Rethinking the Future of Food Packaging: Biobased Edible Films for Powdered Food and Drinks
Abstract
:1. Introduction
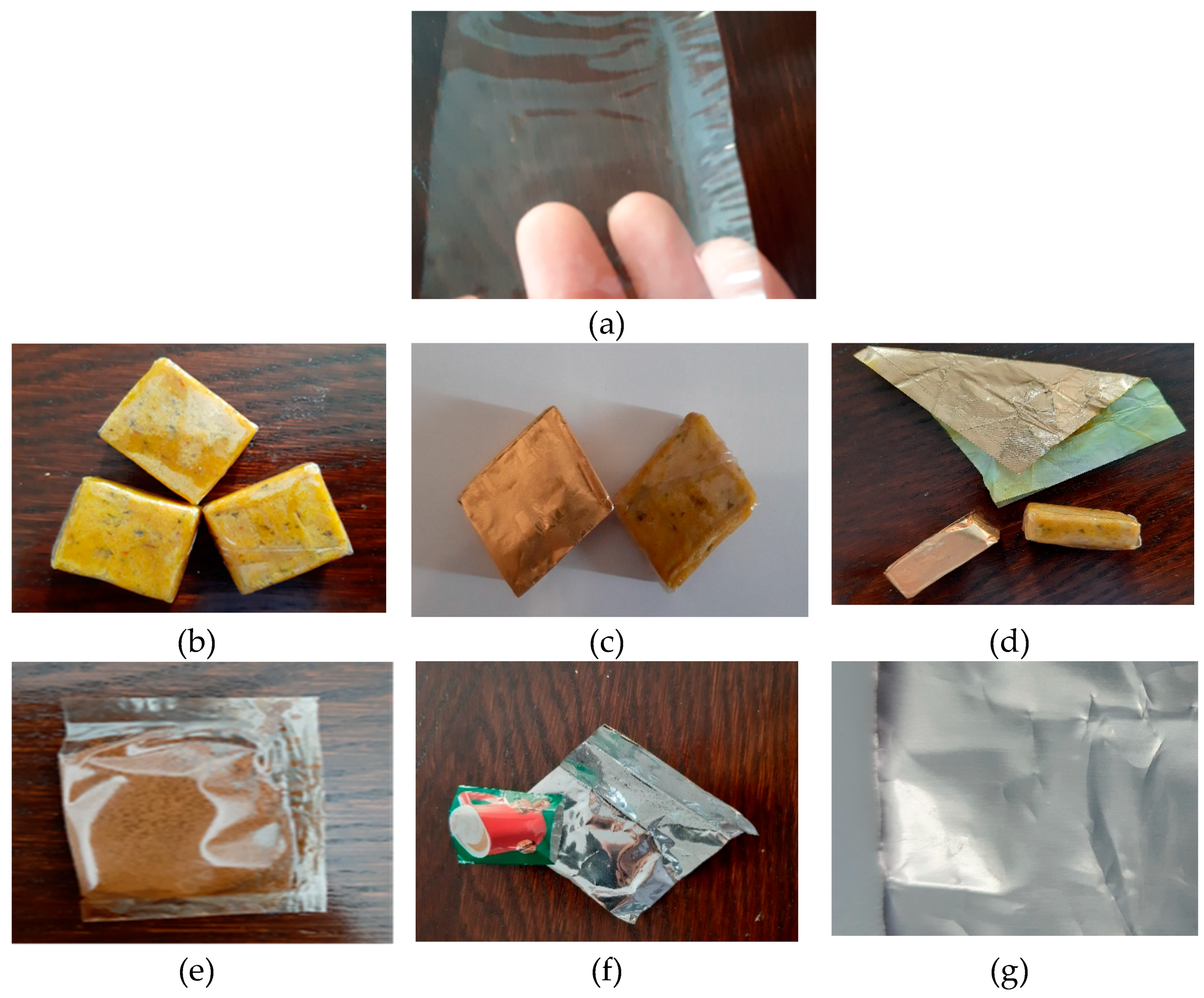
2. Results and Discussions
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Film Development
3.2.2. Determination of Physical and Optical Properties
3.2.3. Determination of Mechanical Properties
3.2.4. Assessment of Solubility
3.2.5. Evaluation of Microbiological Characteristics
3.3. Statistical Analyses
4. Conclusions
Aknowledgments
Author Contributions
Funding
Conflicts of Interest
References
- Thakur, S.; Chaudhary, J.; Sharma, B.; Verma, A.; Tamulevicius, S.; Thakur, V.K. Sustainability of bioplastics: Opportunities and challenges. Curr. Opin. Green Sustain. Chem. 2018, 13, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Giosafatto, V.; Al-Asmar, A.; D’Angelo, A.; Roviello, V.; Esposito, M.; Mariniello, L. Preparation and characterization of bioplastics from grass pea flour cast in the presence of microbial transglutaminaze. Coatings 2018, 8, 435. [Google Scholar] [CrossRef]
- Abdalrazeq, M.; Giosafatto, C.V.; Esposito, M.; Fenderioc, M.; Di Pierro, P.; Porta, R. Glycerol-plasticized films obtained from whey proteins denatured at alkaline pH. Coatings 2019, 9, 322. [Google Scholar] [CrossRef]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, S.; Hosseini, S.V.; Regenstein, J. Edible films and coatings in seafood preservation: A Review. Food Chem. 2018, 240, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Pashova, S.; Radev, R.; Dimitrov, G.; Ivanov, J. Edible coatings in food industry related to circular economy. Quality 2018, 19, 111–117. [Google Scholar]
- Puscaselu, R.; Gutt, G.; Amariei, S. Biopolymer-based films enriched with stevia rebaudiana used for the development of edible and soluble packaging. Coatings 2019, 9, 360. [Google Scholar] [CrossRef]
- Sagnelli, D.; Kirkensgaardb, J.; Giosafatto, C.V.; Ogrodowiczd, N.; Kruczałae, K.; Mikkelsenf, S.; Maigretg, J.E.; Lourding, D.; Mortensenb, K.; Blennowa, A. All-natural bio-plastics using starch-betaglucan composites. Carbohydr. Polym. 2017, 172, 237–245. [Google Scholar] [CrossRef]
- Kumar, P.; Sandeep, K.P.; Alavi, S.; Truong, V.; Gorga, R. Preparation and characterization of bio-nanocomposite films based on soy protein isolate and montmorillonite using melt extrusion. J. Food Eng. 2010, 100, 480–489. [Google Scholar] [CrossRef]
- Basumatary, K.; Daimary, P.; Kumar Das, S.; Thapa, M.; Singh, M.; Mukherjee, A.; Kumar, S. Lagerstroemia speciosa fruit-mediated synthesis of silver nanoparticles and its application as filler in agar-based nanocomposite films for antimicrobial food packaging. Food Packag. Shelf 2018, 17, 99–106. [Google Scholar] [CrossRef]
- Blazquez, I.O.; Grande Burgos, M.J.; Pulido, R.P.; Galvez, A.; Luca, R. Bacterial Inactivation by using plastic materials activated with combinations of natural antimicrobials. Coatings 2018, 8, 460. [Google Scholar] [CrossRef]
