Formation of Nanocomplexes between Carboxymethyl Inulin and Bovine Serum Albumin via pH-Induced Electrostatic Interaction
Abstract
1. Introduction
2. Results
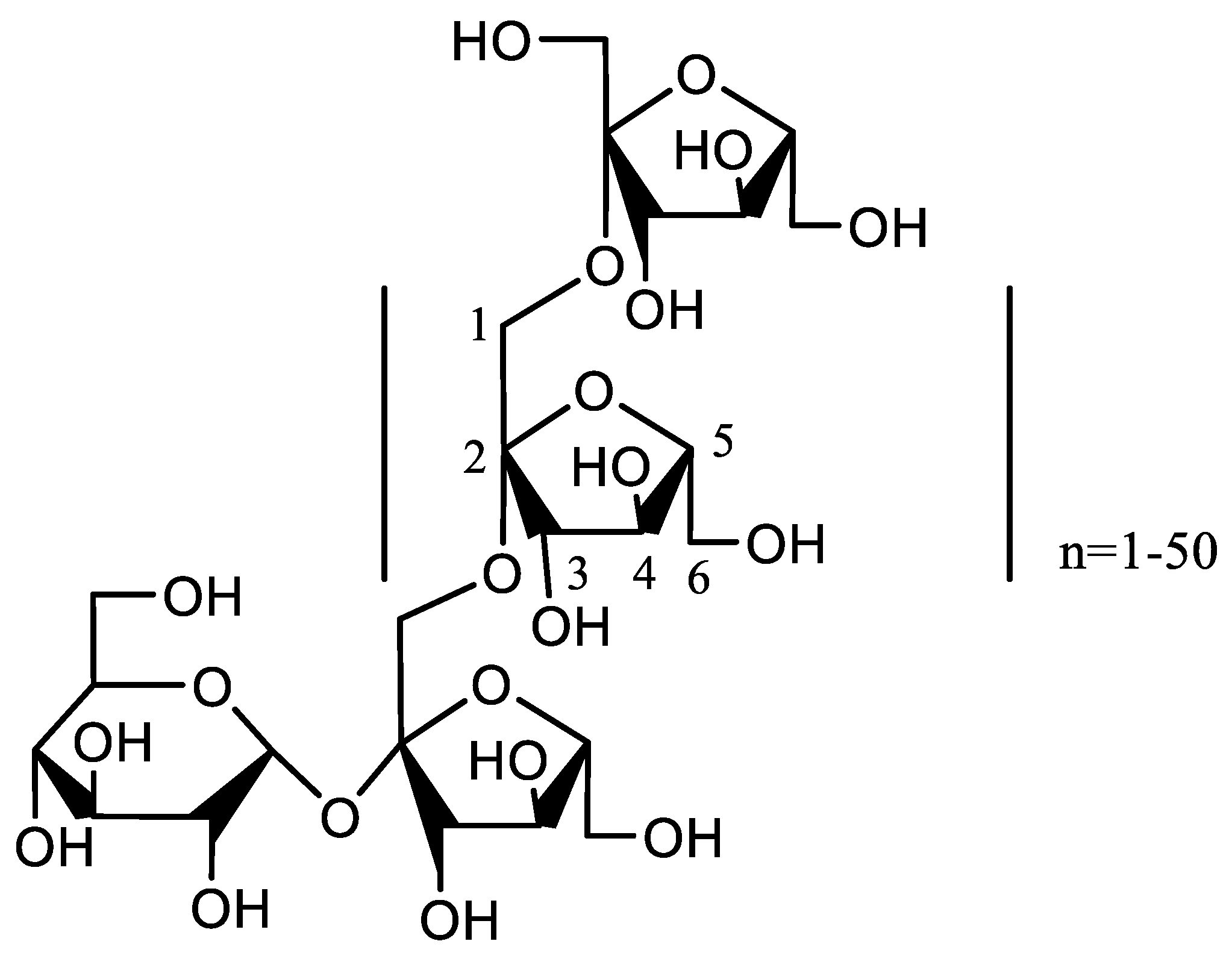
2.1. Characteristics of CMI
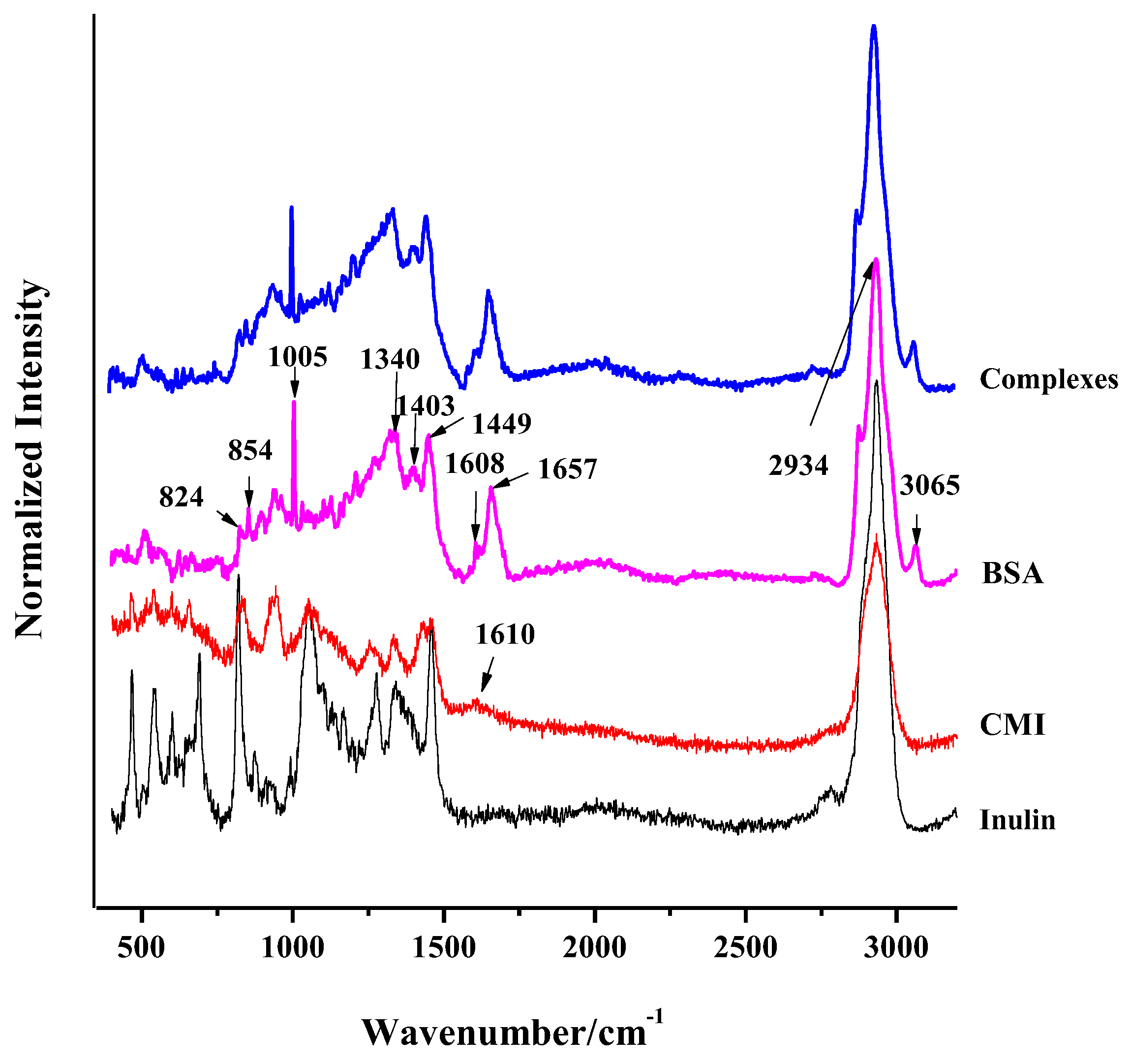
2.1.1. FT-IR Spectra of CMI
2.1.2. Raman Spectra of CMI
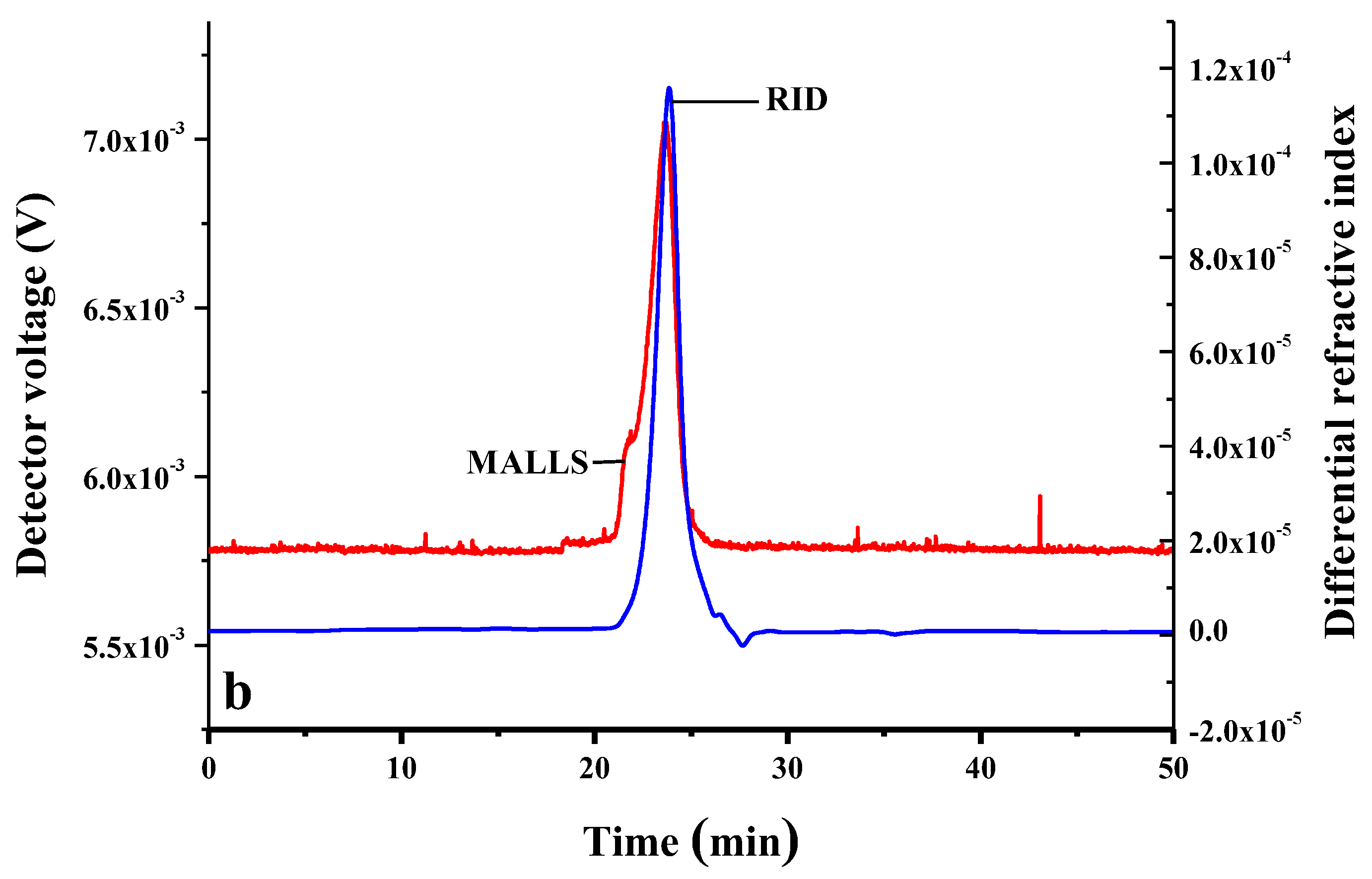
2.1.3. Degree of Substitution (DS) and Molecular Weight (Mw) of CMI
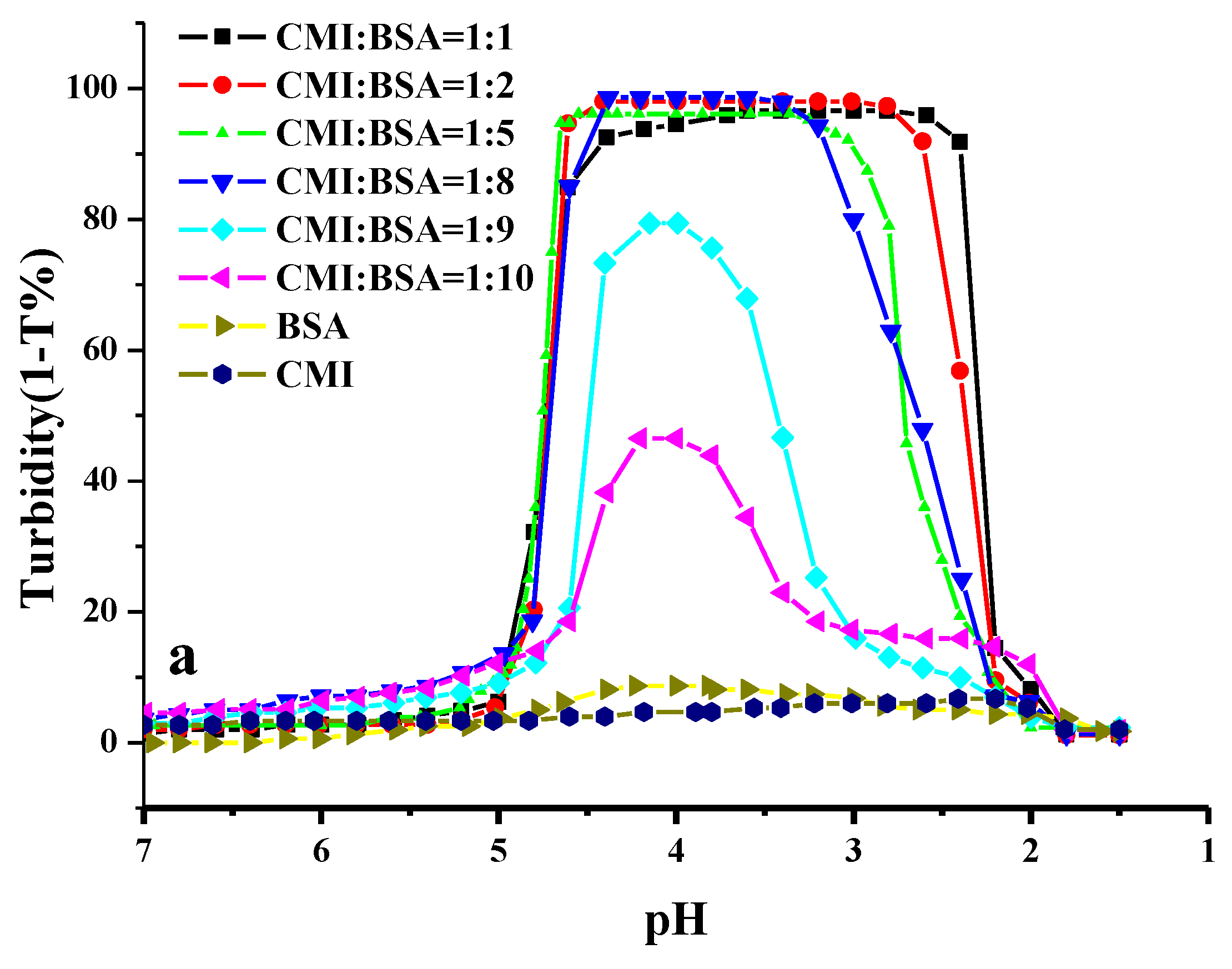
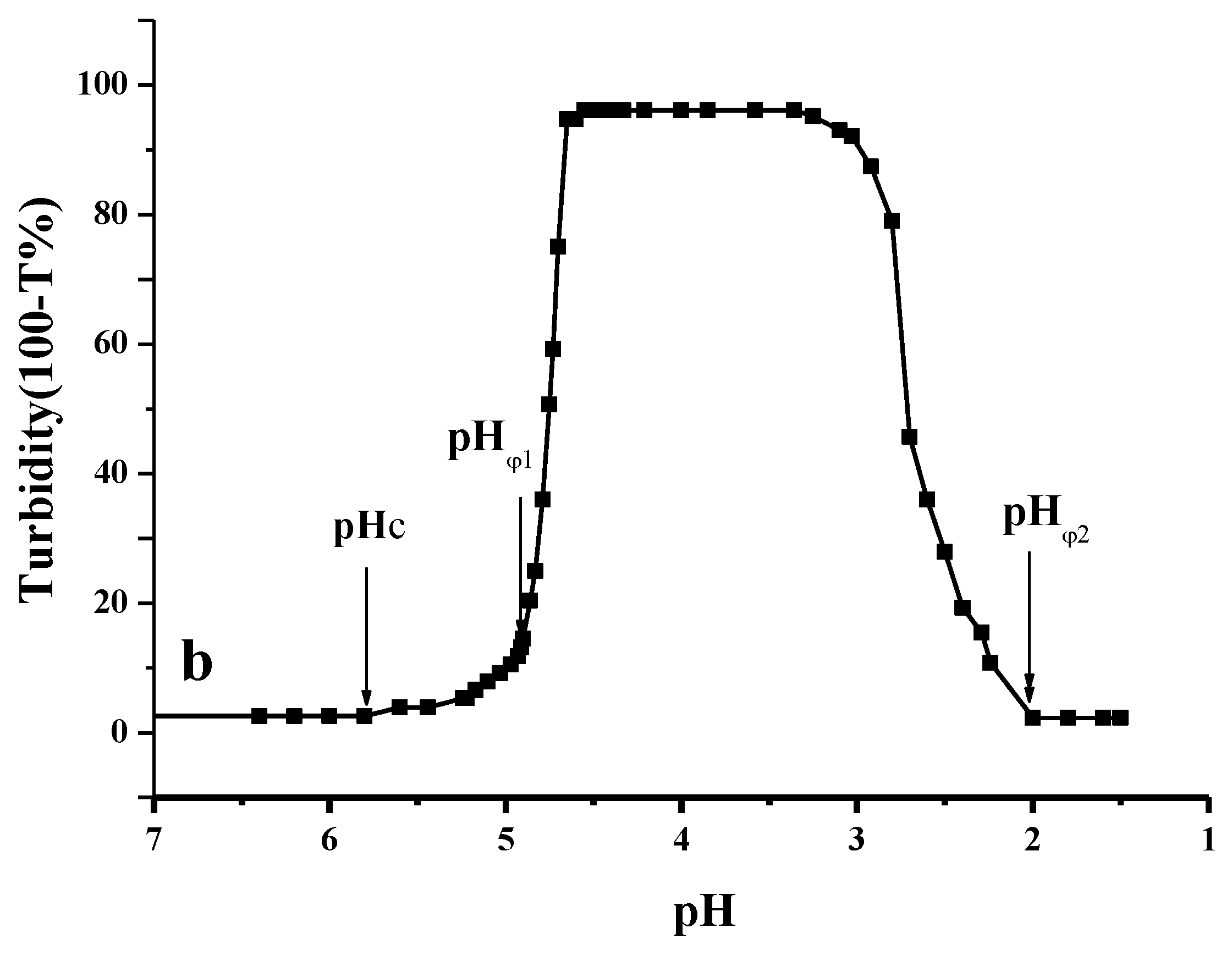
2.2. Effect of pH on the Formation of CMI/BSA Complexes
2.3. Effect of Ionic Strength on the Formation of CMI/BSA Complexes
2.4. Characteristics of CMI/BSA Complexes
2.4.1. FT-IR Spectra of CMI/BSA Complexes
2.4.2. Raman Spectra of CMI
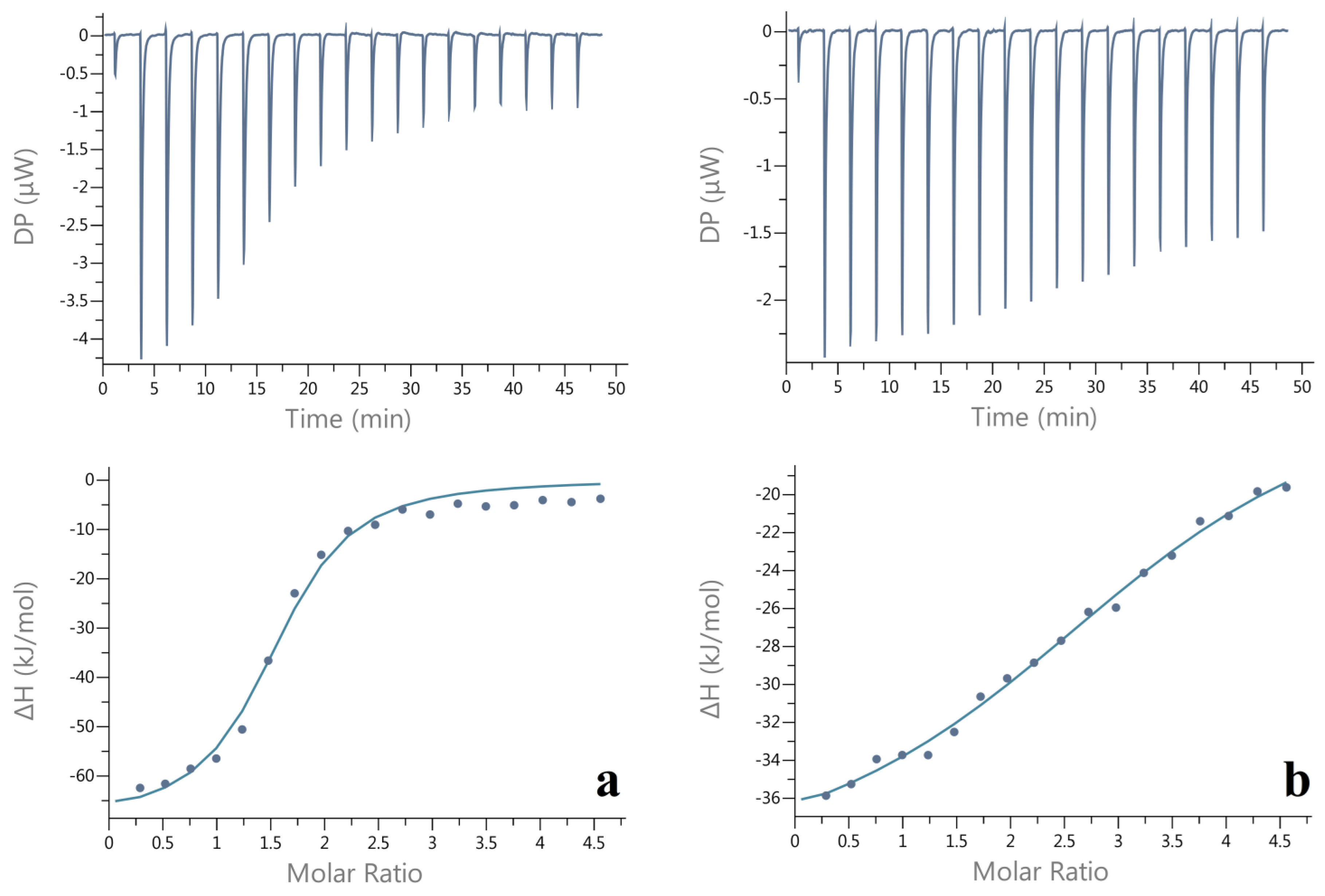
2.5. Isothermal Titration Calorimetry (ITC) of CMI/BSA Interaction
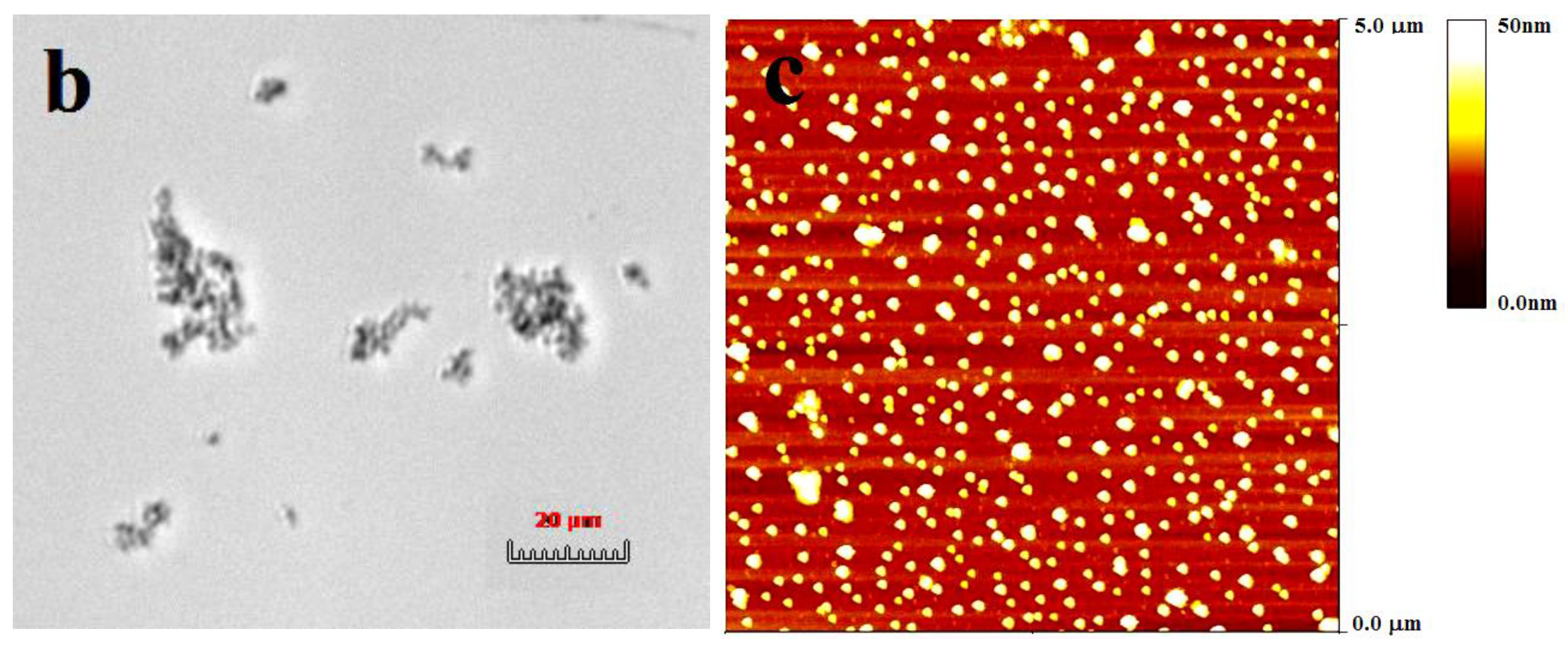
2.6. Morphology of CMI/BSA Complexes
3. Experimental Section
3.1. Materials
3.2. Synthesis of CMI
3.3. Characterization of CMI
3.4. Preparation of CMI and BSA Mixed Solutions
3.5. Turbidimetric Measurements
3.6. Isothermal Titration Calorimetry (ITC) Analysis
3.7. Measurements of the Sizes of Complexes
3.8. ζ-Potential Measurements
3.9. Morphology and Visual Observation of CMI/BSA Complexes
3.9.1. Visual Observation of CMI/BSA Complexes
3.9.2. Morphology of Insoluble Complexes
3.9.3. Morphologies of Soluble Complexes
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2017, 109, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Eghbal, N.; Choudhary, R. Complex coacervation: Encapsulation and controlled release of active agents in food systems. LWT 2018, 90, 254–264. [Google Scholar] [CrossRef]
- Schmitt, C.; Turgeon, S.L. Protein/polysaccharide complexes and coacervates in food systems. Adv. Colloid Interface Sci. 2011, 167, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Gorji, E.G.; Waheed, A.; Ludwig, R.; Tocaherrera, J.L.; Schleining, G.; Gorji, S.G. Complex Coacervation of Milk Proteins with Sodium Alginate. J. Agric. Food Chem. 2018, 66, 3210–3220. [Google Scholar] [CrossRef] [PubMed]
- Gouin, S. Microencapsulation: Industrial appraisal of existing technologies and trends. Trends Food Sci. Technol. 2004, 15, 330–347. [Google Scholar] [CrossRef]
