Optimization of the Electro-Peroxone Process for Micropollutant Abatement Using Chemical Kinetic Approaches
Abstract
:1. Introduction
2. Results and Discussion
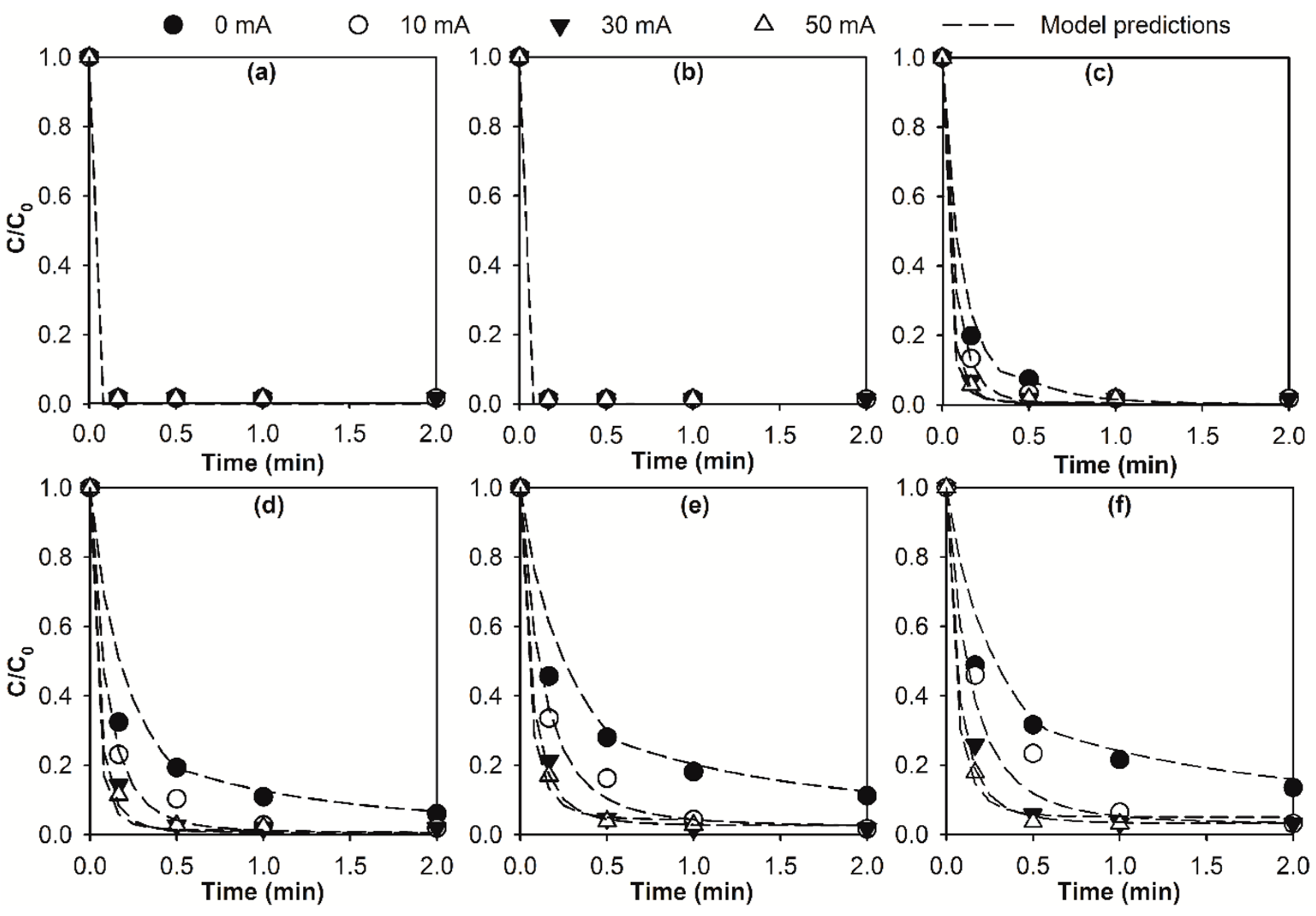
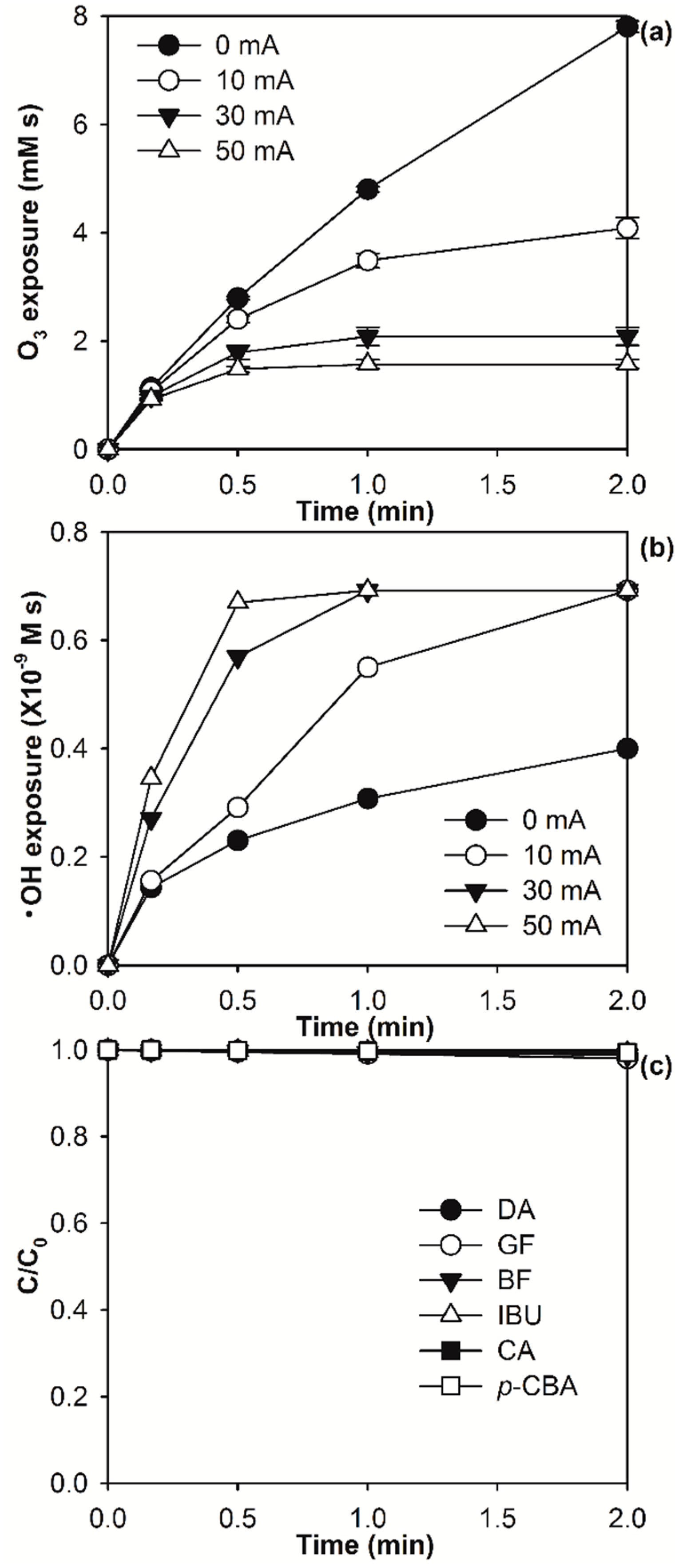
2.1. Kinetic Modelling of Micropollutant Abatement by the E-Peroxone Process
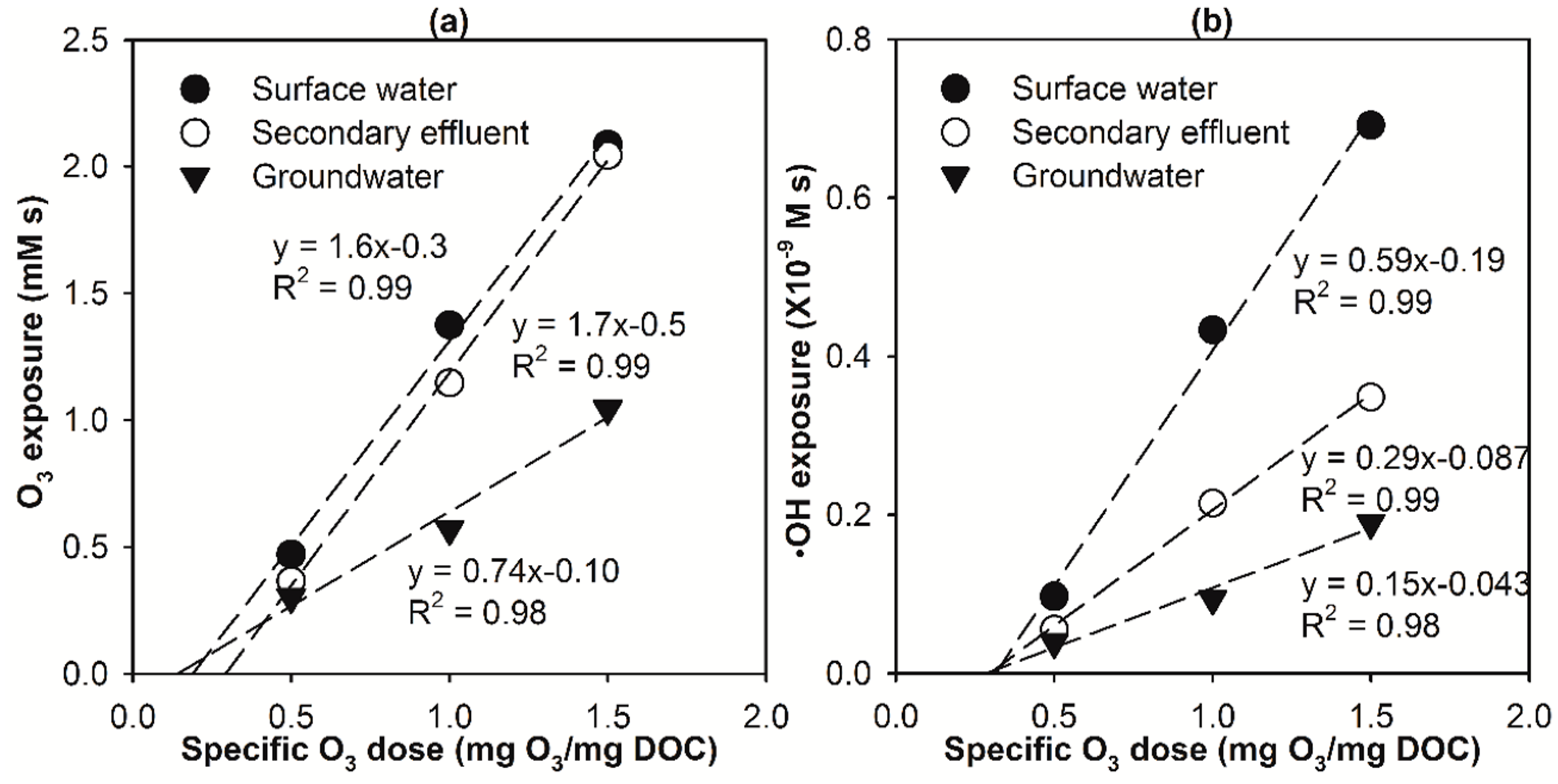
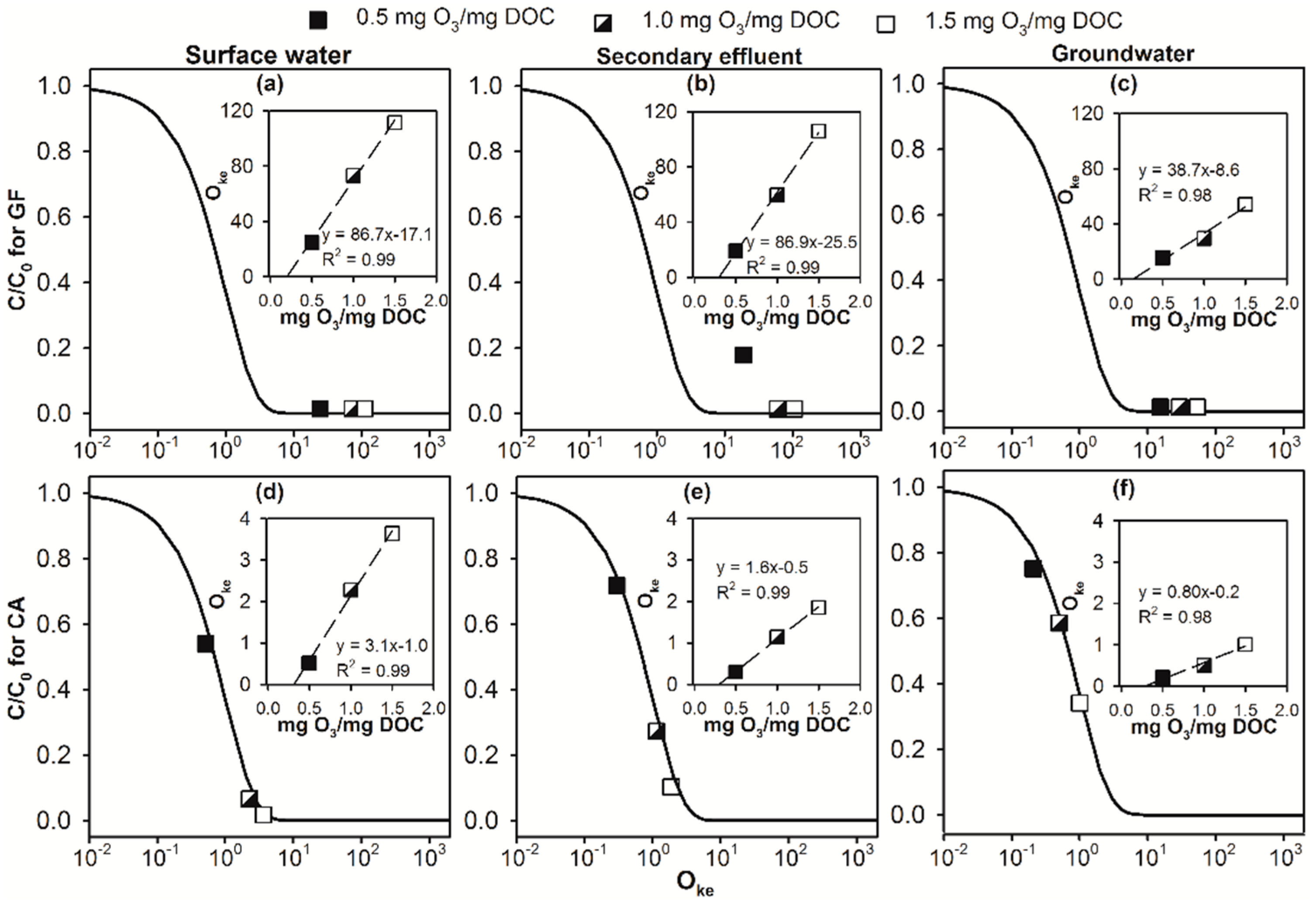
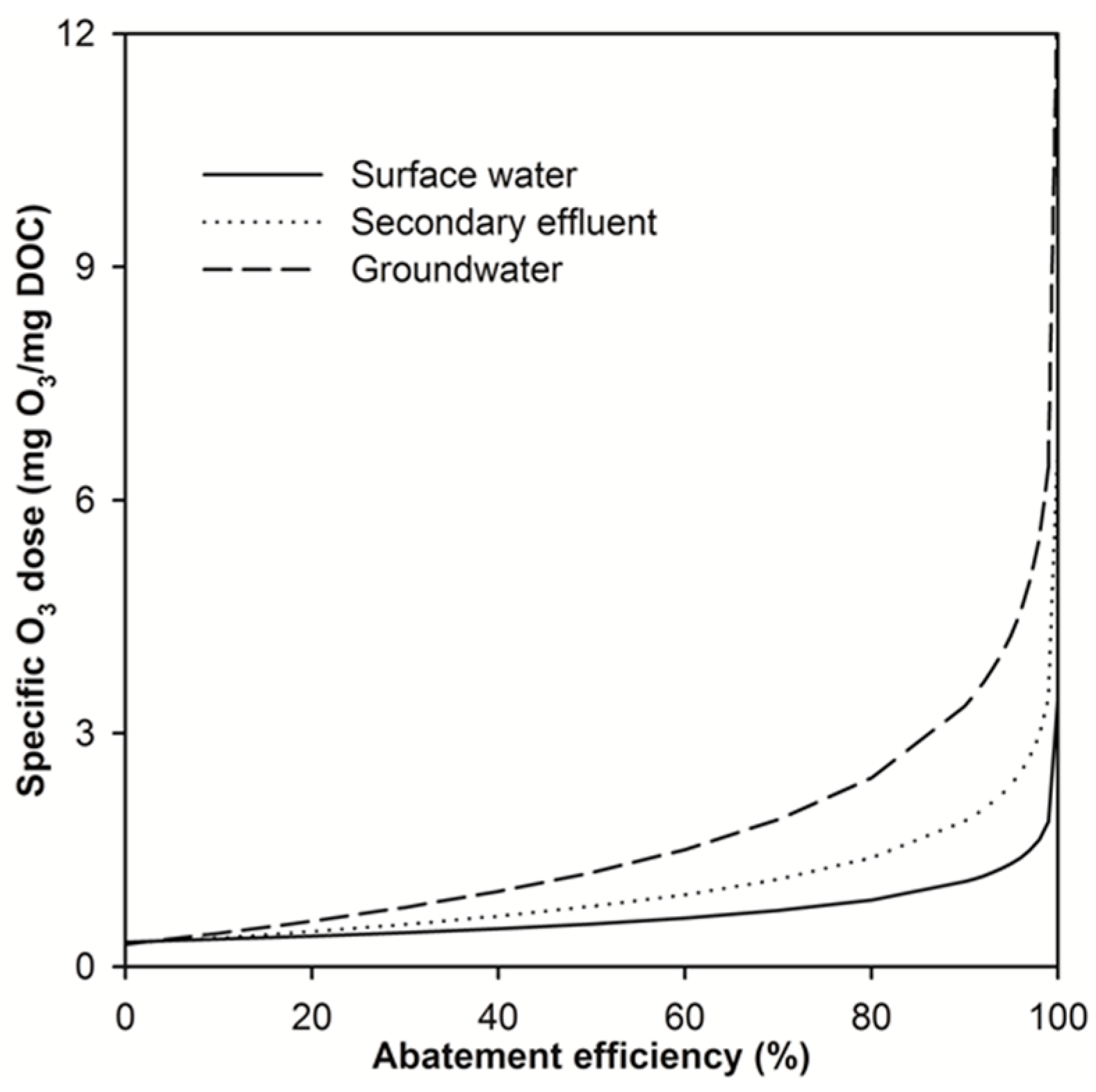
2.2. Modelling the Effect of Ozone Doses on Micropollutant Abatement
2.3. Implications
3. Materials and Methods
3.1. Chemicals and Water Samples
3.2. E-Peroxone Treatment of the Water Samples Containing Micropollutants
3.3. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, X.; Wang, Y.; Yuan, S.; Li, Z.; Wang, B.; Huang, J.; Deng, S.; Yu, G. Degradation of the anti-inflammatory drug ibuprofen by electro-peroxone process. Water Res. 2014, 63, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Y.; Zhao, J.; Wang, H.; Wang, B.; Huang, J.; Deng, S.; Yu, G. Electro-peroxone treatment of the antidepressant venlafaxine: Operational parameters and mechanism. J. Hazard. Mater. 2015, 300, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Wang, X.; Yang, H.; Yu, G.; Deng, S.; Huang, J.; Wang, B.; Wang, Y. Removal of pharmaceuticals from secondary effluents by an electro-peroxone process. Water Res. 2016, 88, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.Q.; Wu, Q.L.; Zhou, X.J.; Cao, H.O.; Du, J.S.; Yin, R.L.; Ren, N.Q. Enhanced amoxicillin treatment using the electro-peroxone process: Key factors and degradation mechanism. Rsc Adv. 2015, 5, 52695–52702. [Google Scholar] [CrossRef]
