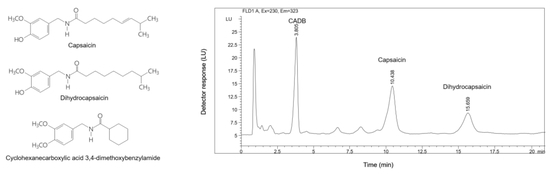
Toxicokinetic Study of a Gastroprotective Dose of Capsaicin by HPLC-FLD Method
Abstract
1. Introduction
2. Methods and Materials
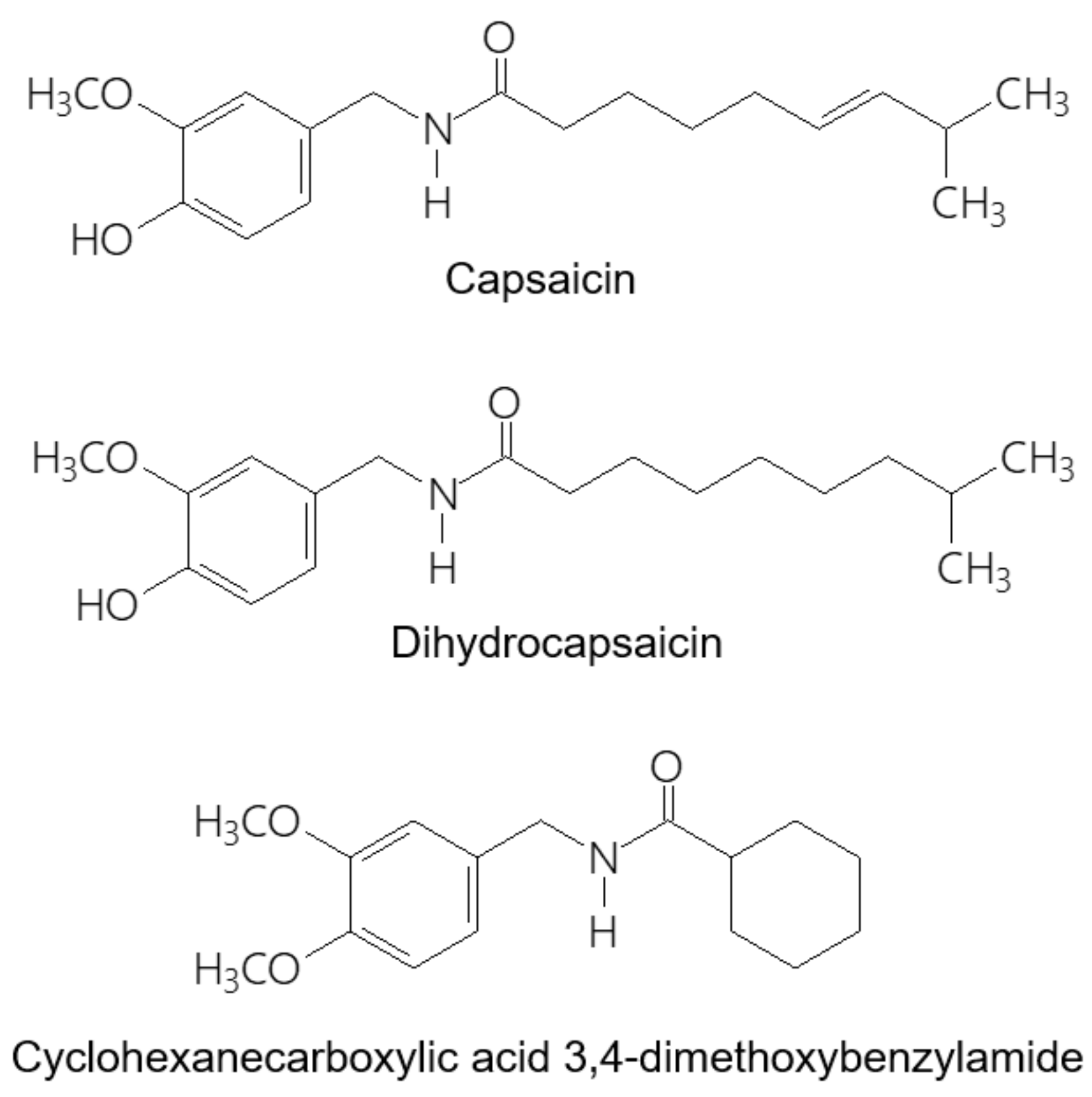
2.1. Chemicals and Reagents
2.2. HPLC Instrumentation and Chromatographic Conditions
2.3. Preparation of the Standard Solutions
2.4. Toxicological Studies
2.5. Sample Preparation
2.5.1. Plasma
2.5.2. Erythrocytes
3. Results
3.1. Extraction of the Plasma Samples
3.2. Extraction of the Erythrocytes
3.3. Method Validation
3.3.1. Specificity
3.3.2. Accuracy
3.3.3. Linearity
3.3.4. System Suitability
3.3.5. Precision
3.3.6. Matrix Effect
3.3.7. Determination of LOD and LOQ
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mózsik, G.; Dömötör, A.; Past, T.; Vas, V.; Perjési, P.; Kuzma, M.; Blazics, G.; Szolcsányi, J. Capsaicinoids, from the Plant Cultivation to the Production of the Human Medical Drug; Academy Publisher: Budapest, Hungary, 2009; pp. 43–80. ISBN 978-963-05-8694-8. [Google Scholar]
- Szállasi, A.; Blumber, M. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol. Rev. 1999, 51, 159–211. [Google Scholar] [PubMed]
- Szolcsányi, J. Forty years in capsaicin research for sensory pharmacology and physiology. Neuropeptides 2004, 38, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Rollyson, W.D.; Stover, C.A.; Brown, K.C.; Perry, H.E.; Stevenson, C.D.; McNees, C.A.; Ball, J.G.; Valentovic, M.A.; Dasgupta, P. Bioavailability of capsaicin and its implications for drug delivery. J. Control. Release 2014, 196, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Deal, C.L.; Schnitzer, T.J.; Lipstein, E.; Seibold, J.R.; Stevens, R.M.; Levy, M.D.; Albert, D.; Renold, F. Treatment of arthritis with topical capsaicin: A double-blind trial. Clin. Ther. 1991, 13, 383–395. [Google Scholar] [PubMed]
- The Capsaicin Study Group. Treatment of painful diabetic neuropathy with topical capsaicin. A multicenter, double-blind, vehicle-controlled study. Arch. Intern. Med. 1991, 151, 2225–2229. [Google Scholar] [CrossRef]
- Altman, R.D.; Aven, A.; Homberg, C.E.; Pfeifer, L.M.; Sack, M.; Young, G.T. Capsaicin cream 0.025% as monotherapy for osteoarthritis: A double-blind study. Semin. Arthritis Rheu. 1994, 23S3, 25–33. [Google Scholar] [CrossRef]
- Morris, G.C.; Gibson, S.J.; Helme, R.D. Capsaicin-induced flare and vasodilatation in patients with post herpetic neuralgia. Clin. J. Pain 1995, 63, 93–101. [Google Scholar] [CrossRef]
