Does Oxygen Feature Chalcogen Bonding?
Abstract
1. Introduction
2. Computational Details
3. Results and Discussion
3.1. Nature of the Intermolecular Distance
3.2. Directionality
3.3. Nature of the Change in the Chalcogen Bond Donor Distance
3.4. Nature of the Change in the Vibrational Frequency of the Chalcogen Bond Donor
3.5. The Molecular Electrostatic Surface Potential Signatures
3.6. The QTAIM Signatures
3.7. Nature of Delocalization Index
3.8. Nature of Reduced Density Gradient Isosurface Domains
3.9. Nature of the Change in the Dipole Moment
3.10. Effect of Polarizability on Complex Formation
3.11. Nature of the Donor-Acceptor Natural Bond Orbital Interactions
3.12. Nature of the Complex Binding Energies
3.13. Factors Contributing to the Binding Energy of the Complexes: A SAPT Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Horowitz, S.; Trievel, R.C. Carbon-Oxygen Hydrogen Bonding in Biological Structure and Function. J. Biol. Chem. 2012, 287, 41576–41582. [Google Scholar] [CrossRef] [PubMed]
- Polander, B.C.; Barry, B.A. A hydrogen-bonding network plays a catalytic role in photosynthetic oxygen evolution. Proc. Natl. Acad. Sci. USA 2012, 109, 6112–6117. [Google Scholar] [CrossRef] [PubMed]
- Szatyłowicz, H.; Sadlej-Sosnowska, N. Characterizing the Strength of Individual Hydrogen Bonds in DNA Base Pairs. J. Chem. Inf. Model. 2010, 50, 2151–2161. [Google Scholar] [CrossRef] [PubMed]
- Yurenko, Y.P.; Zhurakivsky, R.O.; Samijlenko, S.P.; Hovorun, D.M. Intramolecular CH⋯O hydrogen bonds in the AI and BI DNA-like conformers of canonical nucleosides and their Watson-Crick pairs. Quantum chemical and AIM analysis. J. Biomol. Struct. Dyn. 2011, 29, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Pauling, L. The Nature of the Chemical Bond; Cornell University Press: Ithaca, NY, USA, 1940. [Google Scholar]
- Desiraju, G.R. Reflections on the Hydrogen Bond in Crystal Engineering. Cryst. Growth Des. 2011, 114, 896–898. [Google Scholar] [CrossRef]
- Lehn, J.M. Supramolecular chemistry: Receptors, catalysts, and carriers. Science 1985, 227, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Meeuwissen, J.; Reek, J.N.H. Supramolecular catalysis beyond enzyme mimics. Nat. Chem. 2010, 2, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Varadwaj, A.; Varadwaj, P.R. Can a Single Molecule of Water be Completely Isolated within the Subnano-Space Inside the Fullerene C60 Cage? A Quantum Chemical Prospective. Chem. Eur. J. 2012, 18, 15345–15360. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Gong, X.A.; Cheng, J. Cooperativity between OH⋯O and CH⋯O Hydrogen Bonds Involving Dimethyl Sulfoxide−H2O−H2O Complex. J. Phys. Chem. A 2007, 111, 10166–10169. [Google Scholar] [CrossRef]
- Peeters, D. Hydrogen bonds in small water clusters: A theoretical point. J. Mol. Liq. 1995, 67, 49–61. [Google Scholar] [CrossRef]
- Raymo, F.M.; Bartberger, M.D.; Houk, K.N.; Stoddart, J.F. The Magnitude of [C−H⋯O] Hydrogen Bonding in Molecular and Supramolecular Assemblies. J. Am. Chem. Soc. 2001, 123, 9264–9267. [Google Scholar] [CrossRef] [PubMed]
- Zanatta, M.; Dupont, J.; Wentza, G.N.; dos Santos, F.P. Intermolecular hydrogen bonds in water@IL supramolecular complexes. Phys. Chem. Chem. Phys. 2018, 20, 11608–11614. [Google Scholar] [CrossRef] [PubMed]
- Qing, G.; Lu, Q.; Li, X.; Liu, J.; Ye, M.; Liang, X.; Sun, T. Hydrogen bond based smart polymer for highly selective and tunable capture of multiply phosphorylated peptides. Nat. Commun. 2017, 8, 461. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Dehghani-Sanij, A.A.; Blackburn, R.S. IR study on hydrogen bonding in epoxy resin–silica nanocomposites. Prog. Nat. Sci. 2008, 18, 801–805. [Google Scholar] [CrossRef]
- Hutchins, K.M. Functional materials based on molecules with hydrogen-bonding ability: Applications to drug co-crystals and polymer complexes. R. Soc. Open sci. 2018, 5, 180564. [Google Scholar] [CrossRef]
- Głowacki, E.D.; Irimia-Vladu, M.; Bauer, S.; Sariciftci, N.S. Hydrogen-bonds in molecular solids—From biological systems to organic electronics. J. Mater. Chem. B 2013, 1, 3742–3753. [Google Scholar] [CrossRef]
- Vargas, R.; Garza, J.; Dixon, D.A.; Hay, B.P. How Strong Is the Cα−H⋯OC Hydrogen Bond? J. Am. Chem. Soc. 2000, 122, 4750–4755. [Google Scholar] [CrossRef]
- Koch, H.F. Proton-transfer reactions between carbon and oxygen. Acc. Chem. Res. 1984, 174, 137–144. [Google Scholar] [CrossRef]
- Ishikita, H.; Saito, K. Proton transfer reactions and hydrogen-bond networks in protein environments. J. R. Soc. Interface 2014, 11, 20130518. [Google Scholar] [CrossRef]
- Joesten, M.D. Hydrogen bonding and proton transfer. J. Chem. Educ. 1982, 59, 362. [Google Scholar] [CrossRef]
- Pauling, L.; Corey, R.B.; Branson, H.R. The structure of proteins; two hydrogen-bonded helical configurations of the polypeptide chain. Proc. Natl. Acad. Sci. USA 1951, 37, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, D. The discovery of the α-helix and β-sheet, the principal structural features of proteins. Proc. Natl. Acad. Sci. USA 2003, 100, 11207–11210. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Englander, S.W. Hydrogen bond strength and beta-sheet propensities: The role of a side chain blocking effect. Proteins 1994, 18, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Aakeröy, C.B.; Nieuwenhuyzen, M. Hydrogen bonding in crystal engineering: Two-dimensional layers of hydrogen l-malate anions. J. Mol. Struct. THEOCHEM 1996, 374, 223–239. [Google Scholar] [CrossRef]