- Nawrocki, J. Catalytic ozonation in water: Controversies and questions. Discussion paper. Appl. Catal. B Environ. 2013, 142, 465–471. [Google Scholar] [CrossRef]
- Nawrocki, J.; Kasprzyk-Hordern, B. The efficiency and mechanisms of catalytic ozonation. Appl. Catal. B-Environ. 2010, 99, 27–42. [Google Scholar] [CrossRef]
- Beltrán, F.J. Ozone Reaction Kinetics for Water and Wastewater Systems; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Figueredo, M.A.; Rodríguez, E.M.; Checa, M.; Beltran, F.J. Ozone-based advanced oxidation processes for primidone removal in water using simulated solar radiation and TiO2 or WO3 as photocatalyst. Molecules 2019, 24, 1728. [Google Scholar] [CrossRef]
- Beltrán, F.J.; Rey, A. Solar or UVA-visible photocatalytic ozonation of water contaminants. Molecules 2017, 22, 1177. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Chen, C.; Xi, J.; Cao, H.; Duan, X.; Xie, Y.; Song, W.; Wang, S. Occurrence of both hydroxyl radical and surface oxidation pathways in N-doped layered nanocarbons for aqueous catalytic ozonation. Appl. Catal. B Environ. 2019, 254, 283–291. [Google Scholar] [CrossRef]
- Yuan, S.; Li, Z.X.; Wang, Y.J. Effective degradation of methylene blue by a novel electrochemically driven process. Electrochem. Commun. 2013, 29, 48–51. [Google Scholar] [CrossRef]
