Abstract
In spite of significant advancements and success in antiretroviral therapies directed against HIV infection, there is no cure for HIV, which scan persist in a human body in its latent form and become reactivated under favorable conditions. Therefore, novel antiretroviral drugs with different modes of actions are still a major focus for researchers. In particular, novel lead structures are being sought from natural sources. So far, a number of compounds from marine organisms have been identified as promising therapeutics for HIV infection. Therefore, in this paper, we provide an overview of marine natural products that were first identified in the period between 2013 and 2018 that could be potentially used, or further optimized, as novel antiretroviral agents. This pipeline includes the systematization of antiretroviral activities for several categories of marine structures including chitosan and its derivatives, sulfated polysaccharides, lectins, bromotyrosine derivatives, peptides, alkaloids, diterpenes, phlorotannins, and xanthones as well as adjuvants to the HAART therapy such as fish oil. We critically discuss the structures and activities of the most promising new marine anti-HIV compounds.
1. Introduction
Human immunodeficiency virus (HIV) infections pose a global challenge given that in 2017, according to the World Health Organization data, 36.9 million people were living with HIV and additional 1.8 million people were becoming newly infected globally (Table 1). HIV targets immune cells and impairs the human defense against pneumonia, tuberculosis, and shingles as well as certain types of cancer [1]. The most advanced stage of HIV infection is the Acquired Immunodeficiency Syndrome (AIDS), which can take from two to 15 years to develop, depending on the individual [2].

Table 1.
Summary of the global human immunodeficiency virus (HIV) epidemic (2017) according to World Health Organization (WHO) data.
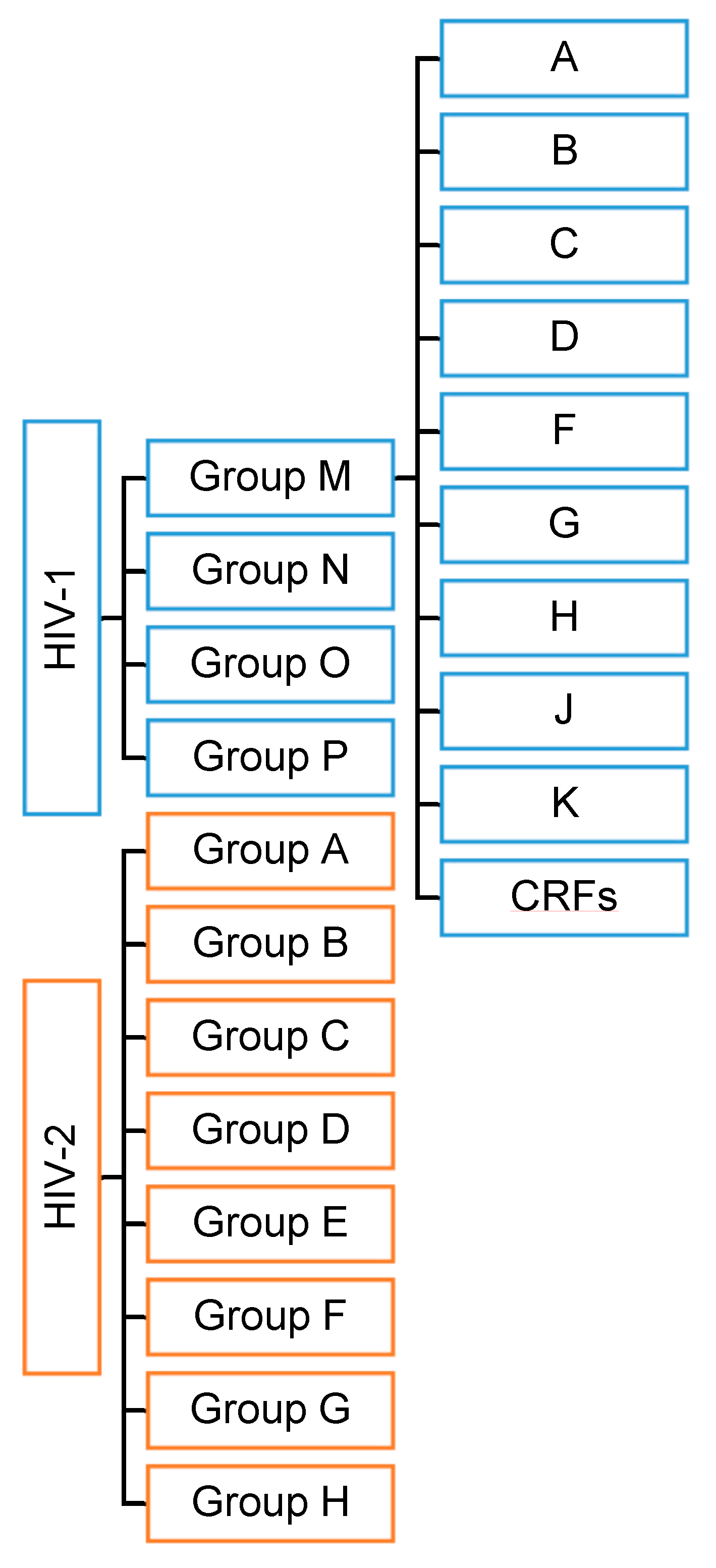
HIV has two viral forms: HIV-1 (the most common form that accounts for around 95% of all infections worldwide) and HIV-2 (relatively uncommon and less infectious). HIV-1 consists of groups M, N, O, and P with at least nine genetically distinct subtypes of HIV-1 within group M (A, B, C, D, F, G, H, J, and K). Additionally, different subtypes can combine genetic material to form a hybrid virus known as the ‘circulating recombinant form’ (CRFs) (Figure 1). HIV-2 consists of eight known groups (A to H). Of these, only groups A and B are pandemic. The HIV-2 mechanism is not clearly defined and neither is its difference from HIV-1. However, the transmission rate is much lower in HIV-2 than in HIV-1. HIV-2 is estimated to be more than 55% genetically distinct from HIV-1.