- Fattori, V.; Hohmann, M.S.N.; Rossaneis, A.C.; Pinho-Ribeiro, F.A.; Verri, W.A., Jr. Capsaicin: Current understanding of its mechanisms and therapy of pain and other pre-clinical and clinical use. Molecules 2016, 21, 844. [Google Scholar] [CrossRef]
- Szolcsányi, J.; Barthó, L. Impaired defense mechanisms to peptic ulcer in the capsaicin-desensitized rat. In Gastrointestinal Defense Mechanisms; Mózsik, G., Hänninan, O., Jávor, T., Eds.; Advances in Physiological Sciences; Pergamon Press: Oxford, UK; Akadémiai Kiadó: Budapest, Hungary, 1981; Volume 29, pp. 39–51. ISBN 978-0-08-027350-1. [Google Scholar]
- Mózsik, G.; Vincze, Á.; Szolcsányi, J. Four response stages of capsaicin-sensitive primary afferent neurons to capsaicin and its analog: Gastric acid secretion, gastric mucosal damage and protection. J. Gastroenterol. Hepatol. 2001, 16, 1093–1097. [Google Scholar] [CrossRef]
- Mózsik, G.; Dömötör, A.; Past, T.; Vas, V.; Perjési, P.; Kuzma, M.; Blazics, G.; Szolcsányi, J. Capsaicinoids, from the Plant Cultivation to the Production of the Human Medical Drug; Academy Publisher: Budapest, Hungary, 2009; pp. 100–119. ISBN 978-963-05-8694-8. [Google Scholar]
- Mózsik, G.; Dömötör, A.; Past, T.; Vas, V.; Perjési, P.; Kuzma, M.; Blazics, G.; Szolcsányi, J. Capsaicinoids, from the Plant Cultivation to the Production of the Human Medical Drug; Academy Publisher: Budapest, Hungary, 2009; pp. 165–206. ISBN 978-963-05-8694-8. [Google Scholar]
- Mózsik, G.; Past, T.; Habon, T.; Keszthelyi, Z.; Perjési, P.; Kuzma, M.; Sándor, B.; Szolcsányi, J.; Abdel-Salam, O.M.E.; Szalai, M. Capsaicin is a New Gastrointestinal Mucosal Protecting Drug Candidate in Humans—Pharmaceutical Development and Production Based on Clinical Pharmacology. In Capsaicin-sensitive Neural Afferentation and the Gastrointestinal Tract: From Bench to Bedside; Mózsik, G., Abdel-Salam, O.M.E., Takeuchi, K., Eds.; InTech: Rijeka, Croatia, 2014; pp. 265–364. ISBN 978-953-51-1631-8. [Google Scholar]
- Szolcsányi, J. Discovery and Mechanism of Gastroprotective Action of Capsaicin. In Capsaicin-Sensitive Neural Afferentation and the Gastrointestinal Tract: From Bench to Bedside; Mózsik, G., Abdel-Salam, O.M.E., Takeuchi, K., Eds.; InTech: Rijeka, Croatia, 2014; pp. 3–17. ISBN 978-953-51-1631-8. [Google Scholar]
- Dray, A. Neuropharmacological mechanisms of capsaicin and related substances. Biochem. Pharmacol. 1992, 44, 611–615. [Google Scholar] [CrossRef]
- Joe, B.; Lokesh, B.R. Prophylactic and therapeutic effects of n-3 polyunsaturated fatty acids, capsaicin, and curcumin on adjuvant induced arthritis in rats. J. Nutr. Biochem. 1997, 8, 397–407. [Google Scholar] [CrossRef]