- Chiabrando, V.; Giacalone, G. Effects of alginate edible coating on quality and antioxidant properties in sweet cherry during postharvest storage. Ital. J. Food Sci. 2015, 27, 173–180. [Google Scholar]
- Tavassoli-Kafrani, E.; Shekarchizadeh, H.; Masoudpour-Behabadi, M. Development of edible films and coatings from alginates and carrageenans. Carbohydr. Polym. 2016, 137, 360–374. [Google Scholar] [CrossRef] [PubMed]
- Apolinario, A.C.; de Lima Damasceno, B.P.G.; de Macedo Beltrao, N.E.; Pessoa, A.; Converti, A.; da Silva, J.A. Inulin-type fructans: A review on different aspects of biological and pharmaceutical technology. Carbohydr. Polym. 2014, 101, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, M.; Shehzad, O.M.; Rakha, A.; Raza, H.; Sharif, H.; Shakeel, A.; Ansari, A.; Niazi, S. Inulin: Properties, health benefits and food applications. Carbohydr. Polym. 2016, 147, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Hala, S.S.; Soha, R.K. Effect of chicory inulin extract as a fat replacer on texture and sensory properties of cookies. Middle East J. Appl. Sci. 2017, 7, 168–177. [Google Scholar]
- Ni, D.; Xu, W.; Zhu, Y.; Zhang, W.; Zhang, T.; Guang, C.; Mu, W. Inulin and its enzymatic production by inulosucrase: Characteristics, structural features, molecular modifications and applications. Biotechnol. Adv. 2019, 37, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.; Singh, T.; Larroche, C. Biotechnological applications of inulin-rich feedstocks. Bioresour. Technol. 2019, 273, 641–653. [Google Scholar] [CrossRef]
- Nieto-Nieto, T.V.; Wang, Y.X.; Ozimek, L.; Chen, L. Inulin at low concentrations significantly improves the gelling properties of oat protein – A molecular mechanism study. Food Hydrocolloids 2015, 50, 116–127. [Google Scholar] [CrossRef]
- Morreale, F.; Benavent-Gil, Y.; Rossel, C. Inulin enrichment of gluten free breads: Interaction between inulin and yeast. Food Chem. 2019, 278, 545–551. [Google Scholar] [CrossRef]
- Blanco Canalis, M.S.; Leon, A.E.; Ribotta, P.D. Incorportaion of dietary fiber on the cookie dough. Effects on thermal properties and water availability. Food Chem. 2019, 271, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Foschia, M.; Perresini, D.; Sensidoni, A.; Brennan, C. The effects of dietary fiber addition on the quality of common cereal products. J. Cereal. Sci. 2013, 58, 216–227. [Google Scholar] [CrossRef]
- Meyer, D.; Bayarri, S.; Tarrega, A.; Costell, E. Inulin as texture modifier in dairy products. Food Hydrolocolloids 2011, 25, 1881–1890. [Google Scholar] [CrossRef]
- Boghadam, M.E.; Keivaninahr, F.; Fouladi, M.; Mokarran, R.; Nazemi, A. Inulin addition to yoghurt; Prebiotic activity, health effects and sensory properties. Int. J. Dairy Technol. 2019. [Google Scholar] [CrossRef]
- Keenan, D.; Resconi, V.; Kerry, J.; Hsmil, R. Modeling the influence of inulin as a fat substitute in comminuted meat products on their physic-chemical characteristics and eating quality using a mixture design approach. Meat Sci. 2014, 96, 1384–1394. [Google Scholar] [CrossRef] [PubMed]