- Wang, H.; Bakheet, B.; Yuan, S.; Li, X.; Yu, G.; Murayama, S.; Wang, Y. Kinetics and energy efficiency for the degradation of 1,4-dioxane by electro-peroxone process. J. Hazard. Mater. 2015, 294, 90–98. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, S.; Zhan, J.; Wang, Y.; Yu, G.; Deng, S.; Huang, J.; Wang, B. Mechanisms of enhanced total organic carbon elimination from oxalic acid solutions by electro-peroxone process. Water Res. 2015, 80, 20–29. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Zhou, L.B.; Cao, H.B.; Xie, Y.B.; Xiao, J.D.; Yang, J.; Zhang, Y. C3N4-Mn/CNT composite as a heterogeneous catalyst in the electro-peroxone process for promoting the reaction between O3 and H2O2 in acid solution. Catal. Sci. Technol. 2018, 8, 6241–6251. [Google Scholar] [CrossRef]
- Turkay, O.; Barisci, S.; Ozturk, B.; Ozturk, H.; Dimoglo, A. Electro-peroxone treatment of phenol: Process comparison, the effect of operational parameters and degradation mechanism. J. Electrochem. Soc. 2017, 164, 180–186. [Google Scholar] [CrossRef]
- Von Gunten, U.; Oliveras, Y. Kinetics of the reaction between hydrogen peroxide and hypobromous acid: Implication on water treatment and natural systems. Water Res. 1997, 31, 900–906. [Google Scholar] [CrossRef]
- Yao, W.; Fu, J.; Yang, H.; Yu, G.; Wang, Y. The beneficial effect of cathodic hydrogen peroxide generation on mitigating chlorinated by-product formation during water treatment by an electro-peroxone process. Water Res. 2019, 157, 209–217. [Google Scholar] [CrossRef]
- Li, Y.K.; Shen, W.H.; Fu, S.J.; Yang, H.W.; Yu, G.; Wang, Y.J. Inhibition of bromate formation during drinking water treatment by adapting ozonation to electro-peroxone process. Chem. Eng. J. 2015, 264, 322–328. [Google Scholar] [CrossRef]