- Vogel, L.; Wonner, P.; Huber, S.M. Chalcogen Bonding: An Overview. Angew. Chem. Int. Ed. 2019, 58, 1880–1891. [Google Scholar] [CrossRef] [PubMed]
- Pascoe, D.J.; Ling, K.B.; Cockroft, S.L. The Origin of Chalcogen-Bonding Interactions. J. Am. Chem. Soc. 2017, 139, 15160–15167. [Google Scholar] [CrossRef] [PubMed]
- Scilabra, P.; Terraneo, G.; Resnati, G. The Chalcogen Bond in Crystalline Solids: A World Parallel to Halogen Bond. Acc. Chem. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Arunan, E.; Desiraju, G.R.; Klein, R.A.; Sadlej, J.; Scheiner, S.; Alkorta, I.; Clary, D.C.; Crabtree, R.H.; Dannenberg, J.J.; Hobza, P.; et al. Definition of the hydrogen bond (IUPAC Recommendations 2011). Pure Appl. Chem. 2011, 83, 1637–1641. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Ho, P.S.; Kloo, L.; Legon, A.C.; Marquardt, R.; Metrangolo, P.; Politzer, P.; Resnati, G.; Rissanen, K. Definition of the halogen bond (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1711–1713. [Google Scholar] [CrossRef]
- Legon, A.C. Tetrel, pnictogen and chalcogen bonds identified in the gas phase before they had names: A systematic look at non-covalent interactions. Phys. Chem. Chem. Phys. 2017, 19, 14884–14896. [Google Scholar] [CrossRef] [PubMed]
- Clark, T.; Hennemann, M.; Murray, J.S.; Politzer, P. Halogen bonding: The σ-hole. J. Mol. Model. 2007, 13, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Varadwaj, P.R.; Varadwaj, A.; Marques, H.M. Halogen Bonding: A Halogen-Centered Noncovalent Interaction Yet to Be Understood. Inorganics 2019, 7, 40. [Google Scholar] [CrossRef]
- Beno, B.R.; Yeung, K.-S.; Bartberger, M.D.; Pennington, L.D.; Meanwell, N.A. A Survey of the Role of Noncovalent Sulfur Interactions in Drug Design. J. Med. Chem. 2015, 58, 4383–4438. [Google Scholar] [CrossRef] [PubMed]
- Bauzá, A.; Mooibroek, T.J.; Frontera, A. The bright future of unconventional σ/π-hole interactions. ChemPhysChem 2015, 16, 2496–2517. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.J.; Zade, S.S.; Singh, H.B.; Sunoj, R.B. Organoselenium chemistry: Role of intramolecular interactions. Chem. Rev. 2010, 110, 4357–4416. [Google Scholar] [CrossRef]
- Huang, H.; Yang, L.; Facchetti, A.; Marks, T.J. Organic and Polymeric Semiconductors Enhanced by Noncovalent Conformational Locks. Chem. Rev. 2017, 117, 10291–10318. [Google Scholar] [CrossRef]
- Benz, S.; Mareda, J.; Besnard, C.; Sakai, N.; Matile, S. Catalysis with chalcogen bonds: Neutral benzodiselenazole scaffolds with high-precision selenium donors of variable strength. Chem. Sci. 2017, 8, 8164–8169. [Google Scholar] [CrossRef]
- Frisch, M.J.; Head-Gordon, M.; Pople, J.A. A Direct MP2 gradient method. Chem. Phys. Lett. 1990, 166, 275–280. [Google Scholar] [CrossRef]
- Murray, S.; Politzer, P. The electrostatic potential: An overview. WIREs Comput. Mol. Sci. 2011, 1, 153–163. [Google Scholar] [CrossRef]
- Varadwaj, A.; Marques, H.M.; Varadwaj, P.R. Nature of halogen-centered intermolecular interactions in crystal growth and design: Fluorine-centered interactions in dimers in crystalline hexafluoropropylene as a prototype. J. Comput. Chem. 2019, 40, 1836–1860. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. An Overview of Strengths and Directionalities of Noncovalent Interactions: σ-Holes and π-Holes. Crystals 2019, 9, 165. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. σ-Holes vs. Buildups of Electronic Density on the Extensions of Bonds to Halogen Atoms. Inorganics 2019, 7, 71. [Google Scholar] [CrossRef]
- Varadwaj, P.R.; Varadwaj, A.; Marques, H.M.; Yamashita, K. Can Combined Electrostatic and Polarization Effects Alone Explain the F⋯F Negative-Negative Bonding in Simple Fluoro-Substituted Benzene Derivatives? A First-Principles Perspective. Computation 2018, 6, 51. [Google Scholar] [CrossRef]
- Varadwaj, A.; Marques, H.M.; Varadwaj, P.R. Is the Fluorine in Molecules Dispersive? Is Molecular Electrostatic Potential a Valid Property to Explore Fluorine-Centered Non-Covalent Interactions? Molecules 2019, 24, 379. [Google Scholar] [CrossRef] [PubMed]
- Esrafili, M.D.; Ghanbari, M.; Mohammadian-sabet, F. Substituent effects on cooperativity of pnicogen bonds. J. Mol. Model. 2014, 20, 2436. [Google Scholar] [CrossRef] [PubMed]
- Mohanand, N.; Suresh, C.H. A Molecular Electrostatic Potential Analysis of Hydrogen, Halogen, and Dihydrogen Bonds. J. Phys. Chem. A 2014, 118, 1697–1705. [Google Scholar] [CrossRef]
- Dong, W.; Li, Q.; Scheiner, S. Comparative Strengths of Tetrel, Pnicogen, Chalcogen, and Halogen Bonds and Contributing Factors. Molecules 2018, 23, 1681. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F. A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Keith, T.A. AIMAll, Version 17.01.25; TK Gristmill Software: Overland Park, KS, USA, 2016; Available online: http://aim.tkgristmill.com (accessed on 8 July 2019).