- Xiao, Z.; Liu, W.; Zhu, G.; Zhou, R.; Niu, Y. A review of the preparation and application of flavour and essential oils microcapsules based on complex coacervation technology. J. Sci. Food Agric. 2014, 94, 1482–1494. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.; Dubin, P.; Kayitmazer, A.; Turksen, S. Polyelectrolyte–protein complexes. Curr. Opin. Colloid Interface Sci. 2005, 10, 52–78. [Google Scholar] [CrossRef]
- Dickinson, E. Interfacial structure and stability of food emulsions as affected by protein–polysaccharide interactions. Soft Matter 2008, 4, 932–942. [Google Scholar] [CrossRef]
- Doublier, J.L.; Garnier, C.; Renard, D.; Sanchez, C. Protein–polysaccharide interactions. Curr. Opin. Colloid Interface Sci. 2000, 5, 202–214. [Google Scholar] [CrossRef]
- Huang, Q. Nanotechnology in the Food, Beverage and Nutraceutical Industries, 1st ed.; Woodhead Publishing Limited: Oxford, UK, 2012. [Google Scholar]
- Kayitmazer, A.B.; Seeman, D.; Minsky, B.B.; Dubin, P.L.; Xu, Y. Protein-polyelectrolyte interactions. Soft Matter 2013, 9, 2553–2583. [Google Scholar] [CrossRef]
- Le, X.T.; Rioux, L.E.; Turgeon, S.L. Formation and functional properties of protein–polysaccharide electrostatic hydrogels in comparison to protein or polysaccharide hydrogels. Adv. Colloid Interface Sci. 2017, 239, 127–135. [Google Scholar] [CrossRef]
- Schmitt, C.; Sanchez, C.; Desobry-Banon, S.; Hardy, J. Structure and technofunctional properties of protein-polysaccharide complexes: A review. Crit. Rev. Food Sci. 1998, 38, 689–753. [Google Scholar] [CrossRef]
- Overbeek, J.T.G.; Voorn, M. Phase separation in polyelectrolyte solutions. Theory of complex coacervation. J. Cell. Physiol. 1957, 49, 7–26. [Google Scholar] [CrossRef]
- Weinbreck, F.; De Vries, R.; Schrooyen, P.; De Kruif, C. Complex coacervation of whey proteins and gum arabic. Biomacromolecules 2003, 4, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ren, Y.; Ding, Y.; Xu, M.; Chen, B. The influence of pH and enzyme cross-linking on protein delivery properties of WPI-beet pectin complexes. Food Res. Int. 2018, 105, 678. [Google Scholar] [CrossRef] [PubMed]