- Yao, W.; Qu, Q.; von Gunten, U.; Chen, C.; Yu, G.; Wang, Y. Comparison of methylisoborneol and geosmin abatement in surface water by conventional ozonation and an electro-peroxone process. Water Res. 2017, 108, 373–382. [Google Scholar] [CrossRef]
- Yao, W.; Ur Rehman, S.W.; Wang, H.; Yang, H.; Yu, G.; Wang, Y. Pilot-scale evaluation of micropollutant abatements by conventional ozonation, UV/O3, and an electro-peroxone process. Water Res. 2018, 138, 106–117. [Google Scholar] [CrossRef]
- Mao, Y.; Guo, D.; Yao, W.; Wang, X.; Yang, H.; Xie, Y.F.; Komarneni, S.; Yu, G.; Wang, Y. Effects of conventional ozonation and electro-peroxone pretreatment of surface water on disinfection by-product formation during subsequent chlorination. Water Res. 2018, 130, 322–332. [Google Scholar] [CrossRef]
- Von Gunten, U. Ozonation of drinking water: Part II. Disinfection and by-product formation in presence of bromide, iodide or chlorine. Water Res. 2003, 37, 1469–1487. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, G.; Deng, S.; Huang, J.; Wang, B. The electro-peroxone process for the abatement of emerging contaminants: Mechanisms, recent advances, and prospects. Chemosphere 2018, 208, 640–654. [Google Scholar] [CrossRef]
- Turkay, O.; Ersoy, Z.G.; Barisci, S. Review—The application of an electro-peroxone process in water and wastewater treatment. J. Electrochem. Soc. 2017, 164, 94–102. [Google Scholar] [CrossRef]
- Von Sonntag, C.; von Gunten, U. Chemistry of Ozone in Water and Wastewater Treatment: From Basic Principles to Applications; IWA Publishing: London, UK, 2012. [Google Scholar]