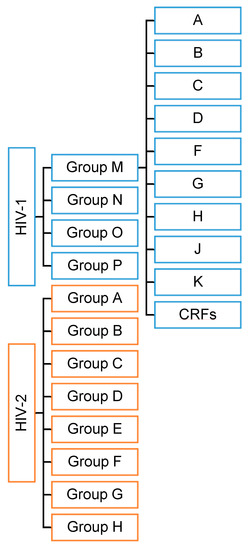
Figure 1.
HIV types and strains classification.
The HIV-1 genome has reading frames coding for structural and regulatory proteins. The gag gene encodes the Pr55Gag precursor of inner structural proteins p24 (capsid protein, CA), p17 (matrix protein, MA), p7 (nucleoprotein, NC), and p6 involved in the virus particle release. The pol gene encodes the Pr160GagPol precursor of the viral enzymes p10 (protease, PR), p51 (reverse transcriptase, RT), p15 (RNase H), and p32 (integrase, IN). The env gene encodes the PrGp160 precursor of the gp120 (surface glycoprotein, SU) and gp41 (transmembrane protein, TM). Other genes include tat, encoding p14 (transactivator protein), rev, encoding p19 (RNA splicing regulator), nef, encoding p27 (negative regulating factor), vif, encoding p23 (viral infectivity protein), vpr, encoding p15 (virus protein r), vpu, encoding p16 (virus protein unique), vpx in HIV2, encoding p15 (virus protein x), and tev, encoding p26 (tat/rev protein) [3].
The HIV infections are extremely problematic as the virus targets the CD4+ memory T-cells population, which is essential for organism immunity. HIV can attach itself to the host cell through 1) a relatively nonspecific interaction with negatively charged cell-surface heparan sulfate proteoglycans [4], 2) specific interactions between the Env and α4β7 integrin [5,6], and/or 3) the interaction with pattern-recognition receptors, such as the dendritic cell-specific intercellular adhesion molecular 3-grabbing non-integrin (DC-SIGN) [7]. The attachment of HIV in any of the abovementioned ways can increase the efficacy of infection because it brings Env, a heavily glycosylated trimer of gp120 and gp41 heterodimers, into close proximity with the viral receptor CD4 and co-receptor [8]. Finally, in order for the viral entry to occur, Env needs to bind itself to the host protein CD4 [9,10].
The binding of the HIV glycoprotein gp120 to the host cell CD4 receptor causes conformational changes of the gp120 glycoprotein, which uncover additional binding sites that interact with distinct proteins on the host cell membrane, known as β-chemokine co-receptors (mainly CCR5 and CXCR4), which facilitate the virus entry into the cell [11].