- Navas, T.; Azam, M.; Ramadhan, A.; Xu, Y.; Xia, W. Impact of wall material on the physicochemical properties and oxidative stability of microencapsulated spray dried silver carp oil. J. Aquat. Food Prod. 2019, 28, 49–63. [Google Scholar] [CrossRef]
- Veereman, G. Pediatric Applications of Inulin and Oligofructose. J. Nutr. 2007, 137, 2585S–2589S. [Google Scholar] [CrossRef] [Green Version]
- Balthazar, C.F.; Silva, H.; Esmerino, E.; Rocha, R.; Moraes, J.; Carmo, M.; Azevedo, L.; Camps, I.; Abud, Y.; Sant’Anna, C.; et al. The addition of inulin and Lactobacillus casei 01 in sheep milk ice cream. Food Chem. 2018, 246, 464–472. [Google Scholar] [CrossRef]
- Tulavi, V.Y.; Londhe, G.K.; Sankpal, S.S. Effect of addition of inulin of chemical, organoleptic, microbiological and rheological properties of burfi. Int. J. Chem. 2018, 6, 2335–2339. [Google Scholar]
- Commission Regulation (EU) No 1129/2011 of 11 November 2011 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council by establishing a Union list of food additives. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:295:0001:0177:RO:PDF (accessed on 22 August 2019).
- Food Additive Status List. Available online: https://www.fda.gov/food/food-additives-petitions/food-additive-status-list#ftnG (accessed on 22 August 2019).
- Podgorna, K.; Szczepanowicz, K.; Piotrowski, M.; Gajdosova, M.; Stepanek, F.; Warszynski, P. Gadolinium alginate nanogels for theranostic applications. Colloids Surf. B 2017, 153, 183–189. [Google Scholar] [CrossRef]
- Hoo, S.J.; Lee, J.H.; Kim, E.J.; Yang, H.J.; Park, J.S.; Hong, S.K. Toxicological evaluation of neoagarooligosaccharides prepared by enzimatic hydrolysis of agar. Regul. Toxicol. Pharmacol. 2017, 90, 9–21. [Google Scholar] [CrossRef]
- Lopes, L.S.; Santos, C.G.; de Almeida Vaucher, R.; Gende, L.; Raffin, R.P.; Santos, R.C.V. Evaluation of antimicrobial activity of glycerol monolaureate nanocapsules against American foulbrood disease agent and toxicity. Microb. Pathog. 2016, 97, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Kuciel, S.; Mazur, K.; Jakubowska, P. Novel biorenewable composites based on poly (3-hydroxibutyrate-co-3-hydroxivalerate) with natural fibers. J. Polym. Environ. 2019, 27, 803–815. [Google Scholar] [CrossRef]
- Guttierez, T.J. Active and intelligent films made from starchy sources/blackberry pulp. J. Polym. Environ. 2018, 26, 2374–2391. [Google Scholar] [CrossRef]
- Phan, T.; Debeaufort, D.; Voilley, A.; Luu, D. Biopolymer interaction affects the functional properties of edible films based on agar, cassava starch and arabynoxylan blends. J. Food Eng. 2009, 90, 548–558. [Google Scholar] [CrossRef]
- ASTM D882 -Standard Test Method for Tensile Properties of Thin Plastic Sheeting. Available online: https://www.astm.org/Standards/D882 (accessed on 21 February 2019).