- Schwarzenbach, R.P.; Escher, B.I.; Fenner, K.; Hofstetter, T.B.; Johnson, C.A.; von Gunten, U.; Wehrli, B. The challenge of micropollutants in aquatic systems. Science 2006, 313, 1072–1077. [Google Scholar] [CrossRef]
- Giannakis, S.; Rtimi, S.; Pulgarin, C. Light-assisted advanced oxidation processes for the elimination of chemical and microbiological pollution of wastewaters in developed and developing countries. Molecules 2017, 22, 1070. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, H.; Wang, B.; Deng, S.; Huang, J.; Yu, G.; Wang, Y. Prediction of micropollutant abatement during homogeneous catalytic ozonation by a chemical kinetic model. Water Res. 2018, 142, 383–395. [Google Scholar] [CrossRef]
- Rivas, F.J.; Solis, R.R.; Beltran, F.J.; Gimeno, O. Sunlight driven photolytic ozonation as an advanced oxidation process in the oxidation of bezafibrate, cotinine and iopamidol. Water Res. 2019, 151, 226–242. [Google Scholar] [CrossRef]
- Cruz-Alcalde, A.; Sans, C.; Esplugas, S. Priority pesticides abatement by advanced water technologies: The case of acetamiprid removal by ozonation. Sci. Total Environ. 2017, 599–600, 1454–1461. [Google Scholar] [CrossRef]
- Lee, Y.; Kovalova, L.; McArdell, C.S.; von Gunten, U. Prediction of micropollutant elimination during ozonation of a hospital wastewater effluent. Water Res. 2014, 64, 134–148. [Google Scholar] [CrossRef]
