A Novel Self-Emulsifying Drug Delivery System (SEDDS) Based on VESIsorb® Formulation Technology Improving the Oral Bioavailability of Cannabidiol in Healthy Subjects
Abstract
1. Introduction
2. Results
2.1. Subject Characteristics
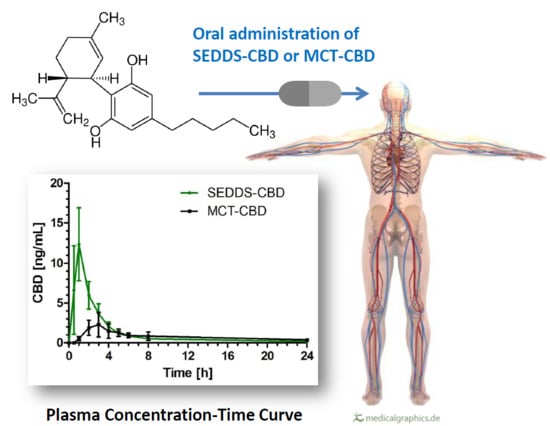
2.2. CBD Plasma Concentration Time Profile
2.3. Pharmacokinetic Parameters
2.4. Effects of Gender on CBD Pharmacokinetics
2.5. Safety Assessment
3. Discussion
4. Materials and Methods
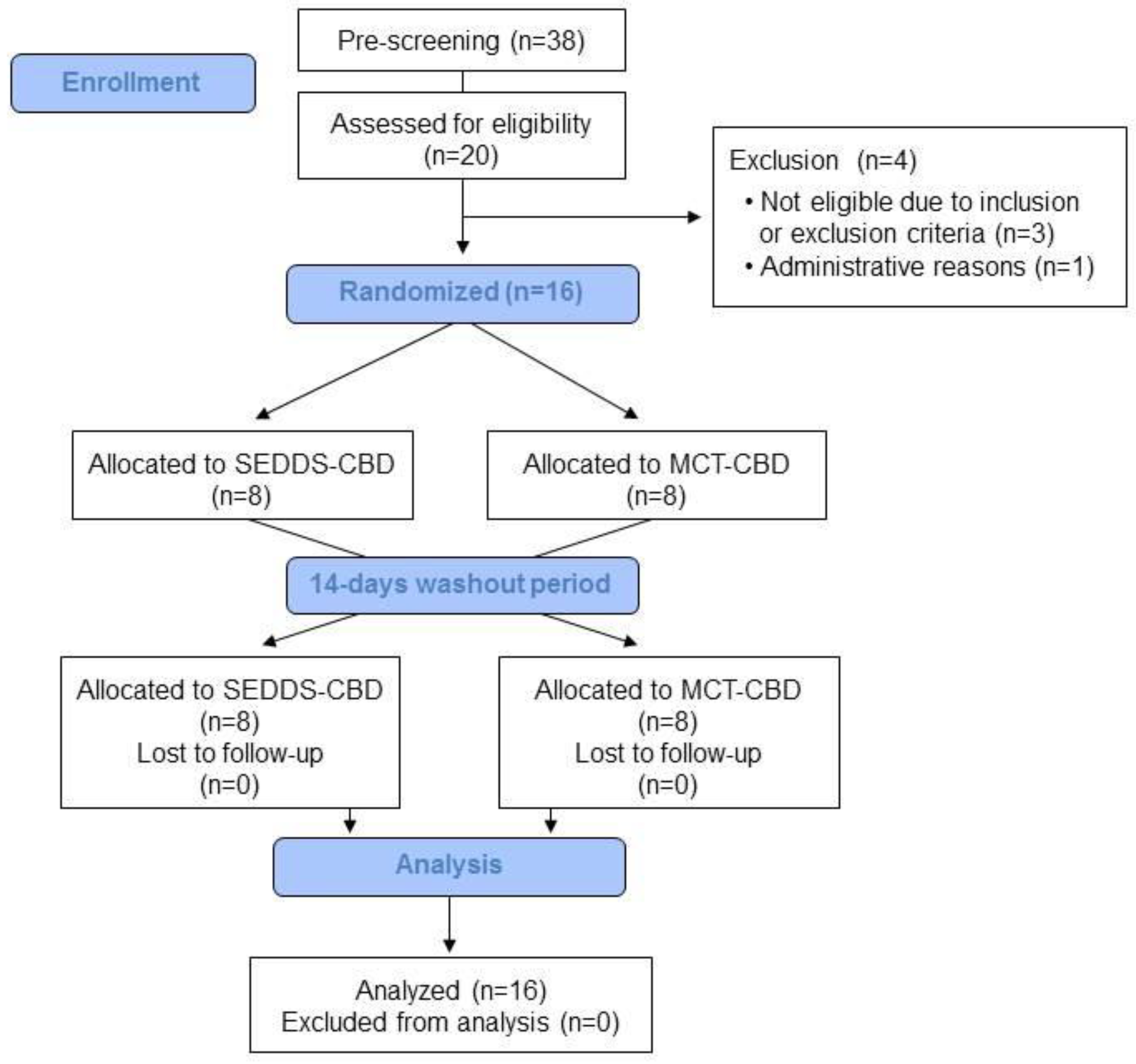
4.1. Study Subjects
4.2. Study Design
4.3. Intervention
4.4. Sample Analysis
4.5. Analysis Software and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Izzo, A.A.; Borrelli, F.; Capasso, R.; Di Marzo, V.; Mechoulam, R. Non-psychotropic plant cannabinoids: New therapeutic opportunities from an ancient herb. Trends Pharmacol. Sci. 2009, 30, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Atsmon, J.; Heffetz, D.; Deutsch, L.; Deutsch, F.; Sacks, H. Single-Dose Pharmacokinetics of Oral Cannabidiol Following Administration of PTL101: A New Formulation Based on Gelatin Matrix Pellets Technology. Clin. Pharmacol Drug Dev. 2018, 7, 751–758. [Google Scholar] [CrossRef] [PubMed]
- McCarberg, B.H.; Barkin, R.L. The future of cannabinoids as analgesic agents: A pharmacologic, pharmacokinetic, and pharmacodynamic overview. Am. J. Ther. 2007, 14, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Malfait, A.M.; Gallily, R.; Sumariwalla, P.F.; Malik, A.S.; Andreakos, E.; Mechoulam, R.; Feldmann, M. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc. Natl. Acad. Sci. USA 2000, 97, 9561–9566. [Google Scholar] [CrossRef] [PubMed]
- Hampson, A.J.; Grimaldi, M.; Axelrod, J.; Wink, D. Cannabidiol and (−) Delta9-tetrahydrocannabinol are neuroprotective antioxidants. Proc. Natl. Acad. Sci. USA 1998, 95, 8268–8273. [Google Scholar] [CrossRef] [PubMed]
- Karst, M.; Salim, K.; Burstein, S.; Conrad, I.; Hoy, L.; Schneider, U. Analgesic effect of the synthetic cannabinoid CT-3 on chronic neuropathic pain: A randomized controlled trial. JAMA 2003, 290, 1757–1762. [Google Scholar] [CrossRef]
- Devinsky, O.; Cilio, M.R.; Cross, H.; Fernandez-Ruiz, J.; French, J.; Hill, C.; Katz, R.; Di Marzo, V.; Jutras-Aswad, D.; Notcutt, W.G.; et al. Cannabidiol: Pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia 2014, 55, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, M.M.; Queiroz, R.H.C.; Zuardi, A.W.; Crippa, J.A.S. Safety and side effects of cannabidiol, a Cannabis sativa constituent. Curr. Drug Saf. 2011, 6, 237–249. [Google Scholar] [CrossRef]