- Puscaselu, R.; Amariei, S. The application of the Peleg model in order to obtain completely soluble materials for food product packaging. J. Appl. Package. Res. 2018, 10, 98–106. [Google Scholar]
- Wang, L.F.; Rhim, J.W. Preparation and application og agar/alginate/collagen ternary blend functional food packaging films. Int. J. Biol. Macromol. 2015, 80, 460–468. [Google Scholar] [CrossRef]
Sample Availability: Samples of the edible materials obtained are available from the authors. |








| Sample | Transmittance, T, (%) | Roughness, Rz, (nm) | Water Activity Index, (aw) |
|---|---|---|---|
| I1 | 82.44 a,d,g ± 2.1 | 144.00 a,c ± 0.05 | 0.376 a,b,c ± 0.07 |
| I2 | 84.42 a,d,h ± 1.01 | 276.40 a,b ± 0.07 | 0.387 a ± 0.05 |
| I3 | 89.68 b ± 0.7 | 211.20 c,g ± 0.02 | 0.371 a,b,c ± 0.01 |
| I4 | 99.43 c ± 0.41 | 371.66 d ± 0.33 | 0.374 a,b,c. ± 0.97 |
| I5 | 85.09 a,b ± 1.7 | 29.37 e ± 0.57 | 0.368 a,b,c ± 0.47 |
| I6 | 97.89 c ± 0.62 | 108.36 c,e,f ± 0.97 | 0.387 a,b ± 1.02 |
| I7 | 80.21 d,g ± 2.2 | 90.36 b,g ± 1.04 | 0.368 a,b,c ± 0.98 |
| I8 | 80.44 a,d,g ± 2.11 | 156.25 b,h ± 0.61 | 0.375 a,b,c ± 0.57 |
| I9 | 89.48 b ± 0.73 | 222.33 i ± 0.84 | 0.375 a,b,c ± 0.78 |
| I10 | 66.30 e ± 2.63 | 207.47 i ± 0.77 | 0.368 a,b,c ± 0.25 |
| I11 | 95.26 c ± 0.52 | 224.25 i ± 0.07 | 0.363 a,b,c ± 0.77 |
| I12 | 73.31 f ± 2.4 | 213.50 b,i ± 0.33 | 0.361 b,c ± 0.68 |
| I13 | 65.36 e ± 2.19 | 195.50 b,i ± 0.04 | 0.356 c ± 0.62 |
| I14 | 68.52 e,f ± 0.97 | 202.25 b,i ± 0.93 | 0.369 a,b,c ± 0.05 |
| I15 | 82.08 a,d,g ± 3.21 | 158.00 c ± 0.61 | 0.369 a,b,c ± 0.21 |
| I16 | 95.89 c ± 1.34 | 171.60 g,h ± 0.05 | 0.364 a,b,c ± 0.41 |
| I17 | 84.61 a,d,h ± 4.77 | 158.25 g ± 0.77 | 0.361 b,c,d ± 0.33 |
| I18 | 78.36 g ± 1.63 | 228.75 i ± 0.33 | 0.357 c, ± 0.18 |
| I19 | 88.89 b,h ± 0.66 | 299.00 d ± 0.57 | 0.367 b,c ± 0.57 |
| I20 | 16.76 i ± 1.88 | 224.00 i ± 0.02 | 0.356 c ± 0.48 |
| MONTH 1 | T | R | TS | E | MC | WS | L* | a* | b* | |
| T | 1 | 0.999 * | 0.490 | 0.675 | −0.353 | −0.644 | −0.224 | −0.145 | 0.003 | |
| R | 1 | −0.780 ** | 0.597 | 0.351 | −0.646 | −0.221 | −0.147 | 0.002 | ||
| TS | 1 | 0.696 ** | −0.434 | −0.410 | −0.547 | 0.347 | 0.596 | |||
| E | 1 | −0.192 | −0.120 | 0.159 | −0.192 | −0.267 | ||||
| MC | 1 | 0.567 | 0.839 * | −0.611 | −0.748 ** | |||||
| WS | 1 | 0.630 | −0.308 | −0.464 | ||||||
| L* | 1 | −0.839 * | −0.949 * | |||||||
| a* | 1 | 0.844 * | ||||||||
| b* | 1 | |||||||||
| MONTH 2 | T | R | TS | E | MC | WS | L* | a* | b* | |
| T | 1 | 0.999 * | −0.524 | −0.472 | −0.590 | −0.609 | −0.381 | 0.366 | 0.138 | |
| R | 1 | −0.520 | −0.473 | −0.570 | −0.615 | −0.388 | 0.362 | 0.140 | ||
