | tr | tr | RI, MS |
| 6 | δ-Terpinene | 1047 | 1049 | 1243 | tr | tr | 0.3 | 0.2 | 0.1 | 0.2 | tr | tr | tr | RI, MS |
| 7 | Nonan-2-one | 1070 | 1076 | 1388 | tr | 0.2 | tr | 0.3 | 0.2 | tr | tr | tr | tr | RI, MS |
| 8 | Terpinolene | 1078 | 1079 | 1280 | tr | tr | 0.2 | 0.1 | 0.2 | tr | tr | tr | tr | RI, MS |
| 9 | Nonanal | 1083 | 1082 | 1394 | 0.1 | 0.1 | 0.1 | tr | 0.1 | 0.1 | tr | tr | 0.1 | RI, MS |
| 10 | Decanal | 1185 | 1184 | 1498 | 0.3 | 0.3 | 0.2 | tr | 0.2 | 0.2 | tr | 0.2 | tr | RI, MS |
| 11 | (E)-2-Decenal | 1248 | 1247 | 1652 | 0.4 | 0.4 | 0.3 | 0.1 | 0.3 | 0.1 | tr | tr | 0.2 | RI, MS |
| 12 | (E)-2-Undecanal | 1343 | 1348 | 1726 | tr | tr | tr | tr | 0.1 | tr | tr | tr | tr | RI, MS |
| 13 | α-Copaene | 1379 | 1375 | 1438 | 0.9 | 0.9 | 0.6 | 0.2 | 1.1 | 0.3 | 0.6 | 0.4 | tr | RI, MS |
| 14 | β-Bourbonene | 1385 | 1383 | 1515 | 0.1 | tr | 0.2 | tr | 0.1 | tr | 0.2 | 0.1 | 0.6 | RI, MS |
| 15 | β-Elemene | 1388 | 1387 | 1589 | 3.0 | 0.4 | 3.7 | 4 | 1.5 | 0.4 | 0.9 | 2.5 | 0.1 | RI, MS |
| 16 | β-Ylangene | 1420 | 1417 | 1562 | 0.6 | 0.1 | 0.7 | 2.1 | 0.8 | 0.5 | 0.7 | 0.7 | tr | RI, MS |
| 17 | (E)-β-Caryophyllene | 1424 | 1426 | 1591 | tr | 0.1 | tr | tr | 0.3 | tr | tr | 0.3 | 0.1 | RI, MS |
| 18 | δ-Elemene | 1431 | 1432 | 1581 | 1.2 | 0.2 | 1.4 | 0.8 | 0.7 | tr | 0.7 | tr | 0.1 | RI, MS |
| 19 | trans-α-Bergamotene | 1432 | 1433 | 1575 | 1.2 | tr | 0.2 | 0.4 | 0.2 | tr | tr | tr | tr | RI, MS |
| 20 | (E)-β-Farnesene | 1448 | 1449 | 1661 | 0.4 | 0.9 | 2.1 | 5.1 | 8.2 | 26.0 | 9.8 | 1.4 | 6.2 | RI, MS |
| 21 | Alloaromadendrene | 1451 | 1454 | 1631 | 0.2 | 0.1 | 0.2 | tr | 0.2 | tr | tr | tr | tr | RI, MS |
| 22 | α-Humulene | 1456 | 1457 | 1665 | 0.3 | 0.1 | tr | tr | 0.1 | tr | 0.2 | 0.4 | tr | RI, MS |
| 23 | 4,5-di-epi-Aristolochene | 1467 | 1465 | 1665 | 0.3 | 1 | 0.3 | tr | tr | 0.1 | tr | tr | tr | RI, MS |