- Wang, H.; Zhan, J.; Yao, W.; Wang, B.; Deng, S.; Huang, J.; Yu, G.; Wang, Y. Comparison of pharmaceutical abatement in various water matrices by conventional ozonation, peroxone (O3/H2O2), and an electro-peroxone process. Water Res. 2018, 130, 127–138. [Google Scholar] [CrossRef]
- Lee, Y.; Gerrity, D.; Lee, M.; Bogeat, A.E.; Salhi, E.; Gamage, S.; Trenholm, R.A.; Wert, E.C.; Snyder, S.A.; von Gunten, U. Prediction of micropollutant elimination during ozonation of municipal wastewater effluents: Use of kinetic and water specific information. Environ. Sci. Technol. 2013, 47, 5872–5881. [Google Scholar] [CrossRef]
- Acero, J.L.; von Gunten, U. Characterization of oxidation processes: Ozonation and the AOP O3/H2O2. J. Am. Water Works Assoc. 2001, 93, 90–100. [Google Scholar] [CrossRef]
- Elovitz, M.S.; von Gunten, U. Hydroxyl radical/ozone ratios during ozonation processes I: The Rct concept. Ozone Sci. Eng. 1999, 21, 239–260. [Google Scholar] [CrossRef]
- Acero, J.L.; Stemmler, K.; Von Gunten, U. Degradation kinetics of atrazine and its degradation products with ozone and OH radicals: A predictive tool for drinking water treatment. Environ. Sci. Technol. 2000, 34, 591–597. [Google Scholar] [CrossRef]
- Cruz-Alcalde, A.; Esplugas, S.; Sans, C. Abatement of ozone-recalcitrant micropollutants during municipal wastewater ozonation: Kinetic modelling and surrogate-based control strategies. Chem. Eng. J. 2018. [Google Scholar] [CrossRef]
- Lee, Y.; von Gunten, U. Advances in predicting organic contaminant abatement during ozonation of municipal wastewater effluent: Reaction kinetics, transformation products, and changes of biological effects. Environ. Sci. Water Res. 2016, 2, 421–442. [Google Scholar] [CrossRef]
