Chiral Recognition of Carboxylate Anions by (R)-BINOL-Based Macrocyclic Receptors
Abstract
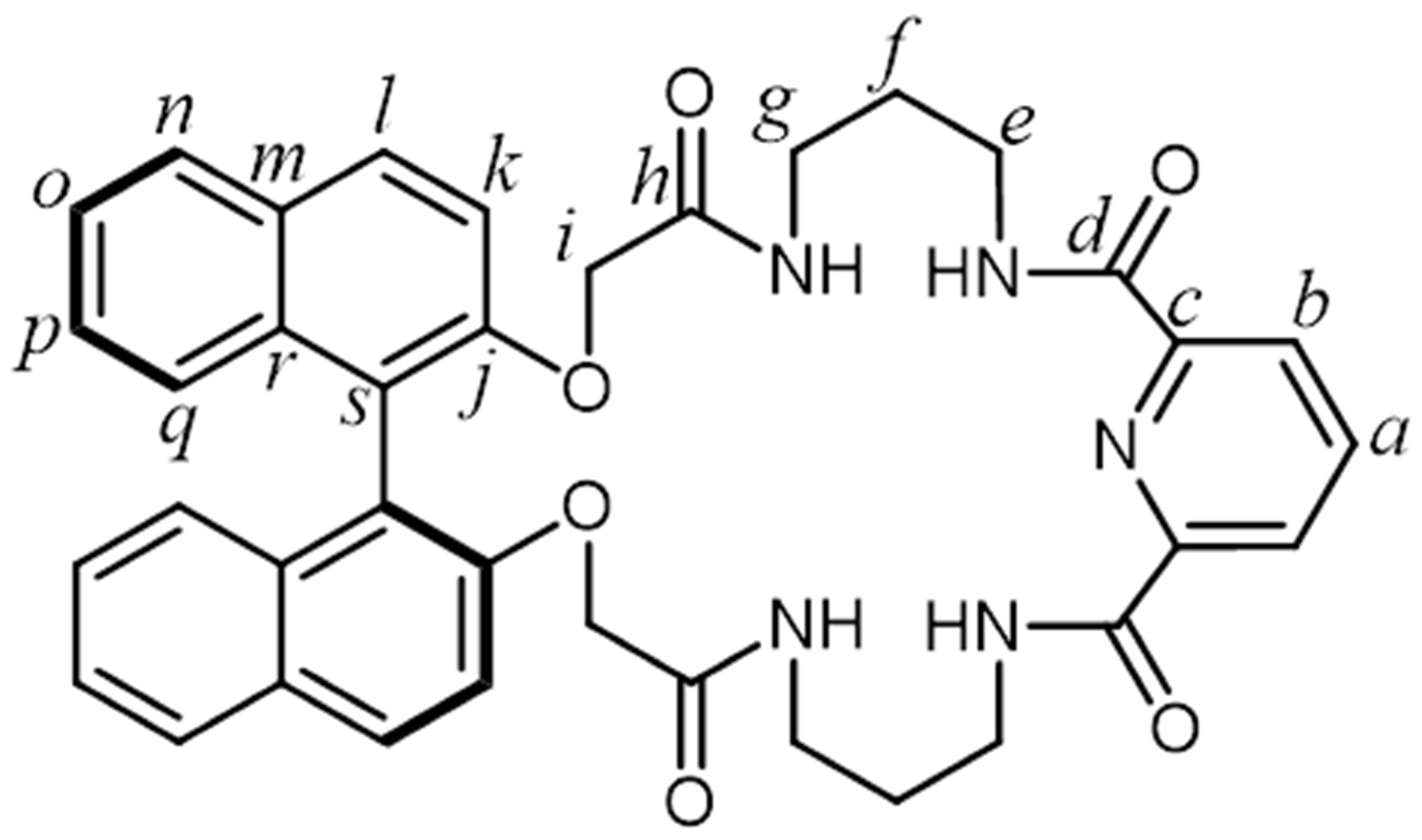
1. Introduction
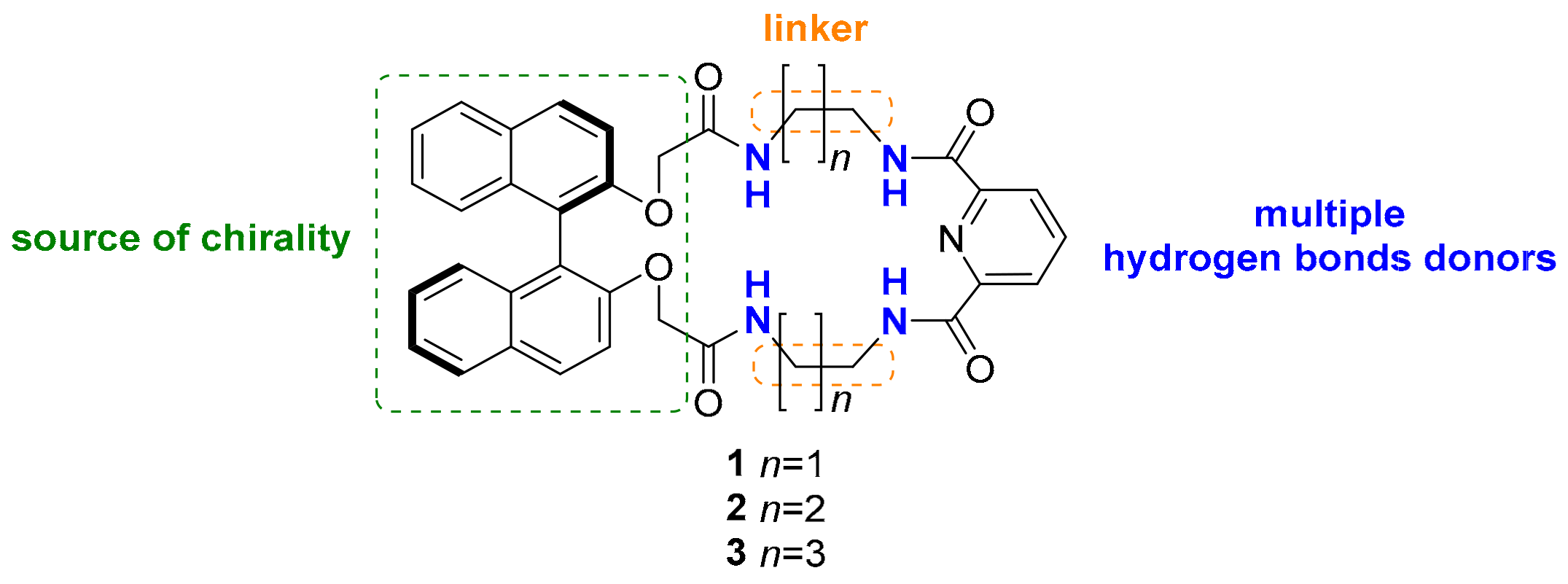
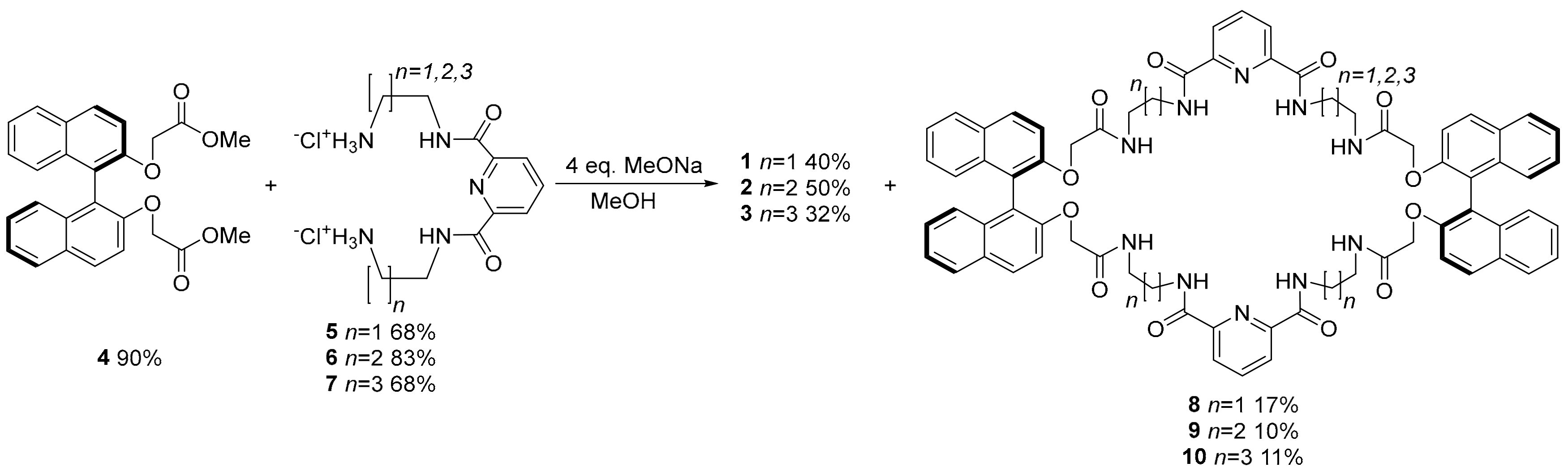
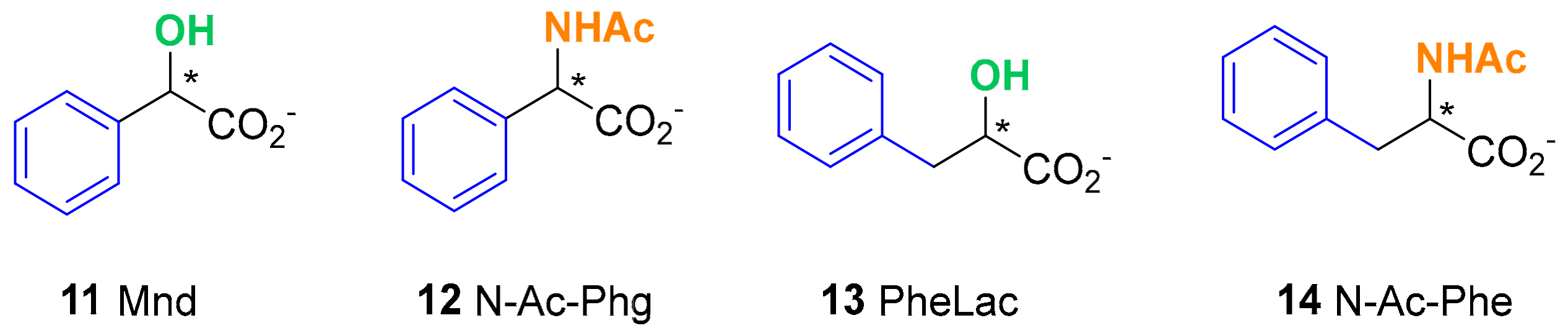
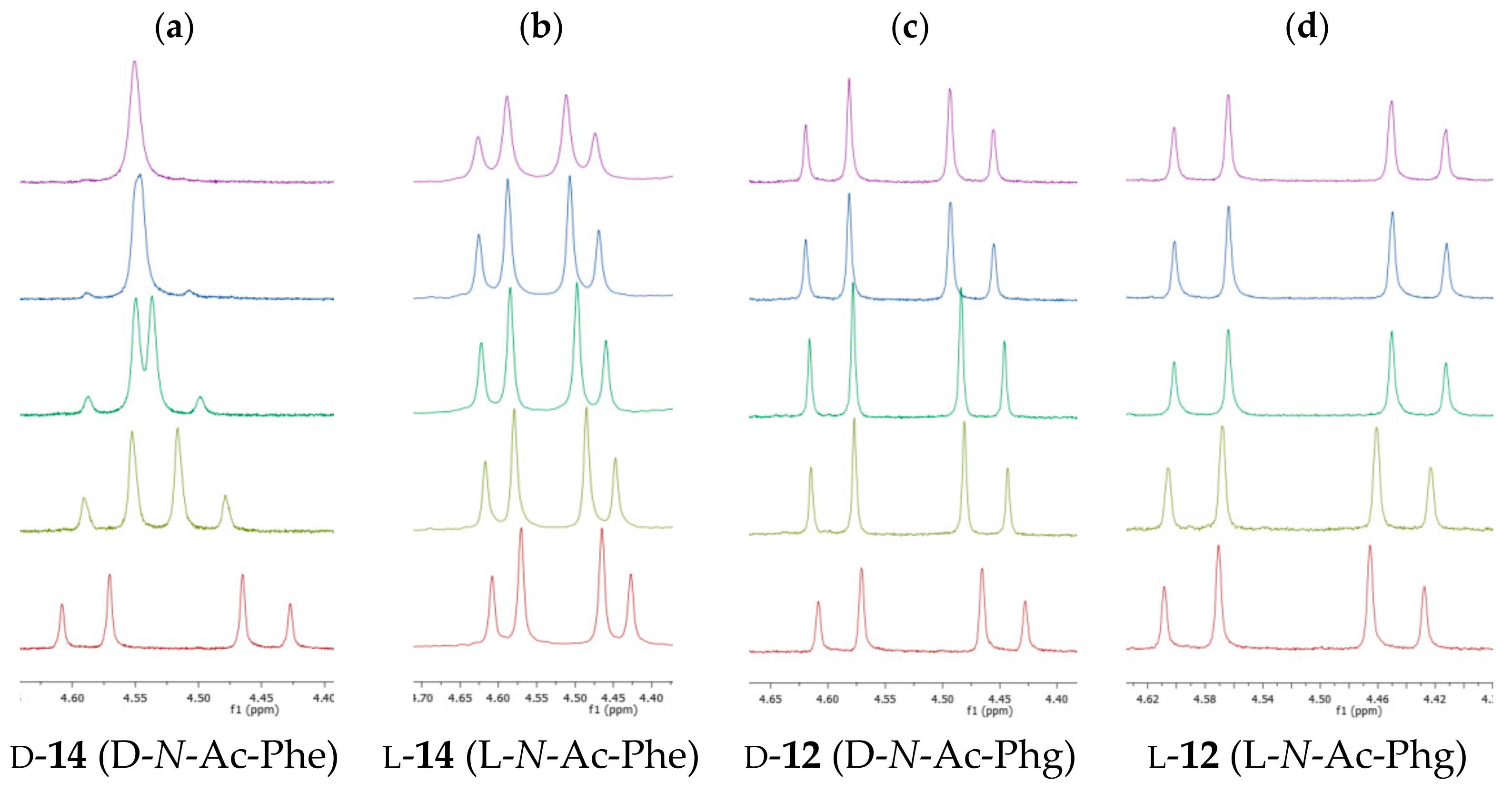
2. Results and Discussion
3. Conclusions
4. Experimental Section
4.1. General Procedure of the Macrocyclization Reaction
4.2. Characterization Data for Products 1–3 and 8–10
4.3. Titration Experiments
4.4. 1H NMR Titration Procedure
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- He, Q.; Vargas-Zúñiga, G.I.; Kim, S.H.; Kim, S.K.; Sessler, J.L. Macrocycles as Ion Pair Receptors. Chem. Rev. 2019, 8b00734. [Google Scholar] [CrossRef] [PubMed]
- Beer, P.D.; Langton, M.J. Anion, Cation and Ion-Pair Recognition by Macrocyclic and Interlocked Host Systems. In Macrocyclic and Supramolecular Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2016; pp. 38–72. [Google Scholar]
- Kean, W.F.; Lock, C.J.L.; Rischke, J.; Butt, R.; Watson Buchanan, W.; Howard-Lock, H. Effect of R and S Enantiomers of Naproxen on Aggregation and Thromboxane Production in Human Platelets. J. Pharm. Sci. 1989, 78, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Cram, D.J.; Helgeson, R.C.; Sousa, L.R.; Timko, J.M.; Newcomb, M.; Moreau, P.; De Jong, F.; Gokel, G.W.; Hoffman, D.H.; Domeier, L.A.; et al. Chiral Recognition in Complexation of Guests by Designed Host Molecules. Org. Synth. 1975, 327–349. [Google Scholar]