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision D 01; Gaussian, Inc.: Wallinford, CT, USA, 2013. [Google Scholar]
- Bader, R.F.W.; Nguyen-Dang, T.T. Advances in Quantum Chemistry; Löwdin, P.-O., Ed.; Academic Press: New York, NY, USA, 1981; Volume 14, pp. 63–124. [Google Scholar]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing noncovalent interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD—Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Weinhold, F.; Landis, C.R. Discovering Chemistry with Natural Bond Orbitals; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012. [Google Scholar]
- SAPT: Symmetry-Adapted Perturbation Theory, A Documentary. Available online: http://www.psicode.org/psi4manual/1.2/sapt.html (accessed on 19 July 2019).
- Turney, J.M.; Simmonett, A.C.; Parrish, R.M.; Hohenstein, E.G.; Evangelista, F.; Fermann, J.T.; Mintz, B.J.; Burns, L.A.; Wilke, J.J.; Abrams, M.L.; et al. Psi4: An open-source ab initio electronic structure program. WIREs Comput. Mol. Sci. 2012, 2, 556. [Google Scholar] [CrossRef]
- Hamilton, G. NO3F, An Explosive Compound. J. Am. Chem. Soc. 1934, 56, 2635–2637. [Google Scholar]
- Casper, B.; Dixon, D.A.; Mack, H.-G.; Ulic, S.E.; Willner, H.; Oberhammer, H. Molecular Structure of Fluorine Nitrate: Dangerous for Experiment and Theory. J. Am. Chem. Soc. 1994, 116, 8317–8321. [Google Scholar] [CrossRef]
- Schmauch, G.E.; Serfass, E.J. The Use of Perchloryl Fluoride in Flame Photometry. Appl. Spectrosc. 1958, 12, 98–102. [Google Scholar] [CrossRef]