| 24 | δ-Muurolene | 1467 | 1469 | 1683 | 0.9 | 0.1 | tr | 1 | tr | tr | tr | tr | tr | RI, MS |
| 25 | α-Curcumene | 1470 | 1471 | 1742 | 1.3 | 0.3 | 0.9 | tr | 0.5 | tr | 0.8 | 0.4 | 0.1 | RI, MS |
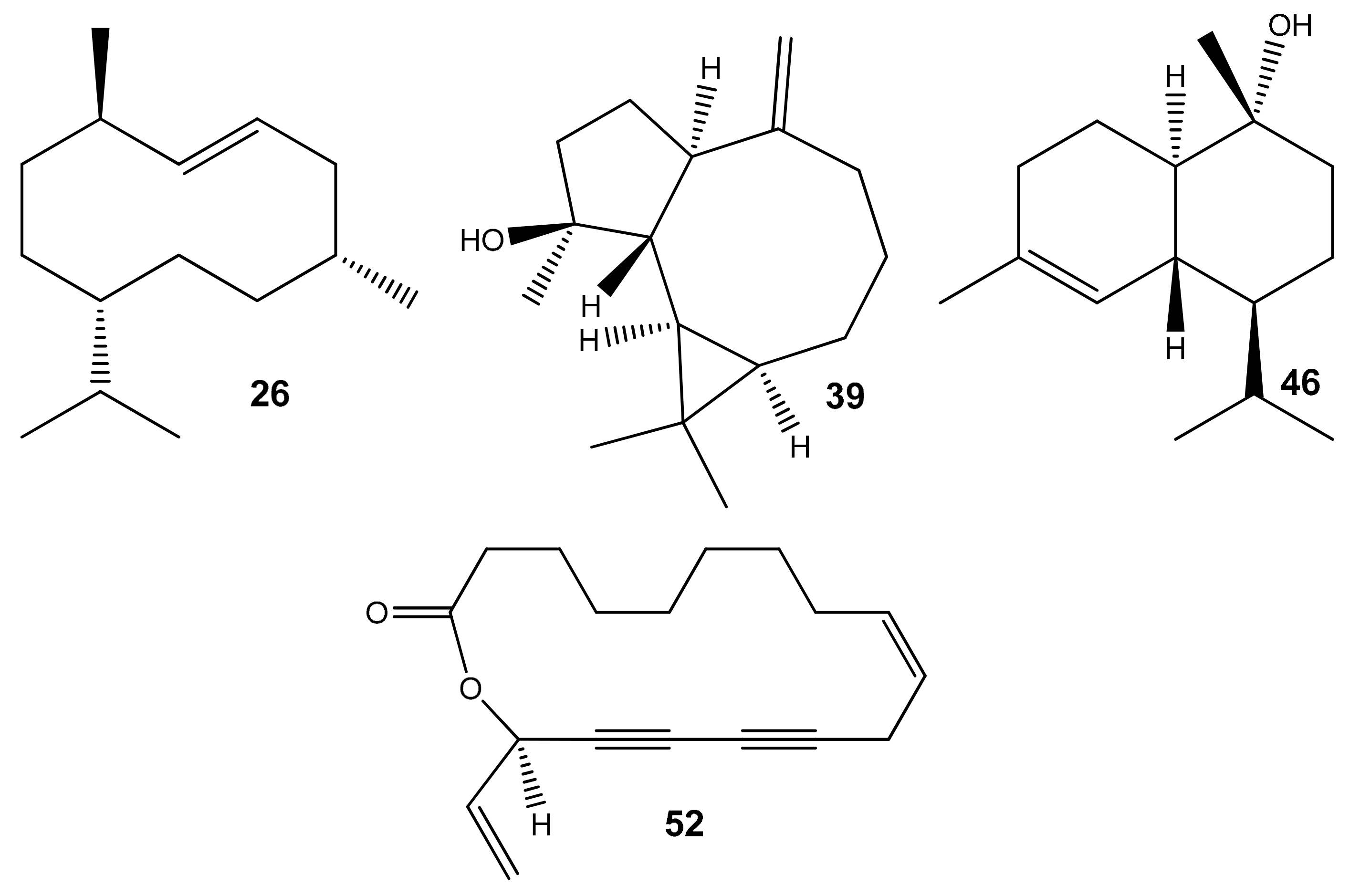
| 26 | Germacrene D | 1476 | 1480 | 1704 | 15.2 | 11.9 | 19.5 | 28.9 | 30.4 | 29.1 | 38.8 | 33.7 | 37.0 | RI, MS |
| 27 | β-Selinene | 1483 | 1484 | 1712 | 0.9 | 0.8 | 0.4 | 0.3 | 0.4 | tr | 0.2 | 0.1 | 0.1 | RI, MS |
| 28 | α-Muurolene | 1496 | 1503 | 1720 | 0.8 | 0.3 | 0.5 | 0.8 | 1.9 | 2 | 1.8 | 1.8 | 0.2 | RI, MS |
| 29 | β-Bisabolene | 1500 | 1500 | 1720 | 0.2 | 0.2 | 0.2 | 0.4 | 1.4 | 0.8 | 0.3 | tr | 0.2 | RI, MS |
| 30 | Sesquicineole | 1505 | 1506 | 1737 | 0.5 | 0.4 | 0.1 | 0.2 | 0.2 | 1.1 | 0.2 | 0.5 | 0.1 | RI, MS |
| 31 | δ-cadinene | 1516 | 1513 | 1752 | 0.3 | 0.1 | tr | tr | 0.2 | tr | tr | tr | tr | RI, MS |
| 32 | β-curcumene | 1509 | 1510 | 1733 | 0.2 | 0.5 | 0.1 | 0.9 | 0.3 | 0.2 | tr | 0.3 | tr | RI, MS |
| 33 | δ-cadinene | 1516 | 1514 | 1752 | 1.5 | 0.2 | tr | 0.3 | 1.5 | tr | 0.2 | 0.3 | tr | RI, MS |
| 34 | (E)-α-bisabolene | 1532 | 1531 | 1753 | 0.5 | 0.7 | 1.5 | tr | tr | 0.6 | 0.8 | 0.9 | 0.5 | RI, MS |
| 35 | β-Elemol | 1535 | 1534 | 2072 | 0.8 | tr | tr | 0.3 | 0.1 | tr | 0.2 | tr | tr | RI, MS |
| 36 | 7-epi-trans-Sesquisabinene hydrate | 1543 | 1547 | 1991 | 1.0 | 0.2 | 2.5 | 3.3 | tr | 0.3 | 0.1 | 0.2 | tr | RI, MS |
| 37 | Salvial-4(14)-ene-1,5-epoxide | 1545 | 1548 | 1941 | 2.1 | 0.9 | tr | 0.2 | 0.2 | 0.6 | tr | 0.8 | 0.6 | RI, MS |
| 38 | Germacrene B | 1553 | 1551 | 1827 | 3.1 | 0.8 | 0.3 | 0.2 | 0.4 | 0.3 | 0.5 | 0.2 | 0.1 | RI, MS |
| 39 | Spathulenol | 1563 | 1562 | 2103 | 4.8 | 0.9 | 0.7 | 9.6 | 6.3 | 0.6 | 2.3 | 0.5 | 1.4 | RI, MS |