- Pirkle, W.H.; Pochapsky, T.C. Considerations of Chiral Recognition Relevant to the Liquid Chromatographic Separation of Enantiomers. Chem. Rev. 1989, 89, 347–362. [Google Scholar] [CrossRef]
- Ulatowski, F.; Jurczak, J. Chiral Recognition of Carboxylates by a Static Library of Thiourea Receptors with Amino Acid Arms. J. Org. Chem. 2015, 80, 4235–4243. [Google Scholar] [CrossRef] [PubMed]
- Chmielewski, M.J.; Jurczak, J. Anion Recognition by Neutral Macrocyclic Amides. Chem. A Eur. J. 2005, 11, 6080–6094. [Google Scholar] [CrossRef]
- Lichosyt, D.; Dydio, P.; Jurczak, J. Azulene-Based Macrocyclic Receptors for Recognition and Sensing of Phosphate Anions. Chem. A Eur. J. 2016, 22, 17673–17680. [Google Scholar] [CrossRef]
- Martí-Centelles, V.; Pandey, M.D.; Burguete, M.I.; Luis, S.V. Macrocyclization Reactions: The Importance of Conformational, Configurational, and Template-Induced Preorganization. Chem. Rev. 2015, 115, 8736–8834. [Google Scholar] [CrossRef]
- Yu, Y.; Bogliotti, N.; Tang, J.; Xie, J. Synthesis and Properties of Carbohydrate-Based BODIPY-Functionalised Fluorescent Macrocycles. Eur. J. Org. Chem. 2013, 2013, 7749–7760. [Google Scholar] [CrossRef]
- Beeren, S.R.; Sanders, J.K.M. Ferrocene-amino Acid Macrocycles as Hydrazone-based Receptors for Anions. Chem. Sci. 2011, 2, 1560. [Google Scholar] [CrossRef]