- Thompson, P.G. Preparation and Characterization of Bis(fluoroxy)perfluoroalkanes. II. Bis(fluoroxy)perfluoromethane. J. Am. Chem. Soc. 1967, 89, 1811–1813. [Google Scholar] [CrossRef]
- Singh, G.S.; Mollet, K.; D’hooghe, M.; De Kimpe, N. Epihalohydrins in Organic Synthesis. Chem. Rev. 2013, 113, 1441–1498. [Google Scholar] [CrossRef] [PubMed]
- Scheffler, D.; Schaper, I.; WillnerHans-Georg, H.; Oberhammer, M. Properties of Fluorocarbonyl Peroxynitrate. Inorg. Chem. 1997, 36, 339–344. [Google Scholar] [CrossRef]
- Ruff, O.; Mensel, W. Neue Sauerstofffluoride: O2F2 und OF. Z. Anorg. Allg. Chem. 1933, 211, 204–208. [Google Scholar] [CrossRef]
- Hohorst, F.A.; DesMarteau, D.D.; Anderson, L.R.; Gould, D.E.; Fox, W.B. Reactions of bis(trifluoromethyl) trioxide. J. Am. Chem. Soc. 1973, 95, 3866–3869. [Google Scholar] [CrossRef]
- Shibue, M.; Mant, C.T.; Hodges, R.S. Effect of anionic ion-pairing reagent hydrophobicity on selectivity of peptide separations by reversed-phase liquid chromatography. J. Chromatogr. A 2005, 1080, 68–75. [Google Scholar] [CrossRef]
- Tyrrell, J.; KarLibero, T.; Bartolotti, J. A Study of the Mechanism of the Reaction between Ozone and the Chlorine Atom Using Density Functional Theory. J. Phys. Chem. A 2001, 105, 4065–4070. [Google Scholar] [CrossRef]
- Zhang, G.; Musgrave, C.B. Comparison of DFT Methods for Molecular Orbital Eigenvalue Calculations. J. Phys. Chem. A 2007, 111, 1554–1561. [Google Scholar] [CrossRef] [PubMed]