- Ru, Q.; Wang, Y.; Lee, J.; Ding, Y.; Huang, Q. Turbidity and rheological properties of bovine serum albumin/pectin coacervates: Effect of salt concentration and initial protein/polysaccharide ratio. Carbohydr. Polym. 2012, 88, 838–846. [Google Scholar] [CrossRef]
- Huang, G.Q.; Du, Y.L.; Xiao, J.X.; Wang, G.Y. Effect of coacervation conditions on the viscoelastic properties of N, O-carboxymethyl chitosan–gum Arabic coacervates. Food Chem. 2017, 228, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Girard, M.; Turgeon, S.L.; Gauthier, S.F. Interbiopolymer complexing between β-lactoglobulin and low- and high-methylated pectin measured by potentiometric titration and ultrafiltration. Food Hydrocoll. 2002, 16, 585–591. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Q. Protein-polysaccharide complexes for effective delivery of bioactive functional food ingredients. In Nano- and Micro-Scale Vehicles for Effective Delivery of Bioactive Ingredients in Functional Foods; Sabliov, C., Chen, H.D., Yada, R., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2015; pp. 224–246. [Google Scholar]
- Ji, N.; Hong, Y.; Gu, Z.; Cheng, L.; Li, Z.; Li, C. Preparation and Characterization of Insulin-Loaded Zein/Carboxymethylated Short-Chain Amylose Complex Nanoparticles. J. Agric. Food Chem. 2018, 66, 9335–9343. [Google Scholar] [CrossRef]
- Ji, N.; Hong, Y.; Gu, Z.; Cheng, L.; Li, Z.; Li, C. The binary and tertiary complex based on short-chain glucan and proanthocyanidins for oral insulin delivery. J. Agric. Food Chem. 2017, 65, 8866–8874. [Google Scholar] [CrossRef]
- Sun, C.; Liu, R.; Liang, B.; Wu, T.; Sui, W.; Zhang, M. Microparticulated whey protein-pectin complex: A texture-controllable gel for low-fat mayonnaise. Food Res. Int. 2018, 108, 151. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Ren, C.; Tian, M.; Yang, X.; Li, J.; Li, B. Complex coacervation of ovalbumin-carboxymethylcellulose assessed by isothermal titration calorimeter and rheology: Effect of ionic strength and charge density of polysaccharide. Food Hydrocoll. 2017, 73, 41–50. [Google Scholar] [CrossRef]