- Kyba, E.P.; Siegel, M.G.; Sousa, L.R.; Sogah, G.D.Y.; Cram, D.J. Chiral, Hinged, and Functionalized Multiheteromacrocycles. J. Am. Chem. Soc. 1973, 95, 2691–2692. [Google Scholar] [CrossRef]
- Yua, S.; Pu, L. Recent Progress on Using BINOLs in Enantioselective Molecular Recognition. Tetrahedron 2015, 71, 745–772. [Google Scholar] [CrossRef]
- Brunel, J.M. BINOL: A Versatile Chiral Reagent. Chem. Rev. 2005, 105, 857–898. [Google Scholar] [CrossRef]
- Lim, J.Y.C.; Marques, I.; Felix, V.; Beer, P.D. Chiral Halogen Bonding Rotaxane for Recognition and Sensing of Biologically−relevant Dicarboxylate. Angew. Chem. Int. Ed. 2018, 57, 548–588. [Google Scholar] [CrossRef]
- Lim, J.Y.C.; Marques, I.; Ferreira, L.; Felix, V.; Beer, P.D. Enhancing the Enantioselective Recognition and Sensing of Chiral Anions by Halogen Bonding. Chem. Commun. 2016, 52, 5527–5530. [Google Scholar] [CrossRef]
- Ema, T.; Okuda, K.; Watanabe, S.; Yamasaki, T.; Minami, T.; Esipenko, N.A.; Anzenbacher, P., Jr. Selective Anion Sensing by Chiral Macrocyclic Receptors with Multiple Hydrogen−Bonding Sites. Org. Lett. 2014, 16, 1302–1305. [Google Scholar] [CrossRef]
- Tyszka, A.; Pikus, G.; Dąbrowa, K.; Jurczak, J. Late-Stage Functionalization of (R)-BINOL-Based Diazacoronands and Their Chiral Recognition of α-Phenylethylamine Hydrochlorides. J. Org. Chem. 2019, 84, 6502–6507. [Google Scholar] [CrossRef]