- Sancho, R.; Lucena, C.; Macho, A.; Calzado, M.A.; Blanco-Molina, M.; Mináis, A.; Appendino, G.; Munoz, E. Immunosuppressive activity of capsaicinoids: Capsiate derived from sweet peppers inhibits NF-κB activation and is a potent antiinflammatory compound in vivo. Eur. J. Immunol. 2002, 32, 1753–1763. [Google Scholar] [CrossRef]
- Reddy, A.C.; Lokesh, B.R. Studies on spice principles as antioxidants in the inhibition of lipid peroxidation of rat liver microsomes. Mol. Cell. Biochem. 1992, 111, 117–124. [Google Scholar]
- Henderson, D.E.; Slickman, A.M. Quantitative HPLC determination of the antioxidant activity of capsaicin on the formation of lipid hydroperoxides of linoleic acid: A comparative study against BHT and melatonin. J. Agric. Food Chem. 1999, 47, 2563–2570. [Google Scholar] [CrossRef] [PubMed]
- Oyagbemi, A.A.; Saba, A.B.; Azeez, O.L. Capsaicin: A novel chemopreventive molecule and its underlying molecular mechanisms of action. Indian J. Cancer 2010, 47, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Kuzma, M.; Past, T.; Mózsik, G.; Perjési, P. Pharmacobotanical analysis and regulatory qualification of Capsicum fruits and Capsicum extracts—A survey. In Capsaicin-Sensitive Neural Afferentation and the Gastrointestinal Tract: From Bench to Bedside; Mózsik, G., Abdel-Salam, O.M.E., Takeuchi, K., Eds.; InTech: Rijeka, Croatia, 2014; pp. 21–74. ISBN 978-953-51-1631-8. [Google Scholar]
- Buck, S.H.; Miller, M.S.; Burks, T.F. Depletion of primary afferent substance P by capsaicin and dihydrocapsaicin without altered thermal sensitivity. Brain Res. 1982, 233, 216–220. [Google Scholar] [CrossRef]
- Kawada, T.; Suzuki, T.; Takahashi, M.; Iwai, K. Gastrointestinal absorption and metabolism of capsaicin and dihydrocapsaicin in rats. Toxicol. Appl. Pharmacol. 1984, 72, 449–456. [Google Scholar] [CrossRef]
- Surh, Y.J.; Lee, S.S. Capsaicin, a double-edged sword: Toxicity, metabolism, and chemopreventive potential. Life Sci. 1995, 56, 1845–1855. [Google Scholar] [CrossRef]
- Reilly, C.A.; Yost, G.S. Metabolism of capsaicinoids by P450 enzymes: A review of recent findings on reaction mechanisms, bio-activation, and detoxification processes. Drug Metabol. Rev. 2006, 38, 685–706. [Google Scholar] [CrossRef]
- Bernard, B.K.; Ubukata, K.; Mihara, R.; Sato, Y.; Nemoto, H. Studies of the toxicological potential of capsinoids, XI: Pharmacokinetic and tissue distribution study of 14C-dihydrocapsiate and metabolites in rats. Int. J. Toxicol. 2010, 29, 3S–14S. [Google Scholar] [CrossRef]