- Kizilay, E.; Kayitmazer, A.B.; Dubin, P.L. Complexation and coacervation of polyelectrolytes with oppositely charged colloids. Adv. Colloid Interface Sci. 2011, 167, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Xu, X.; Li, Z.; Wang, Y.; Xue, Y.; Xue, C. Interactions and phase behaviors in mixed solutions of κ-carrageenan and myofibrillar protein extracted from Alaska Pollock surimi. Food Res. Int. 2018, 105, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Seyrek, E.; Dubin, P.L.; Tribet, C.; Gamble, E.A. Ionic strength dependence of protein-polyelectrolyte interactions. Biomacromolecules 2003, 4, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Grummon, A.H.; Sokol, R.L.; Hecht, C.A.; Patel, A.I. Measuring beverage consumption in US children and adolescents: A systematic review. Obes. Rev. 2018, 19, 1017–1027. [Google Scholar] [CrossRef] [PubMed]
- Fennema, O.R. Food Chemistry, 3rd ed.; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Apolinário, A.C.; De Lima Damasceno, B.P.G.; De Macêdo Beltrão, N.E.; Pessoa, A.; Converti, A.; Da Silva, J.A. Inulin-type fructans: A review on different aspects of biochemical and pharmaceutical technology. Carbohydr. Polym. 2014, 101, 368–378. [Google Scholar] [CrossRef]
- Damodaran, S.; Parkin, K.L.; Fennema, O.R. Fennema’s Food Chemistry, 4th ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Mensink, M.A.; Frijlink, H.W.; Van der Voort Maarschalk, K.; Hinrichs, W.L. Inulin, a flexible oligosaccharide I: Review of its physicochemical characteristics. Carbohydr. Polym. 2015, 130, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Mensink, M.A.; Frijlink, H.W.; Van der Voort Maarschalk, K.; Hinrichs, W.L. Inulin, a flexible oligosaccharide. II: Review of its pharmaceutical applications. Carbohydr. Polym. 2015, 134, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.; Bayarri, S.; Tárrega, A.; Costell, E. Inulin as texture modifier in dairy products. Food Hydrocoll. 2011, 25, 1881–189025. [Google Scholar] [CrossRef]
- Hayashi, M.; Aoshi, T.; Haseda, Y.; Kobiyama, K.; Wijaya, E.; Nakatsu, N.; Igarashi, Y.; Standley, D.M.; Yamada, H.; Honda-Okubo, Y. Advax, a delta inulin microparticle, potentiates in-built adjuvant property of co-administered vaccines. EBioMedicine 2017, 15, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Skwarczynski, M. Inulin: A New Adjuvant with Unknown Mode of Action. EBioMedicine 2017, 15, 8–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, J.F.; Kan, J.; Wen, X.Y.; Jin, C.H. Synthesis, characterization and in vitro anti-diabetic activity of catechin grafted inulin. Int. J. Biol. Macromol. 2014, 64, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, F. Toxicological profile of carboxymethyl inulin. Food Chem. Toxicol. 2003, 41, 49–59. [Google Scholar] [CrossRef]
