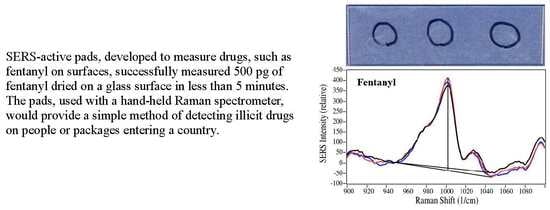
Quantitative Measurements of Codeine and Fentanyl on a Surface-Enhanced Raman-Active Pad
Abstract
:1. Introduction
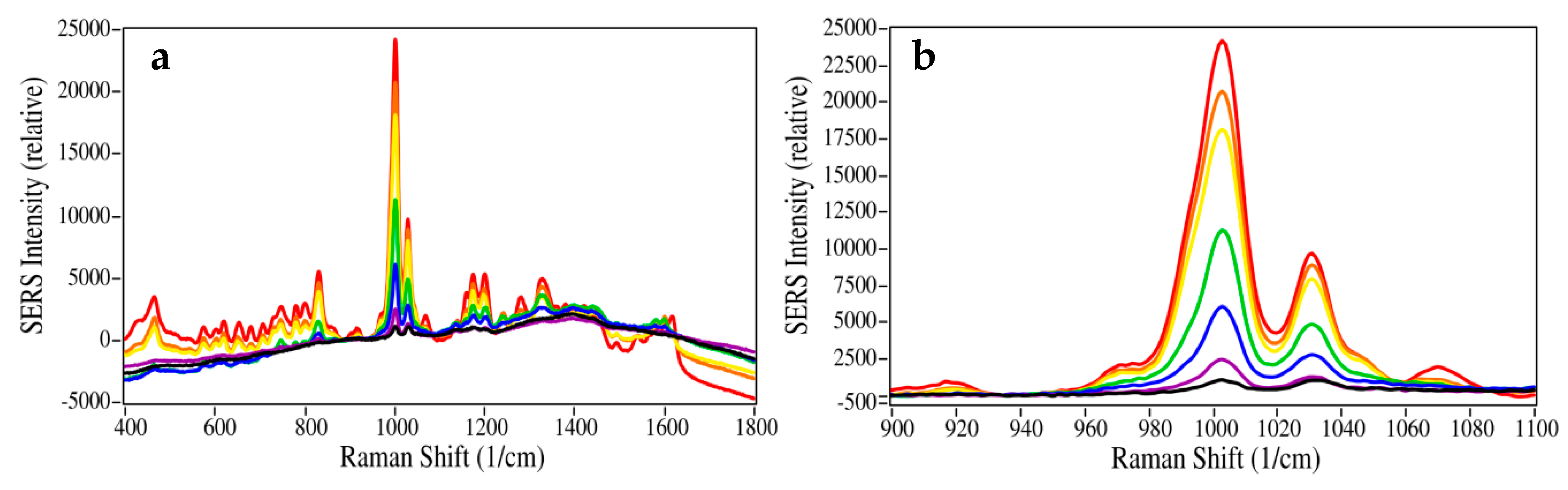
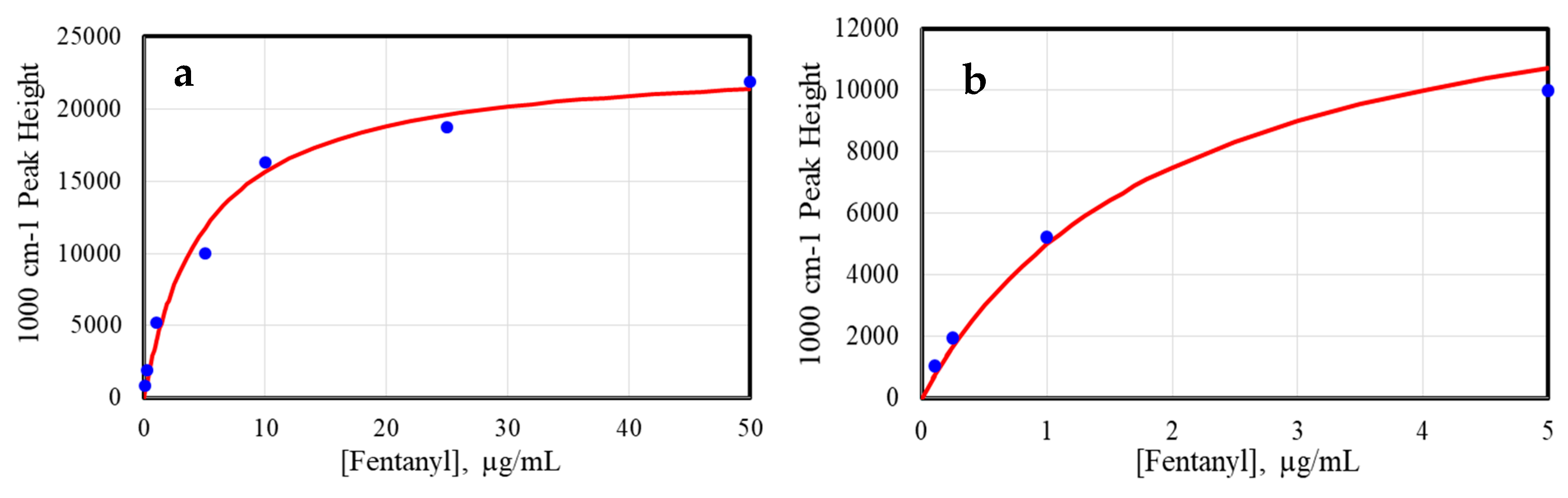
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- NIH National Institute on Drug Abuse, Overdose Death Rates. Available online: www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates (accessed on 18 January 2019).