- Holzer, P.; Lippe, I.T. Stimulation of afferent nerve endings by intragastric capsaicin protects against ethanol-induced damaged of gastric mucosa. Neuroscience 1998, 27, 981–987. [Google Scholar] [CrossRef]
- Holzer, P. Capsaicin cellular targets. Mechanisms of action, as selectivity for thin sensory neurons. Pharmacol. Rev. 1999, 43, 143–201. [Google Scholar]
- Mózsik, G.; Rácz, I.; Szolcsányi, J. Gastroprotection induced by capsaicin in human healthy human subjects. World J. Gastroenterol. 2005, 11, 5180–5184. [Google Scholar] [PubMed]
- Mózsik, G.; Szolcsányi, J.; Dömötör, A. Capsaicin research as a tool to approach of the human gastrointestinal physiology, pathology and pharmacology. Inflammopharmacology 2007, 15, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Mózsik, G.; Past, T.; Abdel-Salam, O.M.E.; Kuzma, M.; Perjési, P. Interdisciplinary review for correlation between the plant origin capsaicinoids, non-steroidal antiinflammatory drugs, gastrointestinal mucosal damage and prevention in animals and human beings. Inflammopharmacology 2009, 17, 113–150. [Google Scholar] [CrossRef] [PubMed]
- Mózsik, G.; Past, T.; Dömötör, A.; Kuzma, M.; Perjési, P. Production of orally applicable new drug or drug combinations from natural origin capsaicinoids for human medical therapy. Curr. Pharm. Des. 2010, 16, 1197–1208. [Google Scholar] [CrossRef]
- Kuzma, M.; Fodor, K.; Maász, G.; Avar, P.; Mózsik, G.; Past, T.; Fischer, E.; Perjési, P. A validated HPLC-FLD method for analysis of intestinal absorption and metabolism of capsaicin and dihydrocapsaicin in the rat. J. Pharm. Biomed. Anal. 2015, 103, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Reilly, C.A.; Crouch, D.J.; Yost, G.S.; Fatah, A.A. Determination of capsaicin, nonivamide, and dihydrocapsaicin in blood and tissue by liquid chromatography-tandem mass spectrometry. J. Anal. Toxicol. 2002, 26, 313–319. [Google Scholar] [CrossRef]
- Thompson, R.Q.; Phinney, K.W.; Welch, M.J.; White, E. Quantitative determination of capsaicinoids by liquid chromatography-electrospray mass spectrometry. Anal. Bioanal. Chem. 2005, 381, 1441–1451. [Google Scholar] [CrossRef]
- Beaudry, F.; Vachon, P. Quantitative determination of capsaicin; a transient receptor potential channel vanilloid 1 agonist; by liquid chromatography quadrupole ion trap mass spectrometry: Evaluation of in vitro metabolic stability. Biomed. Chromatogr. 2009, 23, 204–211. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, J.; Sheng, L.; Li, Y. Simultaneous quantification of capsaicin and dihydrocapsaicin in rat plasma using HPLC coupled with tandem mass spectrometry. J. Chromatogr. B 2010, 878, 2292–2297. [Google Scholar] [CrossRef]