- Naumov, S.; Mark, G.; Jarocki, A.; von Sonntag, C. The Reactions of Nitrite Ion with Ozone in Aqueous Solution—New Experimental Data and Quantum-Chemical Considerations. Ozone Sci. Eng. J. Int. Ozone Assoc. 2010, 32, 430–434. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S.; Clark, T.; Resnati, G. The σ-hole revisited. Phys. Chem. Chem. Phys. 2017, 19, 32166–32178. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, S. A cartography of the van der Waals territories. Dalton Trans. 2013, 42, 8617–8636. [Google Scholar] [CrossRef]
- Murray, J.S.; Resnati, G.; Politzer, P. Close contacts and noncovalent interactions in crystals. Faraday Discuss. 2017, 203, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Ghosh, B.N.; Bauźa, A.; Rissanen, K.; Frontera, A.; Chattopadhyay, S. Observation of novel oxygen/oxygen interactionin supramolecular assembly of cobalt(III) Schiff base complexes: A combined experimental and computational study. RSC Adv. 2015, 5, 73028–73039. [Google Scholar] [CrossRef]
- Hobza, P. In My Element: Hydrogen. Chem. Eur. J. 2018, 25, 1367–1368. [Google Scholar] [CrossRef]
- Joseph, J.; Jemmis, E.D. Red-, Blue-, or No-Shift in Hydrogen Bonds: A Unified Explanation. J. Am. Chem. Soc. 2007, 129, 4620–4632. [Google Scholar] [CrossRef]
- Cremer, D.; Kraka, E. A Description of the Chemical Bond in Terms of Local Propertiesof Electron Density and Energy. Croat. Chem. Acta 1984, 57, 1259–1281. [Google Scholar]