- Jones, C.M.; Einstein, E.B.; Compton, W.M. Changes in synthetic opioid involvement in drug overdose deaths in the United States, 2010–2016. JAMA 2018, 319, 1819–1821. [Google Scholar] [CrossRef] [PubMed]
- Int Narc Control Board, United Nations, Narcotic Drugs, Statistics 2013. Available online: https://www.incb.org/documents/Publications/AnnualReports/AR2014/English/AR_2014.pdf (accessed on 18 January 2019).
- Gorodeizky, C.W.; Martin, W.R. A comparison of fentanyl, droperidol, and morphine. Clin. Pharma Thera. 1965, 7, 731–739. [Google Scholar] [CrossRef] [PubMed]
- U.S. Customs and Border Protection, CBP Officers Seize Largest Amount of Fentanyl in CBP History. Available online: www.cbp.gov/newsroom/local-media-release/cbp-officers-seize-largest-amount-fentanyl-cbp-history (accessed on 15 March 2019).
- Moshtaghian, A.; Chavez, N. Record Fentanyl Bust in Nebraska Finds Enough Doses to Kill an Estimated 26 Million People. CNN. 25 May 2018. Available online: https://www.cnn.com/2018/05/25/health/nebraska-fentanyl-bust/index.html (accessed on 18 January 2019).
- Farquharson, S.; Grigely, L.; Khitrov, V.; Smith, W.W.; Sperry, J.F.; Fenerty, G. Detecting Bacillus cereus spores on a mail sorting system using Raman Spectroscopy. J. Raman Spectrosc. 2004, 35, 82–86. [Google Scholar] [CrossRef]
- Santillán, J.D.; Brown, C.D.; Jalenak, W. Advances in Raman spectroscopy for explosive identification in aviation security. Proc. SPIE 2007, 6540, 1–8. [Google Scholar]
- Donahue, M.; Huang, H.; Brouillette, C.; Smith, W.; Farquharson, S. Detecting explosives by portable Raman analyzers: A comparison of 785, 976, 1064, and 1550 nm (Retina-Safe) laser excitation. Spectroscopy 2011, 26, 24–28. [Google Scholar]
- Bell, S.E.J.; Beattie, J.R.; McGarvey, J.J.; Peters, K.L.; Sirimuthu, N.M.S.; Speers, S.J. Development of sampling methods for Raman analysis of solid dosage forms of therapeutic and illicit drugs. J. Raman Spectrosc. 2004, 35, 409–417. [Google Scholar] [CrossRef]
- De Veij, M.; Deneckere, A.; Vandenabeele, P.; de Kaste, D.; Moens, L. Detection of counterfeit Viagra with Raman spectroscopy. J. Pharm. Biomed. Anal. 2008, 46, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, M.D.; Page, K.; Munshi, T.; Tomsett, R.; Lynch, G.; Edwards, H.G.M. Analysis of seized drugs using portable Raman spectroscopy in an airport environment—a proof of principle study. J. Raman Spectrosc. 2008, 39, 873–880. [Google Scholar] [CrossRef]
- West, M.J.; Went, M.J. The spectroscopic detectionof drugs of abuse in fingerprints after development with powders and recovery with adhesive lifters. Spectrochim. Acta A 2009, 71, 1984–1988. [Google Scholar] [CrossRef]
- Jeanmaire, D.L.; van Duyne, R.P. Surface Raman spectroelectrochemistry. J. Electroanal. Chem. 1977, 84, 1–20. [Google Scholar] [CrossRef]
- Weaver, M.J.; Farquharson, S.; Tadayyoni, M.A. Surface-enhancement factors for Raman scattering at silver electrodes. Role of adsorbate-surface interactions and electrode structure. J. Chem. Phys. 1985, 82, 4867–4874. [Google Scholar] [CrossRef]
- Angel, S.M.; Carter, J.C.; Stratis, D.N.; Marquardt, B.J.; Brewer, W.E. Some new uses for filtered fiber-optic Raman probes: In situ drug identification and in situ and remote Raman imaging. J. Raman Spectrosc. 1999, 30, 795–805. [Google Scholar] [CrossRef]
- Farquharson, S.; Lee, Y.H. Trace drug analysis by surface-enhanced Raman spectroscopy. Proc. SPIE 2000, 4200, 89–95. [Google Scholar]