- Kuzma, M.; Fodor, K.; Boros, B.; Perjési, P. Development and validation of an HPLC-DAD analysis for pharmacopoeial qualification of industrial capsicum extracts. J. Chrom. Sci. 2015, 53, 16–23. [Google Scholar] [CrossRef] [PubMed][Green Version]
- (VIII 19.) EMMI Decree Which Corresponds to the OECD GLP Principles (ENV/MC/CHEM(98)17). 42/2014. Available online: https://net.jogtar.hu/jogszabaly?docid=A1400042.EMM (accessed on 1 July 2019).
- FDA 21 CFR, Part 58—Good Laboratory Practice for Nonclinical Laboratory Studies. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=58 (accessed on 1 July 2019).
- European Medicines Agency. Guideline on Repeated Dose Toxicity, CPMP/SWP/1042/99; European Medicines Agency: London, UK, 1999. [Google Scholar]
- Tiryaki, O.; Baysoyu, D.; Aydin, G.; Secer, E. Setting system suitability parameters for performance optimization of GC-NPD detection for pesticide residue analysis. Gazi Univ. J. Sci. 2009, 22, 149–155. [Google Scholar]
- Chen, X.H.; Franke, J.P.; Wijsbeek, J.; de Zeeuw, R.A. Isolation of acidic, neutral, and basic drugs from whole blood using a single mixed-mode solid-phase extraction column. J. Anal. Toxicol. 1992, 16, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Donnerer, J.; Amann, R.; Schuligoi, R.; Lembeck, F. Absorption and metabolism of capsaieinoids following intragastrie administration in rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1990, 342, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Huggins, R.A.; Deavers, S.; Smith, E.L. Growth in Beagles: Changes in body weight, plasma volume, and venous hematocrit. Pediatr. Res. 1971, 5, 193–198. [Google Scholar] [CrossRef][Green Version]
- Lippe, I.T.; Pabst, M.A.; Holzer, P. Intragastric capsaicin enhances rat gastric acid elimination and mucosal blood flow by afferent nerve stimulation. Br. J. Pharmacol. 1989, 96, 91–100. [Google Scholar] [CrossRef]
- Donnerer, J.; Lembeck, F. Capsaicin-induced reflex fall in rat blood pressure is mediated by afferent substance P-containing neurones via a reflex centre in the brain stem. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1983, 324, 293–295. [Google Scholar] [CrossRef]
- Suresh, D.; Sirinivasan, K. Studies on the in vitro absorption of spice principles—Curcumin, capsaicin and piperine in rat intestines. Food Chem. Toxicol. 2007, 45, 1437–1442. [Google Scholar] [CrossRef]