- Santiago-Rodríguez, L.; Lafontaine, M.M.; Castro, C.; Méndez-Vega, J.; Latorre-Esteves, M.; Juan, E.J.; Mora, E.; Torres-Lugo, M.; Rinaldi, C. Synthesis, stability, cellular uptake, and blood circulation time of carboxymethyl-inulin coated magnetic nanoparticles. J. Mater. Chem. B 2013, 1, 2807–2817. [Google Scholar] [CrossRef]
- Verraest, D.L.; Peters, J.A.; Van Bekkum, H.; Van Rosmalen, G.M. Carboxymethyl inulin: A new inhibitor for calcium carbonate precipitation. J. Am. Oil Chem. Soc. 1996, 73, 55–62. [Google Scholar] [CrossRef]
- Liu, J.; Dong, F.; Xue, Q.; Guo, Z.; Sun, H. The moisture absorption and retention abilities of carboxymethyl inulin and quaternized inulin. In Proceedings of the 2010 4th International Conference on Bioinformatics and Biomedical Engineering, Chengdu, China, 18–20 June 2010; IEEE: Piscataway, NJ, USA, 2010; pp. 1–4. [Google Scholar]
- Greener, I.; Fennema, O. Evaluation of edible, bilayer films for use as moisture barriers for food. J. Food Sci. 1989, 54, 1400–1406. [Google Scholar] [CrossRef]
- Sun, Q.; Luan, L.; Arif, M.; Li, J.; Dong, Q.J.; Gao, Y.; Chi, Z.; Liu, C.G. Redox-sensitive nanoparticles based on 4-aminothiophenol-carboxymethyl inulin conjugate for budesonide delivery in inflammatory bowel diseases. Carbohydr. Polym. 2018, 189, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Szenga, Y.; Siumei, C.; Davidlee, P.; Ma, C.Y. Raman and FTIR spectroscopic study of carboxymethylated non-starch polysaccharides. Food Chem. 2009, 114, 1091–1098. [Google Scholar]
- Liu, W.; Wang, H.; Yu, J.; Liu, Y.; Lu, W.; Chai, Y.; Liu, C.; Pan, C.; Yao, W.; Gao, X. Structure, chain conformation and immunomodulatory activity of the polysaccharide purified from Bacillus Calmette Guerin formulation. Carbohydr. Polym. 2016, 150, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Liu, X.; Guo, H.; Zhang, H.; Zhu, J.; Ren, F. Partial characterization and immunomodulatory activity of polysaccharides from the stem of Dendrobium officinale ( Tiepishihu ) in vitro. J. Funct. Foods 2012, 4, 294–301. [Google Scholar] [CrossRef]