- WHO. Expert Peer Review for Cannabidiol (CBD). In Agenda Item 5.2; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Grotenhermen, F.; Russo, E.; Zuardi, A.W. Even High Doses of Oral Cannabidol Do Not Cause THC-Like Effects in Humans: Comment on Merrick et al. Cannabis and Cannabinoid Research 2016;1(1):102-112; DOI: 10.1089/can.2015.0004. Cannabis. Cannabinoid. Res. 2017, 2, 1–4. [Google Scholar] [CrossRef]
- Shiplo, S.; Asbridge, M.; Leatherdale, S.T.; Hammond, D. Medical cannabis use in Canada: Vapourization and modes of delivery. Harm Reduct. J. 2016, 13, 30. [Google Scholar] [CrossRef]
- Vandrey, R.; Herrmann, E.S.; Mitchell, J.M.; Bigelow, G.E.; Flegel, R.; LoDico, C.; Cone, E.J. Pharmacokinetic Profile of Oral Cannabis in Humans: Blood and Oral Fluid Disposition and Relation to Pharmacodynamic Outcomes. J. Anal. Toxicol. 2017, 41, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Hawksworth, G.; McArdle, K. Metabolism and pharmacokinetics of cannabinoids. In The Medicinal Uses of Cannabis and Cannabinoids; Pharmaceutical Press: London, UK, 2004; pp. 205–228. [Google Scholar]
- Ohlsson, A.; Lindgren, J.E.; Andersson, S.; Agurell, S.; Gillespie, H.; Hollister, L.E. Single-dose kinetics of deuterium-labelled cannabidiol in man after smoking and intravenous administration. Biomed. Environ. Mass Spectrom. 1986, 13, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Millar, S.A.; Stone, N.L.; Yates, A.S.; O’Sullivan, S.E. A Systematic Review on the Pharmacokinetics of Cannabidiol in Humans. Front. Pharmacol. 2018, 9, 1365. [Google Scholar] [CrossRef] [PubMed]
- Stott, C.G.; White, L.; Wright, S.; Wilbraham, D.; Guy, G.W. A phase I study to assess the effect of food on the single dose bioavailability of the THC/CBD oromucosal spray. Eur. J. Clin. Pharmacol. 2013, 69, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Karschner, E.L.; Darwin, W.D.; Goodwin, R.S.; Wright, S.; Huestis, M.A. Plasma cannabinoid pharmacokinetics following controlled oral delta9-tetrahydrocannabinol and oromucosal cannabis extract administration. Clin. Chem. 2011, 57, 66–75. [Google Scholar] [CrossRef] [PubMed]
- McGilveray, I.J. Pharmacokinetics of cannabinoids. Pain Res Manag. 2005, 10, 15A–22A. [Google Scholar] [CrossRef]
- Ujváry, I.; Hanuš, L. Human Metabolites of Cannabidiol: A Review on Their Formation, Biological Activity, and Relevance in Therapy. Cannabis Cannabinoid Res. 2016, 1, 90–101. [Google Scholar] [CrossRef]
- Iversen, L.L. The Science of Marijuana, 2nd ed.; Oxford University Press: Oxford, UK, 2008. [Google Scholar]
- Wang, T.; Collet, J.P.; Shapiro, S.; Ware, M.A. Adverse effects of medical cannabinoids: A systematic review. CMAJ 2008, 178, 1669–1678. [Google Scholar] [CrossRef]