- Chanda, S.; Bashir, M.; Babbar, S.; Koganti, A.; Bley, K. In vitro hepatic and skin metabolism of capsaicin. Drug Metabol. Dispos. 2008, 36, 670–675. [Google Scholar] [CrossRef]
- Park, Y.H.; Lee, S.S. Identification and characterization of capsaicin-hydrolyzing enzymes purified from rat liver microsomes. Biochem. Mol. Biol. Int. 1994, 34, 351–360. [Google Scholar] [PubMed]
- Kawada, T.; Iwai, K. In vivo and in vitro metabolism of dihydrocapsaicin, a pungent principle of hot pepper, in rats. Agric. Biol. Chem. 1985, 49, 441–448. [Google Scholar]
- Marvola, M.; Hannula, A.-M.; Westermarck, E.; Happonen, I.; Kopra, T. Disintegration of hard gelatin capsule formulations in the dog stomach—A radiological study. Int. J. Pharm. 1988, 44, 159–167. [Google Scholar] [CrossRef]
- Chaiyasit, K.; Khovidhunkit, W.; Wittayalertpanya, S. Pharmacokinetic and the effect of capsaicin in Capsicum frutescens on decreasing plasma glucose level. J. Med. Assoc. Thai. 2009, 92, 108–113. [Google Scholar] [PubMed]
- Pannonpharma KFT; Szolcsányi, J.; Mózsik, G.; Perjési, P.; Past, T. Compositions Containing Capsaicinoids. European Patent No. 2219610, 1 May 2019. (WO 2009/068922) 04.06.2009 Gazette 2009/23. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |



| Injections (Standard Solutions) | CADB | Capsaicin RS | Dihydrocapsaicin RS | |||
|---|---|---|---|---|---|---|
| tR(min) | Area | tR(min) | Area | tR(min) | Area | |
| 1 | 3.766 | 284.117 | 10.374 | 202.757 | 15.571 | 190.972 |
| 2 | 3.769 | 284.072 | 10.382 | 200.816 | 15.586 | 191.821 |
| 3 | 3.769 | 284.350 | 10.391 | 201.201 | 15.598 | 189.120 |
| 4 | 3.769 | 285.569 | 10.392 | 200.070 | 15.596 | 190.495 |
| 5 | 3.774 | 283.781 | 10.397 | 201.964 | 15.597 | 190.939 |
| 6 | 3.769 | 283.805 | 10.390 | 200.689 | 15.599 | 189.844 |
| Mean | 3.769 | 284.282 | 10.388 | 201.250 | 15.591 | 190.532 |
| RSD% | 0.068 | 0.234 | 0.080 | 0.481 | 0.070 | 0.497 |
| Compounds | tR(min) | RRT | k′ | T | N | Rs |
| CADB | 3.769 | - | 2.91 | 1.29 | 1109 | - |
| Capsaicin | 10.388 | 2.76 | 9.79 | 1.26 | 1946 | 9.48 |
| Dihydrocapsaicin | 15.591 | 4.14 | 15.19 | 1.20 | 2822 | 4.91 |
| Injections (Standard Solutions) | CADB | Capsaicin RS | Dihydrocapsaicin RS | |||
|---|---|---|---|---|---|---|
| tR(min) | Area | tR(min) | Area | tR(min) | Area | |
| 1 | 3.788 | 286.467 | 10.364 | 285.920 | 15.534 | 168.890 |
| 2 | 3.790 | 286.425 | 10.371 | 285.133 | 15.551 | 170.850 |
| 3 | 3.799 | 286.970 | 10.395 | 285.046 | 15.595 | 172.887 |
| 4 | 3.796 | 287.513 | 10.390 | 287.439 | 15.570 | 172.632 |