| TS | 1 | 0.663 ** | −0.07 | 0.254 | −0.172 | 0.166 | 0.306 | |||
| E | 1 | 0.475 | 0.355 | 0.477 | −0.389 | −0.501 | ||||
| MC | 1 | 0.106 | 0.278 | 0.243 | −0.174 | |||||
| WS | 1 | 0.681 | −0.598 | −0.352 | ||||||
| L* | 1 | −0.523 | −0.794 * | |||||||
| a* | 1 | 0.474 | ||||||||
| b* | 1 | |||||||||
| MONTH 3 | T | R | TS | E | MC | WS | L* | a* | b* | |
| T | 1 | 0.999 * | 0.808 * | −0.408 | −0.610 | −0.655 | −0.224 | 0.197 | 0.090 | |
| R | 1 | 0.812 * | −0.409 | −0.020 | −0.655 | 0.207 | 0.207 | 0.096 | ||
| TS | 1 | −0.248 | 0.143 | −0.124 | −0.114 | 0.207 | 0.097 | |||
| E | 1 | 0.176 | 0.491 | 0.585 | −0.457 | −0.571 | ||||
| MC | 1 | 0.221 | 0.267 | −0.052 | −0.310 | |||||
| WS | 1 | 0.544 | −0.433 | −0.531 | ||||||
| L* | 1 | −0.762 * | −0.895 ** | |||||||
| a* | 1 | 0.574 | ||||||||
| b* | 1 |
| Sample | mALGINATE, (g) | mINULIN, (g) | mAGAR, (g) | mGLYCEROL, (g) | VWATER (mL) |
|---|---|---|---|---|---|
| I1 | 2.50 | 2.00 | 0.00 | 1.00 | 150.00 |
| I2 | 2.00 | 2.50 | 0.00 | 1.00 | |
| I3 | 2.00 | 2.00 | 0.00 | 1.50 | |
| I4 | 3.00 | 1.00 | 0.00 | 1.50 | |
| I5 | 1.00 | 3.00 | 0.00 | 1.50 | |
| I6 | 2.50 | 1.00 | 0.00 | 2.00 | |
| I7 | 1.00 | 2.50 | 0.00 | 2.00 | |
| I8 | 2.50 | 1.00 | 1.00 | 1.00 | |
| I9 | 1.00 | 2.50 | 1.00 | 1.00 | |
| I10 | 1.00 | 1.00 | 2.50 | 1.00 | |
| I11 | 2.00 | 1.00 | 1.00 | 1.50 | |
| I12 | 1.00 | 2.00 | 1.00 | 1.50 | |
| I13 | 1.00 | 1.00 | 2.00 | 1.50 | |
| I14 | 0.00 | 2.00 | 2.50 | 1.00 | |
| I15 | 0.00 | 2.50 | 2.00 | 1.00 | |
| I16 | 0.00 | 2.00 | 2.00 | 1.50 | |
| I17 | 0.00 | 3.00 | 1.00 | 1.50 | |
| I18 | 0.00 | 1.00 | 3.00 | 1.50 | |
| I19 | 0.00 | 1.00 | 2.50 | 2.00 | |
| I20 | 0.00 | 2.50 | 1.00 | 2.00 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puscaselu, R.; Gutt, G.; Amariei, S. Rethinking the Future of Food Packaging: Biobased Edible Films for Powdered Food and Drinks. Molecules 2019, 24, 3136. https://doi.org/10.3390/molecules24173136
Puscaselu R, Gutt G, Amariei S. Rethinking the Future of Food Packaging: Biobased Edible Films for Powdered Food and Drinks. Molecules. 2019; 24(17):3136. https://doi.org/10.3390/molecules24173136
Chicago/Turabian StylePuscaselu, Roxana, Gheorghe Gutt, and Sonia Amariei. 2019. "Rethinking the Future of Food Packaging: Biobased Edible Films for Powdered Food and Drinks" Molecules 24, no. 17: 3136. https://doi.org/10.3390/molecules24173136
APA StylePuscaselu, R., Gutt, G., & Amariei, S. (2019). Rethinking the Future of Food Packaging: Biobased Edible Films for Powdered Food and Drinks. Molecules, 24(17), 3136. https://doi.org/10.3390/molecules24173136


.png)