- Atsmon, J.; Cherniakov, I.; Izgelov, D.; Hoffman, A.; Domb, A.J.; Deutsch, L.; Deutsch, F.; Heffetz, D.; Sacks, H. PTL401, a New Formulation Based on Pro-Nano Dispersion Technology, Improves Oral Cannabinoids Bioavailability in Healthy Volunteers. J. Pharm. Sci. 2018, 107, 1423–1429. [Google Scholar] [CrossRef]
- Cherniakov, I.; Izgelov, D.; Barasch, D.; Davidson, E.; Domb, A.J.; Hoffman, A. Piperine-pro-nanolipospheres as a novel oral delivery system of cannabinoids: Pharmacokinetic evaluation in healthy volunteers in comparison to buccal spray administration. J. Control. Release 2017, 266, 1–7. [Google Scholar] [CrossRef]
- Zgair, A.; Wong, J.C.; Lee, J.; Mistry, J.; Sivak, O.; Wasan, K.M.; Hennig, I.M.; Barrett, D.A.; Constantinescu, C.S.; Fischer, P.M.; et al. Dietary fats and pharmaceutical lipid excipients increase systemic exposure to orally administered cannabis and cannabis-based medicines. Am. J. Transl. Res. 2016, 8, 3448–3459. [Google Scholar] [PubMed]
- Singh, B.; Bandopadhyay, S.; Kapil, R.; Singh, R.; Katare, O.P. Self-emulsifying drug delivery systems (SEDDS): Formulation development, characterization, and applications. Crit. Rev. Ther. Drug Carr. Syst. 2009, 26, 427–521. [Google Scholar] [CrossRef]
- Nigade, P.M.; Patil, S.L.; Tiwari, S.S. Self emulsifying drug delivery system (SEDDS): A Review. IJPBS 2012, 2, 42–52. [Google Scholar]
- Gursoy, R.N.; Benita, S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed. Pharmacother. 2004, 58, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Dokania, S.; Joshi, A.K. Self-microemulsifying drug delivery system (SMEDDS)--challenges and road ahead. Drug Deliv. 2015, 22, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.X.; Artmann, C. Relative bioavailability comparison of different coenzyme Q10 formulations with a novel delivery system. Altern. Ther. Health Med. 2009, 15, 42–46. [Google Scholar] [PubMed]
- Mechoulam, R.; Parker, L.A.; Gallily, R. Cannabidiol: An overview of some pharmacological aspects. J. Clin. Pharmacol. 2002, 42, 11S–19S. [Google Scholar] [CrossRef]
- Huestis, M.A. Human cannabinoid pharmacokinetics. Chem. Biodivers. 2007, 4, 1770–1804. [Google Scholar] [CrossRef]
- Stott, C.G.; White, L.; Wright, S.; Wilbraham, D.; Guy, G.W. A phase I study to assess the single and multiple dose pharmacokinetics of THC/CBD oromucosal spray. Eur. J. Clin. Pharmacol. 2013, 69, 1135–1147. [Google Scholar] [CrossRef]
- FDA. EPIDIOLEX (Cannabidiol) Oral Solution 2018; Food and Drug Administration: Carlsbad, CA, USA, 2018. [Google Scholar]