| 5 | 3.799 | 287.942 | 10.400 | 287.673 | 15.608 | 172.359 |
| 6 | 3.798 | 286.939 | 10.403 | 288.441 | 15.599 | 172.231 |
| Mean | 3.795 | 287.043 | 10.387 | 286.609 | 15.576 | 171.642 |
| RSD% | 0.127 | 0.207 | 0.154 | 0.500 | 0.189 | 0.887 |
| Compounds | tR(min) | RRT | k′ | T | N | Rs |
| CADB | 3.795 | - | 3.10 | 1.49 | 1453 | - |
| Capsaicin | 10.387 | 2.74 | 10.23 | 1.44 | 2486 | 10.69 |
| Dihydrocapsaicin | 15.576 | 4.10 | 15.84 | 1.25 | 3121 | 5.32 |
| CADB | Capsaicin RS | Dihydrocapsaicin RS | ||||||
|---|---|---|---|---|---|---|---|---|
| cspiked plasma (ng/mL) | Area | Recovery % | cspiked plasma (ng/mL) | Area | Recovery % | cspiked plasma (ng/mL) | Area | Recovery % |
| 20 | 191.35 | 67.11 | 10 | 77.42 | 74.84 | 10 | 92.86 | 91.73 |
| 20 | 218.95 | 76.75 | 10 | 86.85 | 83.91 | 10 | 97.98 | 96.64 |
| 20 | 186.92 | 65.56 | 10 | 84.02 | 81.19 | 10 | 76.49 | 76.03 |
| 20 | 222.89 | 78.12 | 10 | 81.84 | 79.09 | 10 | 89.01 | 88.04 |
| 20 | 211.78 | 74.24 | 20 | 171.74 | 82.79 | 20 | 183.61 | 89.39 |
| 20 | 190.08 | 66.66 | 20 | 159.88 | 77.08 | 20 | 180.82 | 88.05 |
| 20 | 166.62 | 58.47 | 20 | 134.45 | 64.85 | 20 | 152.89 | 74.66 |
| 20 | 191.31 | 67.09 | 20 | 130.54 | 62.97 | 20 | 157.25 | 76.75 |
| 20 | 184.59 | 64.74 | 40 | 348.6 | 83.93 | 40 | 368.06 | 88.93 |
| 20 | 216.36 | 75.84 | 40 | 354.28 | 85.29 | 40 | 367.99 | 88.91 |
| 20 | 192.02 | 67.34 | 40 | 353.13 | 85.02 | 40 | 352.48 | 85.19 |
| 20 | 177.84 | 62.39 | 40 | 344.44 | 82.93 | 40 | 349.48 | 84.47 |
| Mean recovery % | 68.69 | 78.52 | 86.30 | |||||
| RSD % | 8.956 | 9.888 | 8.101 | |||||
| Weighting/Dilution (Standard Solution) | CADB | Capsaicin RS | Dihydrocapsaicin RS | |||
|---|---|---|---|---|---|---|
| c (ng/ml) | Area | c (ng/ml) | Area | c (ng/ml) | Area | |
| 1/1 | 200 | 281.645 | 200 | 206.907 | 200 | 209.446 |
| 1/2 | 200 | 282.931 | 200 | 208.611 | 200 | 209.324 |
| 1/3 | 200 | 284.115 | 200 | 207.578 | 200 | 209.066 |
| 2/1 | 200 | 297.110 | 200 | 219.010 | 200 | 210.680 |
| 2/2 | 200 | 298.515 | 200 | 220.277 | 200 | 211.036 |
| 2/3 | 200 | 299.517 | 200 | 218.821 | 200 | 211.225 |
| Mean | 290.639 | 213.534 | 210.130 | |||
| RSD % | 2.942 | 3.013 | 0.455 | |||
| Day | Dilution (Standard Solution) | CADB | Capsaicin RS | Dihydrocapsaicin RS |
|---|---|---|---|---|
| Area | Area | Area | ||
| 1 | 1. | 282.931 | 208.611 | 209.324 |
| 2. | 284.115 | 207.578 | 209.066 | |
| 3. | 298.515 | 220.277 | 211.036 | |
| 2 | 1. | 280.698 | 207.174 | 207.810 |
| 2. | 281.373 | 206.958 | 206.943 | |
| 3. | 295.956 | 217.797 | 210.953 | |