| 40 | Caryophyllene oxide | 1576 | 1570 | 1980 | 0.2 | 3.1 | 1.1 | 0.8 | 2.6 | 0.2 | 0.3 | 0.7 | 1.2 | RI, MS |
| 41 | Salvial-4(14)-en-1-one | 1583 | 1577 | 2005 | 1.8 | 1.7 | 0.4 | 0.5 | 1 | 0.8 | 1.9 | 4.2 | 4.5 | RI, MS |
| 42 | Ledol | 1600 | 1602 | 2030 | 1.6 | 0.8 | 0.4 | 0.4 | 0.5 | 0.2 | 0.4 | 0.5 | 0.6 | RI, MS |
| 43 | 1,10-di-epi-Cubenol | 1610 | 1611 | 2054 | 1.4 | tr | 1.8 | 2.2 | 0.1 | 0.5 | 0.2 | 1.4 | 0.3 | RI, MS |
| 44 | Caryophylla-4(14),8(15)-dien-5-α-ol | 1626 | 1624 | 2285 | 0.3 | 0.2 | 2.3 | 2 | 0.5 | 0.3 | tr | 0.5 | 1.9 | RI, MS |
| 45 | τ-Cadinol | 1632 | 1638 | 2169 | 2.3 | 0.3 | 1.1 | 1.6 | 1 | 0.2 | 1.6 | 0.2 | 0.4 | RI, MS |
| 46 | α-Cadinol | 1645 | 1645 | 2231 | 5.5 | tr | tr | tr | 1.2 | 1.7 | 1.4 | 1.2 | 1.3 | RI, MS |
| 47 | Eudesma-4(15)-7-dien-1-β-ol | 1663 | 1672 | 2199 | 3.0 | tr | 0.5 | 0.2 | 0.3 | 0.3 | 0.5 | 0.6 | 1.1 | RI, MS |
| 48 | α-Bisabolol | 1681 | 1667 | 2333 | 0.6 | 1.7 | 1.1 | 1.8 | 1 | 2.7 | 1.8 | 3.9 | 8 | RI, MS |
| 49 | 14-Hydroxy-α-muurolene | 1755 | 1759 | 2599 | 0.5 | 1.6 | 0.4 | 0.5 | 0.3 | 0.5 | 0.5 | 0.5 | 0.9 | RI, MS |
| 50 | 14-Hydroxy-δ-cadinene | 1788 | 1784 | 2607 | 1.0 | 0.2 | 0.1 | 0.6 | 0.4 | 0.6 | 0.4 | 0.6 | 0.3 | RI, MS |
| 51 | Hexadecanoic acid | 1942 | 1941 | 2930 | 1.2 | 0.2 | 0.5 | 0.4 | 0.1 | 0.8 | 0.1 | 0.9 | 0.4 | RI, MS |
| 52 | Campestrolide | 2142 | 2143 | 2970 | 10.3 | 50.9 | 32.1 | 16.3 | 8.1 | 9.5 | 14.6 | 16.3 | 4.5 | RI, MS |
| Total Identification | 73.1 | 84.4 | 81.6 | 91.8 | 84 | 85.1 | 84.9 | 78.6 | 78.0 | |||||
| Yields | 0.22 | 0.19 | 0.17 | 0.16 | 0.14 | 0.20 | 0.33 | 0.17 | ||||||
| Hydrocarbon compounds | 33.4 | 20.3 | 35.9 | 50.5 | 59.2 | 63.8 | 58.4 | 44.9 | 50.2 | |||||
| Oxygenated compounds | 39.7 | 64.1 | 45.7 | 41.3 | 24.8 | 21.3 | 26.5 | 33.7 | 27.8 | |||||
| Sesquiterpene compounds | 60.5 | 31.7 | 45.3 | 69.6 | 65.9 | 70.9 | 68.3 | 59.8 | 67.9 | |||||