- Waxman, D.J.; Holloway, M.G. Sex differences in the expression of hepatic drug metabolizing enzymes. Mol. Pharmacol. 2009, 76, 215–228. [Google Scholar] [CrossRef]
- Anderson, G.D. Sex and racial differences in pharmacological response: Where is the evidence? Pharmacogenetics, pharmacokinetics, and pharmacodynamics. J. Womens. Health 2005, 14, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Lane, H.Y.; Chang, Y.C.; Chang, W.H.; Lin, S.K.; Tseng, Y.T.; Jann, M.W. Effects of gender and age on plasma levels of clozapine and its metabolites: Analyzed by critical statistics. J. Clin. Psychiatry 1999, 60, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Freire, A.C.; Basit, A.W.; Choudhary, R.; Piong, C.W.; Merchant, H.A. Does sex matter? The influence of gender on gastrointestinal physiology and drug delivery. Int. J. Pharm. 2011, 415, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, M.; Aweeka, F.; Greenblatt, R.M.; Blaschke, T.F. Sex differences in pharmacokinetics and pharmacodynamics. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 499–523. [Google Scholar] [CrossRef] [PubMed]
- Vahl, N.; Moller, N.; Lauritzen, T.; Christiansen, J.S.; Jorgensen, J.O. Metabolic effects and pharmacokinetics of a growth hormone pulse in healthy adults: Relation to age, sex, and body composition. J. Clin. Endocrinol. Metab. 1997, 82, 3612–3618. [Google Scholar] [CrossRef] [PubMed]
- Berg, U.B. Differences in decline in GFR with age between males and females. Reference data on clearances of inulin and PAH in potential kidney donors. Nephrol. Dial. Transplant. 2006, 21, 2577–2582. [Google Scholar] [CrossRef] [PubMed]
- Nadulski, T.; Pragst, F.; Weinberg, G.; Roser, P.; Schnelle, M.; Fronk, E.-M.; Stadelmann, A.M. Randomized, double-blind, placebo-controlled study about the effects of cannabidiol (CBD) on the pharmacokinetics of Delta9-tetrahydrocannabinol (THC) after oral application of THC verses standardized cannabis extract. Ther. Drug Monit. 2005, 27, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Fadiran, E.O.; Zhang, L. Effects of sex differences in the pharmacokinetics of drugs and their impact on the safety of medicines in women. In Medicines for Women; Springer International Publishing: Basel Switzerland, 2015; pp. 41–68. [Google Scholar]
Sample Availability: Samples of the compounds SEDDS-CBD or MCT-CBD are not available from the authors. |


| Variable | Men | Women | ||
|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | |
| Age (years) | 27.8 | (23.7–31.8) | 27.9 | (26.4–29.3) |
| BMI (kg/m2) | 25.0 | (23.4–26.5) | 21.3 | (19.1–23.4) |