- Chaplin, B.P. Critical review of electrochemical advanced oxidation processes for water treatment applications. Environ. Sci. Process. Impacts 2014, 16, 1182–1203. [Google Scholar] [CrossRef]
- Katsoyiannis, I.A.; Canonica, S.; von Gunten, U. Efficiency and energy requirements for the transformation of organic micropollutants by ozone, O3/H2O2 and UV/H2O2. Water Res. 2011, 45, 3811–3822. [Google Scholar] [CrossRef]
- Beltrán, F.J.; Rey, A. Free radical and direct ozone reaction competition to remove priority and pharmaceutical water contaminants with single and hydrogen peroxide ozonation systems. Ozone Sci. Eng. 2018, 40, 251–265. [Google Scholar] [CrossRef]
- Huber, M.M.; Canonica, S.; Park, G.-Y.; von Gunten, U. Oxidation of pharmaceuticals during ozonation and advanced oxidation processes. Environ. Sci. Technol. 2003, 37, 1016–1024. [Google Scholar] [CrossRef]
- Huber, M.M.; Gobel, A.; Joss, A.; Hermann, N.; Loffler, D.; McArdell, C.S.; Ried, A.; Siegrist, H.; Ternes, T.A.; von Gunten, U. Oxidation of pharmaceuticals during ozonation of municipal wastewater effluents: A pilot study. Environ. Sci. Technol. 2005, 39, 4290–4299. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Zhen, L.; Zhang, H.; Zhang, Y.; Wang, C. Electro-Fenton treatment of concentrates generated in nanofiltration of biologically pretreated landfill leachate. J. Hazard. Mater. 2012, 229–230, 115–121. [Google Scholar] [CrossRef]
- Sellers, R.M. Spectrophotometric determination of hydrogen-peroxide using potassium titanium (IV) oxalate. Analyst 1980, 105, 950–954. [Google Scholar] [CrossRef]