- Fradera, X.; Austen, M.A.; Bader, R.F.W. The Lewis Model and Beyond. J. Phys. Chem. A 1999, 103, 304–314. [Google Scholar] [CrossRef]
- Poater, J.; Duran, M.; Solà, M.; Silvi, B. Theoretical evaluation of electron delocalization in aromatic molecules by means of atoms in molecules (AIM) and electron localization function (ELF) topological approaches. Chem. Rev. 2005, 105, 3911–3947. [Google Scholar] [CrossRef] [PubMed]
- Bartashevicha, E.V.; Troitskaya, E.A.; Tsirelson, V.G. The N⋯I halogen bond in substituted pyridines as viewed by the source function and delocalization indices. Chem. Phys. Lett. 2014, 601, 144–148. [Google Scholar] [CrossRef]
- Hobza, P.; Müller-Dethlefs, K. Non-Covalent Interactions: Theory and Experiment; RSC Publishing: Cambridge, UK, 2009; p. 139. ISBN 978-1-84755-853-4. [Google Scholar]
- Kemp, D.D.; Gordon, M.S. An Interpretation of the Enhancement of the Water Dipole Moment Due to the Presence of Other Water Molecules. J. Phys. Chem. A 2008, 112, 4885–4894. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wang, X.; Yang, M.; Krishtal, A.; van Alsenoy, C.; Delarue, P.; Senet, P. Effect of hydrogen bonds on polarizability of a water molecule in (H2O)N (N = 6, 10, 20) isomers. Phys. Chem. Chem. Phys. 2010, 12, 9239–9248. [Google Scholar] [CrossRef]
- Pople, J.A. The Lennard-Jones lecture. Intermolecular binding. Faraday Discuss. 1982, 73, 7–17. [Google Scholar] [CrossRef]