| Oxygenated sesquiterpenes | 27.4 | 12 | 12.5 | 24.2 | 15.7 | 10.6 | 11.8 | 16.3 | 22.6 | |||||
| Hydrocarbon sesquiterpenes | 33.1 | 19.7 | 32.8 | 45.4 | 50.2 | 60.3 | 56.5 | 43.5 | 45.3 | |||||
| Monoterpene compounds | 0.3 | 0.6 | 3.1 | 5.1 | 9.0 | 3.5 | 1.9 | 1.4 | 4.9 | |||||
| Non terpenic compounds | 12.3 | 52.1 | 33.2 | 17.1 | 9.1 | 10.7 | 14.7 | 17.4 | 5.2 | |||||
| Diameters (mm) | MIC a (μg/mL) | |||||
|---|---|---|---|---|---|---|
| Strains | EC-CO | ATB b: GENT | ATB b: AmB | EC-CO | ATB b: GENT | ATB b: AmB |
| Yeasts | ||||||
| Candida albicans ATCC 10231 | 6 | ND | 22 | - | ND | 1 |
| Candida albicans IP 444 | 6 | ND | 20 | - | ND | 1 |
| Gram + | ||||||
| Bacillus subtilis ATCC 6633 | 6 | 20 | ND | - | 4 | ND |
| Bacillus cereus ATCC 11778 | 23 | 19 | ND | 250 | 4 | ND |
| Staphylococcus aureus ATCC 25923 | 35 | 21 | ND | 125 | 2 | ND |
| Staphylococcus aureus ATCC 33862 | 21 | 20 | ND | 250 | 2 | ND |
| Staphylococcus aureus ATCC 29213 | 20 | 20 | ND | 250 | 2 | ND |
| Enterococcus faecalis ATCC 29212 | 35 | 10 | ND | 125 | 2 | ND |
| Listeria monocytogenes ATCC 19115 | 6 | 19 | ND | - | 2 | ND |
| Gram − | ||||||
| Pseudomonas aeruginosa ATCC 27853 | 6 | 11 | ND | - | 4 | ND |
| Pseudomonas fluorescens ATCC 13525 | 6 | 11 | ND | - | 4 | ND |
| Salmonella enteritidis ATCC 2453 | 6 | 20 | ND | - | 4 | ND |
| Escherichia coli ATCC 25922 | 6 | 15 | ND | - | 4 | ND |
| Klebsiella pneumoniae ATCC 70603 | 6 | 11 | ND | - | 4 | ND |
| Cytotoxicity | Antiparasitic Activity | Selectivity Index | ||||
|---|---|---|---|---|---|---|
| IC50 ± SD in µg/mL (µM for Pure Compound) | IC50 WI38/IC50 Parasite | |||||
| WI38 | J774 | Tbb | Lmm | Tbb | Lmm | |
| EC-CO | >25 | 20.09 ± 2.00 | 0.86 ± 0.15 | >25 | >28.9 | ND |
| S31 | 9.79 ± 1.95 | 8.29 ± 1.79 | 1.87 ± 0.73 | 7.40 ± 0.28 | 5.2 | 1.3 |