- Verraest, D.L.; Peters, J.A.; Batelaan, J.G.; Van Bekkum, H. Carboxymethylation of inulin. Carbohydr. Res. 1995, 271, 101–112. [Google Scholar] [CrossRef]
- Verraest, D.L.; Peters, J.A.; Kuzee, H.C.; Raaijmakers, H.W.; Van Bekkum, H. Distribution of substituents in O-carboxymethyl and O-cyanoethyl ethers of inulin. Carbohydr. Res. 1997, 302, 203–212. [Google Scholar] [CrossRef]
- Xia, J.; Dubin, P.L.; Dautzenberg, H. Light scattering, electrophoresis, and turbidimetry studies of bovine serum albumin-poly (dimethyldiallylammonium chloride) complex. Langmuir 1993, 9, 2015–2019. [Google Scholar] [CrossRef]
- Souza, C.J.F.; Garcia-Rojas, E.E. Interpolymeric complexing between egg white proteins and xanthan gum: Effect of salt and protein/polysaccharide ratio. Food Hydrocoll. 2016, 66, 268–275. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.W.; Ruengruglikit, C.; Huang, Q. Effects of salt concentration on formation and dissociation of β-lactoglobulin/pectin complexes. J. Agric. Food Chem. 2007, 55, 10432–10436. [Google Scholar] [CrossRef] [PubMed]
- Laos, K.; Brownsey, G.J.; Ring, S.G. Interactions between furcellaran and the globular proteins bovine serum albumin and β-lactoglobulin. Carbohydr. Polym. 2007, 67, 116–123. [Google Scholar] [CrossRef]
- De Kruif, C.G.; Weinbreck, F.; De Vries, R. Complex coacervation of proteins and anionic polysaccharides. Curr. Opin. Colloid Interface Sci. 2004, 9, 340–349. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Q. Influence of protein self-association on complex coacervation with polysaccharide: A monte carlo study. J. Phys. Chem. B 2013, 117, 2615–2624. [Google Scholar] [CrossRef] [PubMed]
- Anirudhan, T.S.; Christa, J.; Binusreejayan. pH and magnetic field sensitive folic acid conjugated protein–polyelectrolyte complex for the controlled and targeted delivery of 5-fluorouracil. J. Ind. Eng. Chem. 2017, 57, 199–207. [Google Scholar] [CrossRef]
- Meng, G.; Chan, J.C.K.; Rousseau, D.; Li, C.; Eunice, C.Y. Study of Protein−Lipid Interactions at the Bovine Serum Albumin/Oil Interface by Raman Microspectroscopy. J. Agric. Food Chem. 2005, 53, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Niu, F.; Niu, D.; Zhang, H.; Chang, C.; Gu, L.; Su, Y.; Yang, Y. Ovalbumin/gum arabic-stabilized emulsion: Rheology, emulsion characteristics, and Raman spectroscopic study. Food Hydrocoll. 2016, 52, 607–614. [Google Scholar] [CrossRef]