- Boys, S.F.; Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Longansen, A.V. Direct proportionality of the hydrogen bonding energy and the intensification of the stretching ν(XH) vibration in infrared spectra. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1999, 55, 1585–1612. [Google Scholar]
- Varadwaj, P.R.; Varadwaj, A.; Marques, H.M.; MacDougall, P.J. The Chalcogen bond: Can it be Formed by Oxygen? Phys. Chem. Chem. Phys. 2019. [Google Scholar] [CrossRef]
- Forni, A.; Moretti, I.; Torre, G.; Bruckner, S.; Malpezzi, L. X-Ray Structure and Stereochemical Properties of (S,S)-(−)-2-Methylsulphonyl-3-Phenyloxaziridine and of (S,S)-(−)-2-Methylsulphonyl-3-(2-Chloro-5-Nitrophenyl)Oxaziridine. J. Chem. Soc. Perkin Trans. 1987, 2, 699–704. [Google Scholar] [CrossRef]
- Sitzmann, M.E.; Bichay, M.; Fronabarger, J.W.; Williams, M.D.; Sanborn, W.B.; Gilardi, R. Hydroxynitrobenzodifuroxan and Its Salts. J. Heterocycl. Chem. 2005, 42, 1117–1125. [Google Scholar] [CrossRef]
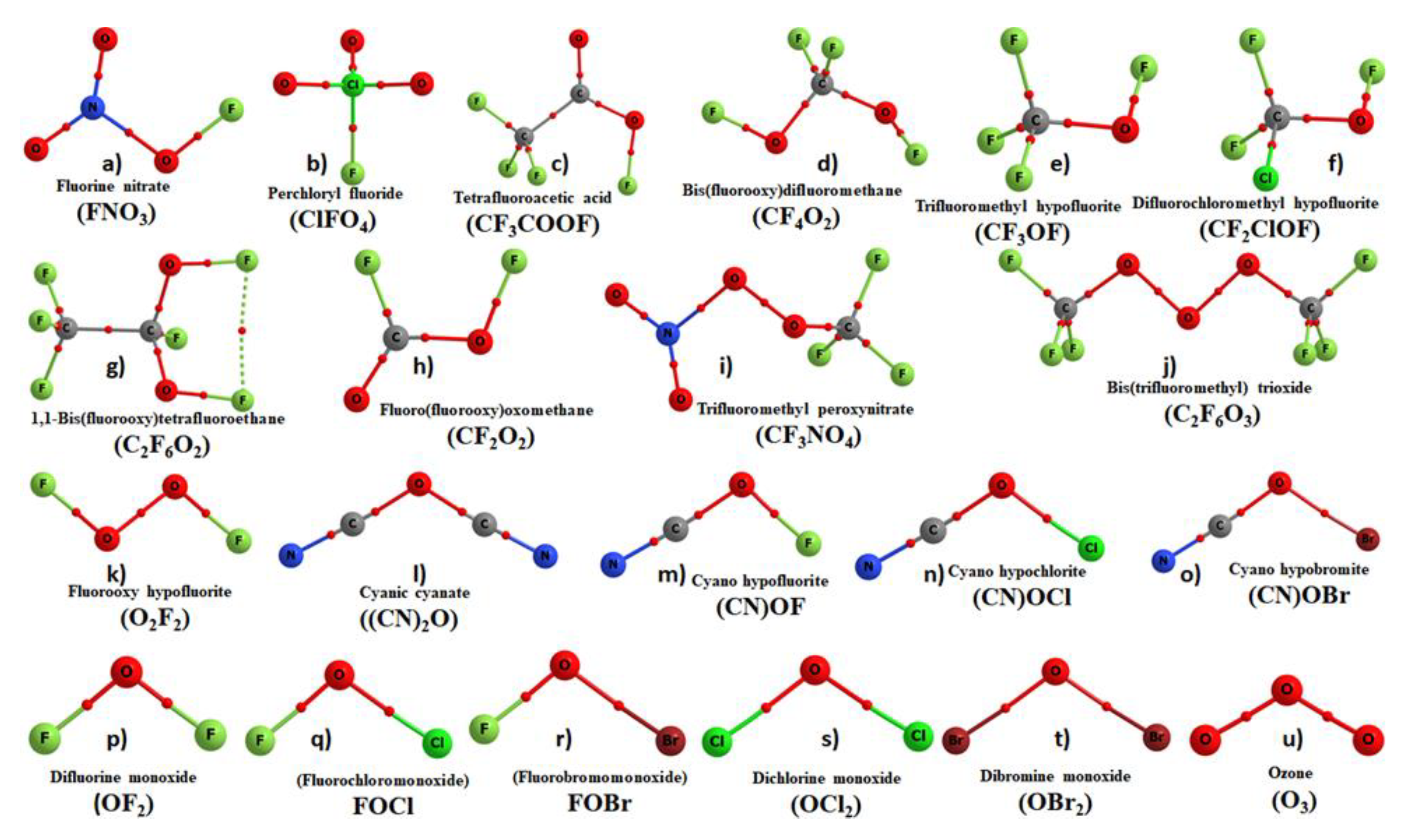
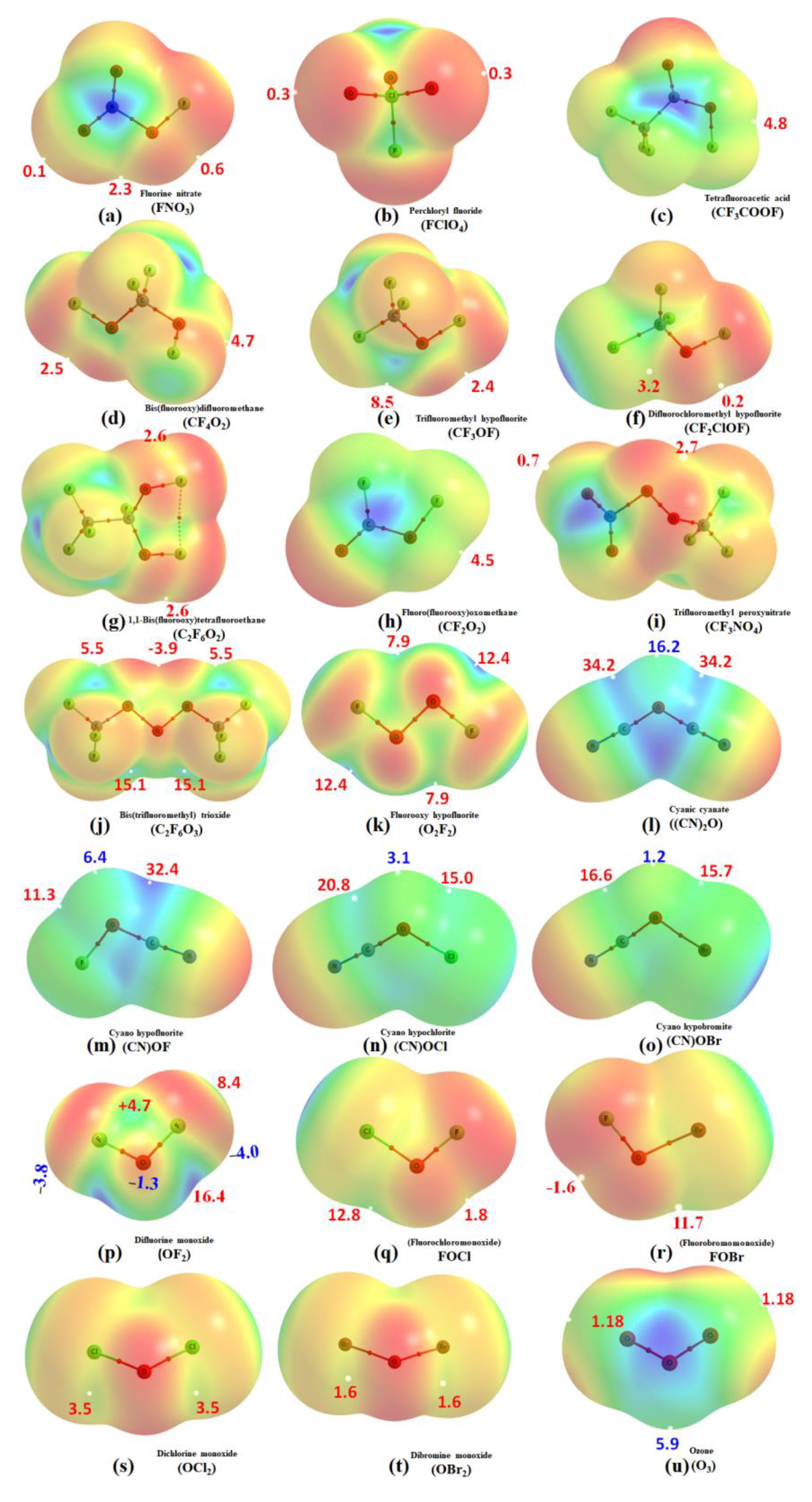
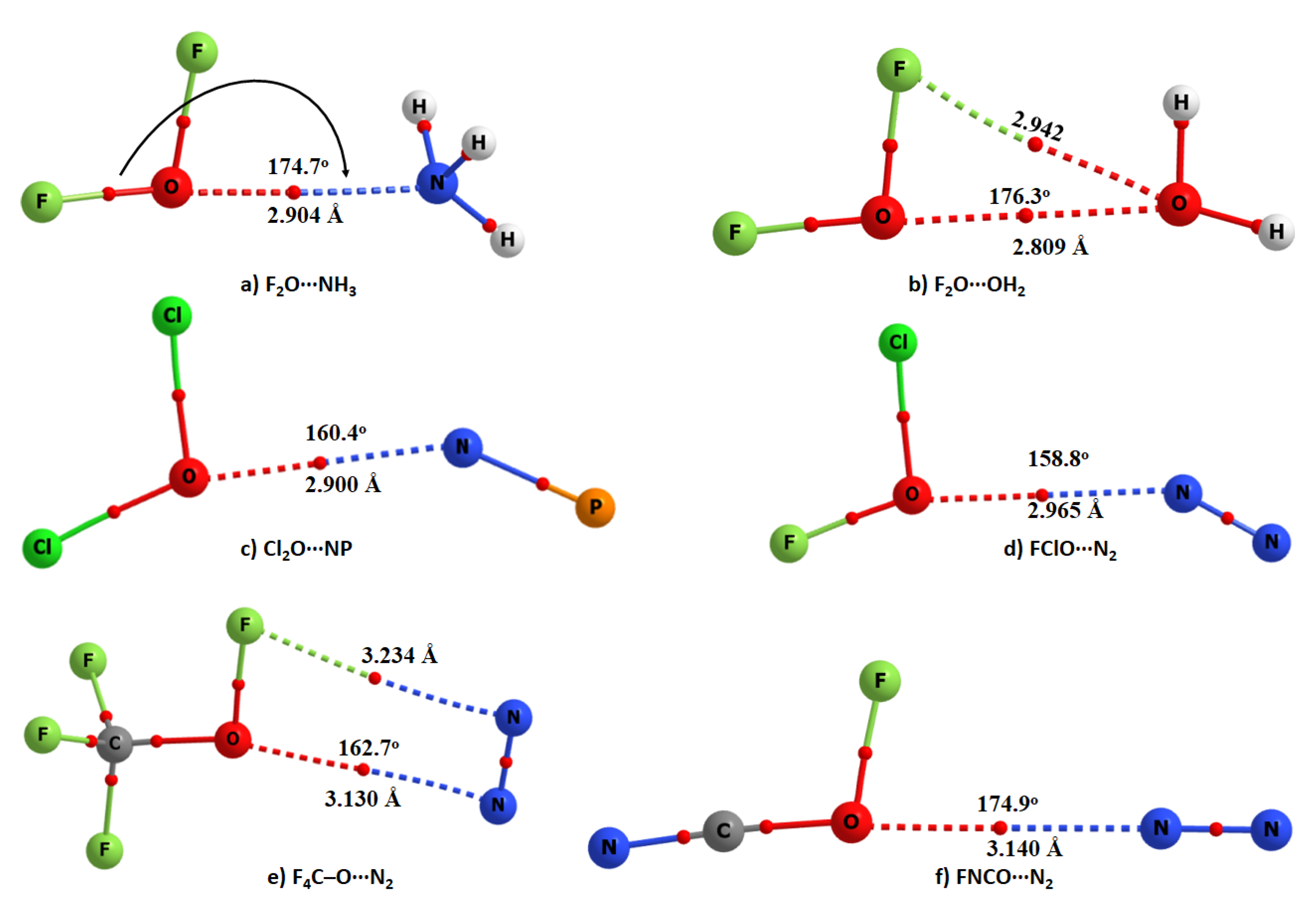
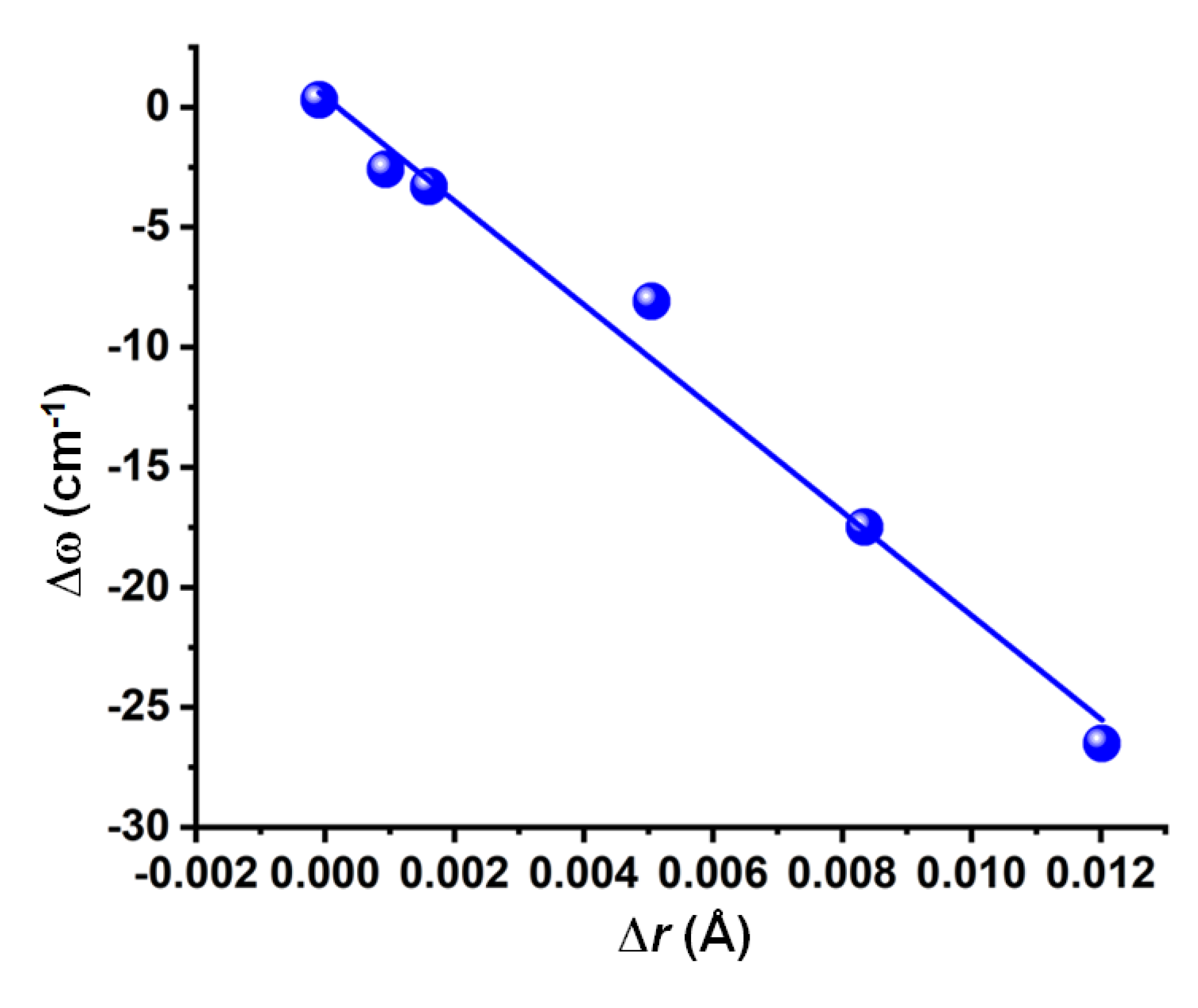

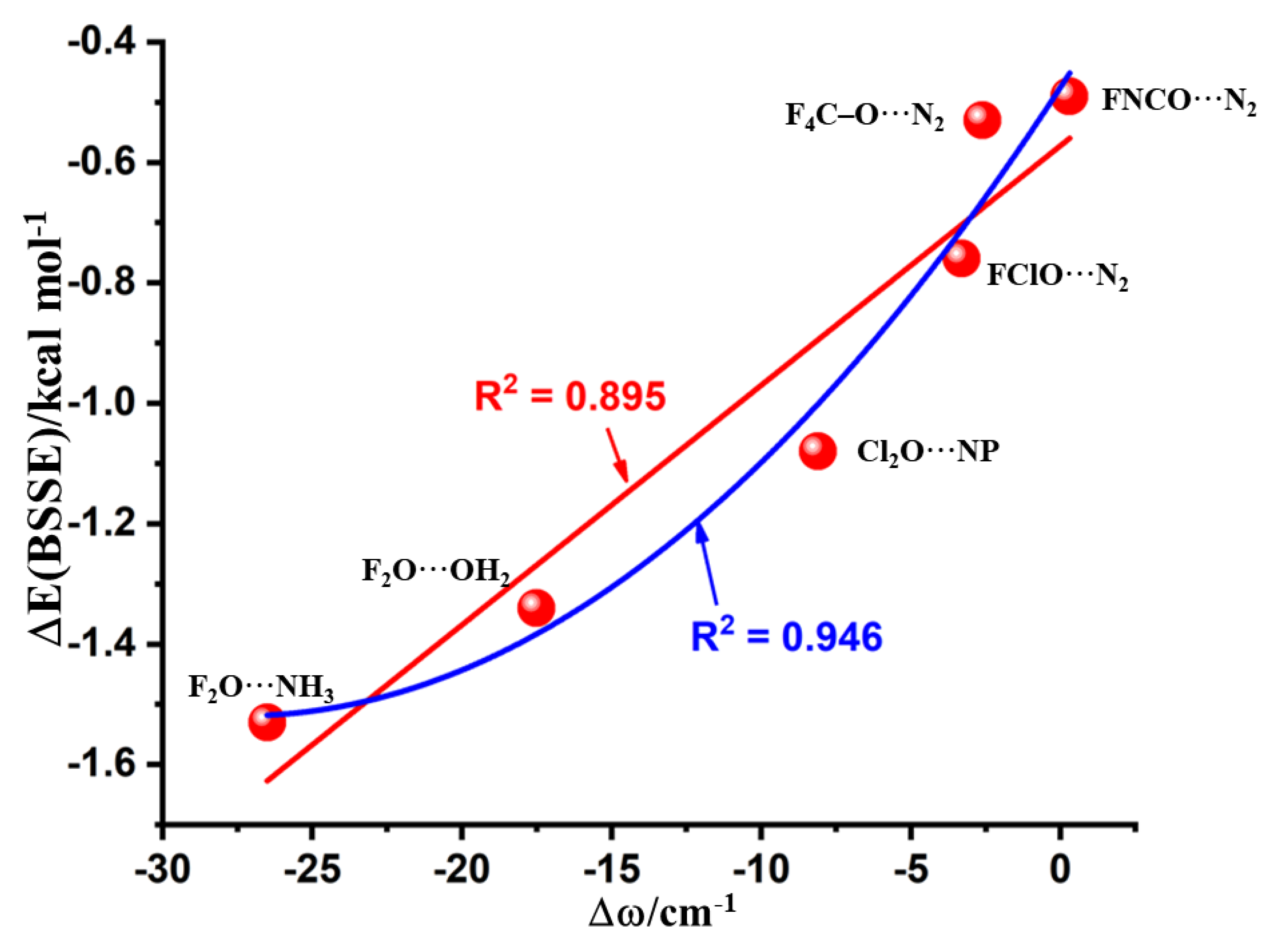
| Complex | Bond | Distance (r) | Δr | ω | I | Δω | ΔI | μ | α | Δμ | Δα |
|---|---|---|---|---|---|---|---|---|---|---|---|
| F2O⋯NH3 | F–O | 1.4109 | 0.0120 | 954.3 | 14.4 | −26.50 | 1.64 | 1.82 | 28.18 | 0.003 | 0.53 |
| F2O⋯OH2 | F–O | 1.4072 | 0.0084 | 963.3 | 12.0 | −17.50 | 1.36 | 2.02 | 23.40 | −0.150 | 0.31 |
| Cl2O⋯NP | Cl–O | 1.7129 | 0.0051 | 645.2 | 0.4 | −8.10 | 0.27 | 2.32 | 67.08 | −0.878 | 1.62 |