- Bell, S.E.; Burns, D.T.; Dennis, A.C.; Speers, J.S. Rapid analysis of ecstasy and related phenethylamines in seized tablets by Raman spectroscopy. Analyst 2000, 125, 541–544. [Google Scholar] [CrossRef]
- Sagmuller, B.; Schwarze, B.; Brehm, G.; Schneider, S. Application of SERS spectroscopy to the identification of (3,4-methylenedioxy)amphetamine in forensic samples utilizing matrix stabilized silver halides. Analyst 2001, 126, 2066–2071. [Google Scholar] [CrossRef] [PubMed]
- Faulds, K.; Smith, W.E.; Graham, D.; Lacey, R.J. Assessment of silver and gold substrates for the detection of amphetamine sulfate by surface enhanced Raman scattering (SERS). Analyst 2002, 127, 282. [Google Scholar] [CrossRef]
- Farquharson, S.; Shende, C.; Sengupta, A.; Huang, H.; Inscore, F. Rapid detection and identification of overdose drugs in saliva by surface-enhanced Raman scattering using fused gold colloids. Pharmaceutics 2011, 3, 425–439. [Google Scholar] [CrossRef] [PubMed]
- Mabbott, S.; Correa, E.; Cowcher, D.P.; Allwood, J.W.; Goodacre, R. Optimization of Parameters for the Quantitative Surface-Enhanced Raman Scattering Detection of Mephedrone Using a Fractional Factorial Design and a Portable Raman Spectrometer. Anal. Chem. 2013, 85, 923–931. [Google Scholar] [CrossRef]
- Lanzarotta, A.; Lorenz, L.; JaCinta, S.; Flurer, C. Development and implementation of a Pass/Fail field-friendly method for detecting sildenafil in suspect pharmaceutical tablets using a handheld Raman spectrometer and silver colloids. J. Pharma. Biomedl. Anal. 2017, 146, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Haddad, A.; Comanescu, M.A.; Green, O.; Kubic, T.A.; Lombardi, J.R. Detection and Quantitation of Trace Fentanyl in Heroin by Surface- Enhanced Raman Spectroscopy. Anal. Chem. 2018, 90, 12678–12685. [Google Scholar] [CrossRef] [PubMed]
- Rana, V.; Canamares, M.V.; Kubic, T.; Leona, M.; Lombardi, J.R. Surface-enhanced Raman spectroscopy for trace identification of controlled substances: Morphine, codeine, and hydrocodone. J. Forensic. Sci. 2010, 56, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Leonard, J.; Haddad, A.; Green, O.; Birke, R.L.; Kubic, T.; Kocak, A.; Lombardi, J.R. SERS, Raman, and DFT analyses of fentanyl and carfentanil: Toward detection of trace samples. J. Raman Spectrosc. 2017, 48, 1323–1329. [Google Scholar] [CrossRef]
- Langmuir, I. The Adsorption of Gases on Plane Surface of Glass, Mica and Platinum. Res. Lab. GE Co. 1918, 40, 1361–1402. [Google Scholar] [CrossRef]
Sample Availability: No samples are available. |






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shende, C.; Farquharson, A.; Brouillette, C.; Smith, W.; Farquharson, S. Quantitative Measurements of Codeine and Fentanyl on a Surface-Enhanced Raman-Active Pad. Molecules 2019, 24, 2578. https://doi.org/10.3390/molecules24142578
Shende C, Farquharson A, Brouillette C, Smith W, Farquharson S. Quantitative Measurements of Codeine and Fentanyl on a Surface-Enhanced Raman-Active Pad. Molecules. 2019; 24(14):2578. https://doi.org/10.3390/molecules24142578
Chicago/Turabian StyleShende, Chetan, Amelia Farquharson, Carl Brouillette, Wayne Smith, and Stuart Farquharson. 2019. "Quantitative Measurements of Codeine and Fentanyl on a Surface-Enhanced Raman-Active Pad" Molecules 24, no. 14: 2578. https://doi.org/10.3390/molecules24142578
APA StyleShende, C., Farquharson, A., Brouillette, C., Smith, W., & Farquharson, S. (2019). Quantitative Measurements of Codeine and Fentanyl on a Surface-Enhanced Raman-Active Pad. Molecules, 24(14), 2578. https://doi.org/10.3390/molecules24142578