- Gangopadhyay, M.; Maity, A.; Dey, A.; Rajamohanan, P.R.; Ravindranathan, S.; Das, A. Chiral Discrimination through 1H NMR and Luminescence Spectroscopy: Dynamic Processes and Solid Strip for Chiral Recognition. Chem. Eur. J. 2017, 23, 18303–18313. [Google Scholar] [CrossRef]
- Zeng, C.; Zhang, X.; Pu, L. Enhanced Enantioselectivity in the Fluorescent Recognition of a Chiral Diamine by Using a Bisbinaphthyl Dialdehyde. ACS Omega 2018, 3, 12545–12548. [Google Scholar] [CrossRef]
- Muralidharan, V.P.; Sathiyanarayanan, K.I. Naphthalimide Based Chiral Fluorescence Sensor Employing (S)-BINOL Unit for Highly Enantioselective Recognition of α—Amino Alcohols with Opposite Chiral Selectivity. Chem. Sel. 2018, 3, 3111–3117. [Google Scholar] [CrossRef]
- Dąbrowa, K.; Pawlak, M.; Duszewski, P.; Jurczak, J. Unclosed Cryptands: A Point of Departure for Developing Potent Neutral Anion Receptors. Org. Lett. 2012, 14, 6298–6301. [Google Scholar] [CrossRef]
- Hofmeister, F. Zur Lehre von der Wirkung der Salze. Arch. Exp. Pathol. Pharmakol. 1888, 24, 247–260. [Google Scholar] [CrossRef]
- Granda, J.M.; Jurczak, J. Exploration of the Chiral Recognition of Sugar-Based Diindolylmethane Receptors: Anion and Receptor Structures. Chem. A Eur. J. 2015, 21, 16585–16592. [Google Scholar] [CrossRef]
- Lichosyt, D.; Wasiłek, S.; Jurczak, J. Exploring the Chiral Recognition of Carboxylates by C2-Symmetric Receptors Bearing Glucosamine Pendant Arms. J. Org. Chem. 2016, 81, 7342–7348. [Google Scholar] [CrossRef]