- Nawrocka, A.; Szymańska-Chargot, M.; Miś, A.; Wilczewska, A.Z.; Markiewicz, K.H. Effect of dietary fibre polysaccharides on structure and thermal properties of gluten proteins–A study on gluten dough with application of FT-Raman spectroscopy, TGA and DSC. Food Hydrocoll. 2017, 69, 410–421. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Zhao, Y.; Ding, J.; Lin, S. Investigation on complex coacervation between fish skin gelatin from cold-water fish and gum arabic: Phase behavior, thermodynamic, and structural properties. Food Res. Int. 2018, 107, 596–604. [Google Scholar] [CrossRef]
- Duhoranimana, E.; Yu, J.; Mukeshimana, O.; Habinshuti, I.; Karangwa, E.; Xu, X.; Bertrand, M.; Shuqin, X.; Xiaoming, Z. Thermodynamic characterization of gelatin-sodium carboxymethyl cellulose complex coacervation encapsulating conjugated linoleic acid (CLA). Food Hydrocoll. 2018, 80, 149–159. [Google Scholar] [CrossRef]
- Maldonado, L.; Chough, S.; Bonilla, J.; Kim, K.H.; Kokini, J. Mechanism of fabrication and nano-mechanical properties of α-lactalbumin/chitosan and BSA/κ-carrageenan nanotubes through layer-bylayer assembly for curcumin encapsulation and determination of in vitro cytotoxicity. Food Hydrocoll. 2019, 93, 293–307. [Google Scholar] [CrossRef]
- Jones, O.G.; McClements, D.J. Recent progress in biopolymer nanoparticle and microparticle formation by heat-treating electrostatic protein–polysaccharide complexes. Adv. Colloid Interface Sci. 2011, 167, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kimura, K.; Huang, Q.; Dubin, P.L.; Jaeger, W. Effects of salt on polyelectrolyte–micelle coacervation. Macromolecules 1999, 32, 7128–7134. [Google Scholar] [CrossRef]
- Hu, B.; Ting, Y.; Zeng, X.; Huang, Q. Bioactive peptides/chitosan nanoparticles enhance cellular antioxidant activity of (−)-epigallocatechin-3-gallate. J. Agric. Food Chem. 2013, 61, 875–881. [Google Scholar] [CrossRef]
- Xiao, J.; Nian, S.; Huang, Q. Assembly of kafirin/carboxymethyl chitosan nanoparticles to enhance the cellular uptake of curcumin. Food Hydrocoll. 2015, 51, 166–175. [Google Scholar] [CrossRef]