| Systolic BP (mmHg) | 129.1 | (121.5–136.7) | 118.6 | (109.1–128.1) |
| Diastolic BP (mmHg) | 76.9 | (73.2–80.5) | 73.1 | (65.5–80.7) |
| Hemoglobin (g/dL) | 15.3 | (14.1–16.1) | 13.0 | (12.3–13.6) |
| Total Cholesterol (mg/dL) | 171.8 | (148.0–195.5) | 180.3 | (156.5–204.0) |
| Subjects | Pharmacokinetic Parameters | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cmax [ng/mL] | AUC0-8h [ng/mL*h] | AUC0-24h [ng/mL*h] | Tmax [h] | |||||||||
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Median (25th–75th percentile) | |||||||||
| MCT-CBD | SEDDS-CBD | p | MCT-CBD | SEDDS-CBD | p | MCT-CBD | SEDDS-CBD | p | MCT-CBD | SEDDS-CBD | p | |
| All (n = 16) | 3.05 (1.57–4.54) | 13.53 (7.96–19.10) | <0.0001 | 9.51 (5.73–13.30) | 27.15 (18.68–35.63) | <0.0001 | 19.23 (13.03–25.42) | 32.63 (23.18–42.08) | 0.0021 | 3.0 (2.0–5.0) | 1.0 (1.0–1.0) | 0.0007 |
| Men (n = 8) | 1.93 (0.89–2.96) | 13.75 (2.83–24.68) | 0.0013 | 5.54 (3.87–7.22) | 24.86 (8.05–41.66) | 0.0005 | 15.10 (4.51–25.70) | 28.95 (10.68–47.22) | 0.0112 | 4.0 (2.0–5.0) | 1.0 (0.5–1.0) | 0.0202 |
| Women (n = 8) | 4.18 (1.25–7.11) | 13.32 (6.66–19.97) | 0.0033 | 13.48 (6.59–20.38) | 29.44 (20.06–38.82) | 0.0095 | 23.35 (15.40–31.29) | 36.31 (25.56–47.06) | 0.0042 | 2.5 (2.0–3.0) | 1.0 (1.0–1.8) | 0.0187 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knaub, K.; Sartorius, T.; Dharsono, T.; Wacker, R.; Wilhelm, M.; Schön, C. A Novel Self-Emulsifying Drug Delivery System (SEDDS) Based on VESIsorb® Formulation Technology Improving the Oral Bioavailability of Cannabidiol in Healthy Subjects. Molecules 2019, 24, 2967. https://doi.org/10.3390/molecules24162967
Knaub K, Sartorius T, Dharsono T, Wacker R, Wilhelm M, Schön C. A Novel Self-Emulsifying Drug Delivery System (SEDDS) Based on VESIsorb® Formulation Technology Improving the Oral Bioavailability of Cannabidiol in Healthy Subjects. Molecules. 2019; 24(16):2967. https://doi.org/10.3390/molecules24162967
Chicago/Turabian StyleKnaub, Katharina, Tina Sartorius, Tanita Dharsono, Roland Wacker, Manfred Wilhelm, and Christiane Schön. 2019. "A Novel Self-Emulsifying Drug Delivery System (SEDDS) Based on VESIsorb® Formulation Technology Improving the Oral Bioavailability of Cannabidiol in Healthy Subjects" Molecules 24, no. 16: 2967. https://doi.org/10.3390/molecules24162967
APA StyleKnaub, K., Sartorius, T., Dharsono, T., Wacker, R., Wilhelm, M., & Schön, C. (2019). A Novel Self-Emulsifying Drug Delivery System (SEDDS) Based on VESIsorb® Formulation Technology Improving the Oral Bioavailability of Cannabidiol in Healthy Subjects. Molecules, 24(16), 2967. https://doi.org/10.3390/molecules24162967