- Frassineti, C.; Ghelli, S.; Gans, P.; Sabatini, A.; Moruzzi, M.S.; Vacca, A. Nuclear Magnetic Resonance as a Tool for Determining Protonation Constants of Natural Polyprotic Bases in Solution. Anal. Biochem. 1995, 231, 374–382. [Google Scholar] [CrossRef]
- Frassineti, C.; Alderighi, L.; Gans, P.; Sabatini, A.; Vacca, A.; Ghelli, S. Determination of Protonation Constants of Some Fluorinated Polyamines by Means of 13C NMR Data Processed by the New Computer Program HypNMR2000. Protonation Sequence in Polyamines. Anal. Bioanal. Chem. 2003, 376, 1041–1052. [Google Scholar]
- Rodríguez-Barrientos, D.; Rojas-Hernández, A.; Gutiérrez, A.; Moya-Hernández, R.; Gómez-Balderas, R.; Ramírez-Silva, M.T. Determination of pKa Values of Tenoxicam from 1H NMR Chemical Shifts and of Oxicams from Electrophoretic Mobilities (CZE) with the Aid of Programs SQUAD and HYPNMR. Talanta 2009, 80, 754–762. [Google Scholar] [CrossRef]
Sample Availability: All samples are available from the authors. |


| Host | Macro Ring Size | Binding Constants [M−1] | ||||
|---|---|---|---|---|---|---|
| DMSO–d6 + 0.5% H2O | Acetone–d6 + 0.5% H2O | |||||
| Cl− | H2PO4− | AcO− | BzO− | BzO− | ||
| 1 | 23 | 12 | 121 | 90 | 25 | 916 |
| 2 | 25 | 35 | 136 | 82 | 29 | 916 |
| 3 | 27 | 8 | 148 | 60 | 25 | 972 |
| Host | Chiral Guests (b) | |||||||