- Bader, H.; Hoigne, J. Determination of ozone in water by the indigo method. Water Res. 1981, 15, 449–456. [Google Scholar] [CrossRef]
- Von Gunten, U.; Hoigne, J. Bromate formation during ozonization of bromide-containing waters: Interaction of ozone and hydroxyl radical reactions. Environ. Sci. Technol. 1994, 28, 1234–1242. [Google Scholar] [CrossRef]
Sample Availability: Not available. |


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Su, L.; Zhu, S.; Zhu, W.; Han, X.; Cheng, Y.; Yu, G.; Wang, Y. Optimization of the Electro-Peroxone Process for Micropollutant Abatement Using Chemical Kinetic Approaches. Molecules 2019, 24, 2638. https://doi.org/10.3390/molecules24142638
Wang H, Su L, Zhu S, Zhu W, Han X, Cheng Y, Yu G, Wang Y. Optimization of the Electro-Peroxone Process for Micropollutant Abatement Using Chemical Kinetic Approaches. Molecules. 2019; 24(14):2638. https://doi.org/10.3390/molecules24142638
Chicago/Turabian StyleWang, Huijiao, Lu Su, Shuai Zhu, Wei Zhu, Xia Han, Yi Cheng, Gang Yu, and Yujue Wang. 2019. "Optimization of the Electro-Peroxone Process for Micropollutant Abatement Using Chemical Kinetic Approaches" Molecules 24, no. 14: 2638. https://doi.org/10.3390/molecules24142638
APA StyleWang, H., Su, L., Zhu, S., Zhu, W., Han, X., Cheng, Y., Yu, G., & Wang, Y. (2019). Optimization of the Electro-Peroxone Process for Micropollutant Abatement Using Chemical Kinetic Approaches. Molecules, 24(14), 2638. https://doi.org/10.3390/molecules24142638