| EC1 | 5.95 ± 0.87 | 6.70 ± 1.65 | 1.89 ± 0.69 | 3.90 ± 0.41 | 3.1 | 1.5 |
| ECC1 | 24.74 ±0.05 | 12.23 ± 1.42 | 0.57 ± 0.06 | 14.74 ± 1.11 | 43.7 | 1.7 |
| campestrolide | 5.20 ± 0.24 | 4.84 ± 0.10 | 0.59 ± 0.08 | 3.43 ± 0.02 | 8.9 | 1.5 |
| (19.24 ± 0.87) | (17.89 ± 0.35) | (2.17 ± 0.28) | (12.67 ± 0.09) | |||
| Positive control | 0.036 ± 0.022 (0.103 ± 0.062) a | 0.007 ± 0.005 (0.021 ± 0.013) a | 0.031 ± 0.012 (0.022 ± 0.008) b | 0.057 ± 0.008 (0.097 ± 0.014) c | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medbouhi, A.; Benbelaïd, F.; Djabou, N.; Beaufay, C.; Bendahou, M.; Quetin-Leclercq, J.; Tintaru, A.; Costa, J.; Muselli, A. Essential Oil of Algerian Eryngium campestre: Chemical Variability and Evaluation of Biological Activities. Molecules 2019, 24, 2575. https://doi.org/10.3390/molecules24142575
Medbouhi A, Benbelaïd F, Djabou N, Beaufay C, Bendahou M, Quetin-Leclercq J, Tintaru A, Costa J, Muselli A. Essential Oil of Algerian Eryngium campestre: Chemical Variability and Evaluation of Biological Activities. Molecules. 2019; 24(14):2575. https://doi.org/10.3390/molecules24142575
Chicago/Turabian StyleMedbouhi, Ali, Fethi Benbelaïd, Nassim Djabou, Claire Beaufay, Mourad Bendahou, Joëlle Quetin-Leclercq, Aura Tintaru, Jean Costa, and Alain Muselli. 2019. "Essential Oil of Algerian Eryngium campestre: Chemical Variability and Evaluation of Biological Activities" Molecules 24, no. 14: 2575. https://doi.org/10.3390/molecules24142575
APA StyleMedbouhi, A., Benbelaïd, F., Djabou, N., Beaufay, C., Bendahou, M., Quetin-Leclercq, J., Tintaru, A., Costa, J., & Muselli, A. (2019). Essential Oil of Algerian Eryngium campestre: Chemical Variability and Evaluation of Biological Activities. Molecules, 24(14), 2575. https://doi.org/10.3390/molecules24142575