| 3 | 1. | 284.195 | 211.165 | 209.74 |
| 2. | 286.657 | 210.898 | 210.037 | |
| 3. | 299.469 | 221.129 | 215.672 | |
| Mean | 288.212 | 212.399 | 210.065 | |
| RSD % | 2.629 | 2.713 | 1.186 | |
| CADB | Capsaicin RS | Dihydrocapsaicin RS | |||
|---|---|---|---|---|---|
| c (ng/mL) | Area | c (ng/mL) | Area | c (ng/mL) | Area |
| 200 | 309.861 | 20 | 17.580 | 20 | 18.341 |
| 200 | 309.816 | 20 | 18.680 | 20 | 18.181 |
| 200 | 307.434 | 20 | 17.852 | 20 | 18.770 |
| 200 | 310.107 | 20 | 18.329 | 20 | 18.086 |
| 200 | 304.773 | 100 | 93.517 | 100 | 95.614 |
| 200 | 310.350 | 100 | 93.559 | 100 | 96.691 |
| 200 | 319.699 | 100 | 96.824 | 100 | 98.475 |
| 200 | 313.438 | 100 | 93.435 | 100 | 98.698 |
| 200 | 309.791 | 200 | 181.260 | 200 | 193.176 |
| 200 | 310.974 | 200 | 193.205 | 200 | 211.009 |
| 200 | 309.756 | 200 | 190.162 | 200 | 203.587 |
| 200 | 315.218 | 200 | 187.609 | 200 | 205.103 |
| 200 | 320.154 | 400 | 390.450 | 400 | 384.022 |
| 200 | 311.768 | 400 | 380.979 | 400 | 387.807 |
| 200 | 329.947 | 400 | 393.093 | 400 | 404.482 |
| 200 | 334.889 | 400 | 406.833 | 400 | 402.899 |
| Compounds | c (ng/mL) | Fcalculated | Fcritical |
|---|---|---|---|
| CADB | 200 | 4.3096 | 4.4139 |
| Capsaicin RS | 20 | 4.0824 | 5.9874 |
| Capsaicin RS | 100 | 4.7856 | 5.9874 |
| Capsaicin RS | 200 | 2.8410 | 5.9874 |
| Capsaicin RS | 400 | 0.0702 | 5.9874 |
| Dihydrocapsaicin RS | 20 | 0.3378 | 5.9874 |
| Dihydrocapsaicin RS | 100 | 0.7692 | 5.9874 |
| Dihydrocapsaicin RS | 200 | 0.2914 | 5.9874 |
| Dihydrocapsaicin RS | 400 | 3.0580 | 5.9874 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuzma, M.; Fodor, K.; Almási, A.; Mózsik, G.; Past, T.; Perjési, P. Toxicokinetic Study of a Gastroprotective Dose of Capsaicin by HPLC-FLD Method. Molecules 2019, 24, 2848. https://doi.org/10.3390/molecules24152848
Kuzma M, Fodor K, Almási A, Mózsik G, Past T, Perjési P. Toxicokinetic Study of a Gastroprotective Dose of Capsaicin by HPLC-FLD Method. Molecules. 2019; 24(15):2848. https://doi.org/10.3390/molecules24152848
Chicago/Turabian StyleKuzma, Mónika, Krisztina Fodor, Attila Almási, Gyula Mózsik, Tibor Past, and Pál Perjési. 2019. "Toxicokinetic Study of a Gastroprotective Dose of Capsaicin by HPLC-FLD Method" Molecules 24, no. 15: 2848. https://doi.org/10.3390/molecules24152848
APA StyleKuzma, M., Fodor, K., Almási, A., Mózsik, G., Past, T., & Perjési, P. (2019). Toxicokinetic Study of a Gastroprotective Dose of Capsaicin by HPLC-FLD Method. Molecules, 24(15), 2848. https://doi.org/10.3390/molecules24152848