| FClO⋯N2 | F–O | 1.4421 | 0.0016 | 858.8 | 21.5 | −3.30 | 1.04 | 1.01 | 36.94 | −0.001 | 0.35 |
| F4C–O⋯N2 | C–O | 1.3911 | 0.0009 | 1240.1 | 358.6 | −2.60 | 1.06 | 0.33 | 36.68 | 0.042 | −0.11 |
| FNCO⋯N2 | C–O | 1.3009 | −0.0001 | 1039.5 | 1.3 | 0.30 | 1.6 | 1.69 | 37.98 | −0.175 | 0.48 |
| Complex | Figure 2 | Eeles | Erep | Epol | Edisp | ΔE(SAPT0) | ΔE(MP2) | ΔE(MP2(BSSE)) |
|---|---|---|---|---|---|---|---|---|
| F2O⋯NH3 | a | −2.65 | 3.14 | −0.65 | −1.61 | −1.77 | −1.86 | −1.53 |
| F2O⋯OH2 | b | −1.81 | 1.87 | −0.37 | −1.35 | −1.66 | −1.66 | −1.34 |
| Cl2O⋯NP | c | −0.69 | 3.02 | −0.57 | −2.66 | −0.91 | −1.56 | −1.08 |
| FClO⋯N2 | d | −0.64 | 1.40 | −0.14 | −1.42 | −0.80 | −1.12 | −0.76 |
| F4C–O⋯N2 | e | −0.34 | 1.05 | −0.08 | −1.22 | −0.59 | −0.88 | −0.53 |
| FNCO⋯N2 | f | −0.31 | 0.71 | −0.04 | −0.90 | −0.54 | −0.81 | −0.49 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varadwaj, P.R. Does Oxygen Feature Chalcogen Bonding? Molecules 2019, 24, 3166. https://doi.org/10.3390/molecules24173166
Varadwaj PR. Does Oxygen Feature Chalcogen Bonding? Molecules. 2019; 24(17):3166. https://doi.org/10.3390/molecules24173166
Chicago/Turabian StyleVaradwaj, Pradeep R. 2019. "Does Oxygen Feature Chalcogen Bonding?" Molecules 24, no. 17: 3166. https://doi.org/10.3390/molecules24173166
APA StyleVaradwaj, P. R. (2019). Does Oxygen Feature Chalcogen Bonding? Molecules, 24(17), 3166. https://doi.org/10.3390/molecules24173166