- Liu, J.; Lu, J.F.; Kan, J.; Tang, Y.Q.; Jin, C.H. Preparation, characterization and antioxidant activity of phenolic acids grafted carboxymethyl chitosan. Int. J. Biol. Macromol. 2013, 62, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Chourpa, I.; Ducel, V.; Richard, J.; Dubois, P.; Boury, F. Conformational modifications of α gliadin and globulin proteins upon complex coacervates formation with gum arabic as studied by Raman microspectroscopy. Biomacromolecules 2006, 7, 2616–2623. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Ge, H.; Wang, Y.; Du, X.; Li, B. Molecular migration of konjac glucomannan and gum Arabic phase separation and its application in oil-water interfaces. Food Hydrocoll. 2016, 61, 868–876. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |




| CNaCl (mM) | Size (nm) | PDI | ζ-Potential (mv) |
|---|---|---|---|
| 0 | 227.0 ± 0.8 | 0.178 | 6.9 ± 0.2 |
| 10 | 146.8 ± 0.1 | 0.241 | 7.5 ± 0.3 |
| 50 | 373.7 ± 7.6 | 0.299 | 5.0 ± 0.2 |
| 100 | 300.4 ± 8.4 | 0.302 | - |
| 200 | 275.5 ± 13.4 | 0.368 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, G.; Liu, J.; Jin, W.; Wei, Z.; Ho, C.-T.; Zhao, S.; Zhang, K.; Huang, Q. Formation of Nanocomplexes between Carboxymethyl Inulin and Bovine Serum Albumin via pH-Induced Electrostatic Interaction. Molecules 2019, 24, 3056. https://doi.org/10.3390/molecules24173056
Huang G, Liu J, Jin W, Wei Z, Ho C-T, Zhao S, Zhang K, Huang Q. Formation of Nanocomplexes between Carboxymethyl Inulin and Bovine Serum Albumin via pH-Induced Electrostatic Interaction. Molecules. 2019; 24(17):3056. https://doi.org/10.3390/molecules24173056
Chicago/Turabian StyleHuang, Guiying, Jun Liu, Weiping Jin, Zihao Wei, Chi-Tang Ho, Suqing Zhao, Kun Zhang, and Qingrong Huang. 2019. "Formation of Nanocomplexes between Carboxymethyl Inulin and Bovine Serum Albumin via pH-Induced Electrostatic Interaction" Molecules 24, no. 17: 3056. https://doi.org/10.3390/molecules24173056
APA StyleHuang, G., Liu, J., Jin, W., Wei, Z., Ho, C.-T., Zhao, S., Zhang, K., & Huang, Q. (2019). Formation of Nanocomplexes between Carboxymethyl Inulin and Bovine Serum Albumin via pH-Induced Electrostatic Interaction. Molecules, 24(17), 3056. https://doi.org/10.3390/molecules24173056