|---|---|---|---|---|---|---|---|---|
| 11 Mnd | 12 N-Ac-Phg | 13 Phelac | 14 N-Ac-Phe | |||||
| Ka | α (c) | Ka | α | Ka | α | Ka | α | |
| 1 | KR = 215 | 1.29 | KD = 229 | 1.04 | KR = 233 | 1.42 | KD = 318 | 1.70 |
| KS = 167 | KL = 221 | KS = 164 | KL = 187 | |||||
| 2 | KR = 96 | 0.95 | KD = 124 | 0.65 | KR = 100 | 1.10 | KD = 140 | 1.15 |
| KS = 101 | KL = 192 | KS = 91 | KL = 122 | |||||
| 3 | KR = 227 | 1.19 | KD = 329 | 1.11 | KR = 169 | 1.48 | KD = 221 | 1.32 |
| KS = 191 | KL = 297 | KS = 114 | KL = 167 | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tyszka-Gumkowska, A.; Pikus, G.; Jurczak, J. Chiral Recognition of Carboxylate Anions by (R)-BINOL-Based Macrocyclic Receptors. Molecules 2019, 24, 2635. https://doi.org/10.3390/molecules24142635
Tyszka-Gumkowska A, Pikus G, Jurczak J. Chiral Recognition of Carboxylate Anions by (R)-BINOL-Based Macrocyclic Receptors. Molecules. 2019; 24(14):2635. https://doi.org/10.3390/molecules24142635
Chicago/Turabian StyleTyszka-Gumkowska, Agata, Grzegorz Pikus, and Janusz Jurczak. 2019. "Chiral Recognition of Carboxylate Anions by (R)-BINOL-Based Macrocyclic Receptors" Molecules 24, no. 14: 2635. https://doi.org/10.3390/molecules24142635
APA StyleTyszka-Gumkowska, A., Pikus, G., & Jurczak, J. (2019). Chiral Recognition of Carboxylate Anions by (R)-BINOL-Based Macrocyclic Receptors. Molecules, 24(14), 2635. https://doi.org/10.3390/molecules24142635