After the infection, a progressive decline of CD4 + cells consequently leads to the failure of the immune system function and the development of opportunistic infections that usually lead to death [1]. In HIV-infected patients, immunodeficiency develops both as a result of the viral replication and the failure of the patients’ homeostatic mechanisms. The continuous viral presence in the patients after the application of therapy is attributed to the CD4 + T-cell homeostasis owing to a pool of latently infected and resting CD4 + T-cells, macrophages, and follicular dendritic cells that remain in the organism. Indeed, the complex interactions of the patient’s immune system with the virus, and vice versa after the viral suppression, are thought to be crucial for the control of disease progression [12,13].
Current therapeutic approaches mainly target proteins that are vital for the viral cycle. One of the prominent examples is the linear 36-amino acid synthetic peptide enfuviritide (T20, Fuzeon), developed by Hoffmann-La Roche, and the first FDA approved fusion inhibitor for the treatment of HIV-1/AIDS acting through the binding to the gp41 subunit of the HIV-1 envelope glycoprotein. This induces a conformational change that brings the viral and cellular membranes into close enough proximity for the fusion and the subsequent viral entry into the host-cell to occur. Nevertheless, several restrictions, such as a low genetic barrier for drug resistance and a short in vivo half-life, limit its clinical use [14,15,16,17].
Other various FDA-approved antiretroviral drugs from seven mechanistic classes of inhibitors of the HIV replication are also available for the treatment of infected patients, namely, the nucleoside reverse-transcriptase inhibitors NRTIs, non-nucleoside reverse-transcriptase (RT) inhibitors NNRTIs, protein inhibitors PIs, fusion inhibitors, entry inhibitors—CCR5 co-receptor antagonists, HIV integrase strand transfer inhibitors, and multi-class combinations. None of the mentioned drug classes alone or in combination, the latter being known as the highly active antiretroviral therapy (HAART), can eradicate the HIV infection, and effective vaccines remain unavailable.
The difficulties of HIV-1 vaccine research are, in part, a result of 1) the unavailability of a model for natural immunity related to HIV; 2) the existence of genetically distinct subtypes of HIV and frequent mutations; 3) unidentified correlates of specific immune response to HIV; 4) lack of a reliable, non-human animal model for HIV infection (SIV in monkeys vs. HIV in humans).
The established latent pro-viral reservoirs in the patient’s body can stochastically begin to reproduce viral particles, which makes the HIV disease practically incurable. From over 160 compounds identified so far as latency-reversing agents (LRAs), none have led to a promising cure [18].
Several rare and long-term remissions of HIV cases are described in the literature. For example, Berlin, London, and Düsseldorf’s patients underwent bone marrow transplantation with stem cells from a donor with a rare genetic mutation of the CCR5. The Mississippi baby received a very early antiretroviral therapy that extended the time of the viral rebound for more than 27 months. There undoubtedly remains a lot to be learned from these cases, and further investigation of stem-cell transplantation in people living with HIV is required [19,20,21].
The currently used antiretroviral treatment, alone or in combination, extends the quality and life expectancy of HIV-infected individuals but does not cure them. Drug resistance, along with the emergence of drug-resistant virus strains, a high-cost of the lifetime treatment regimen, cell toxicity, and serious side effects of currently used anti-HIV drugs [2] underlie the need for a synthetic development of new drugs or the search for active anti-HIV molecules in natural sources. Mother Nature has been perfecting its chemistry for three billion years, and most of it has been done in water. Intense competition and feeding pressure as well as non-static marine environmental conditions yield compounds with chemical and structural features generally not found in terrestrial natural products.
New efficient molecules directed against HIV should demonstrate better performance in comparison with the currently approved drugs and suppress the HIV virus and/or eliminate the latent HIV reservoirs present in the human body.
Around 60% of drugs currently available on the market are derived or inspired by nature [22]. Turning to nature for drug development holds great potential, especially when it comes to marine organisms. Only few marine-derived drugs have been approved on the market so far but many are in the preclinical or clinical stage of development [23]. Marine organisms make up to two-thirds of Earth’s species and produce, as a consequence of living in a highly competitive environment, unique and structurally diverse metabolites. Over the last 40 years, bioprospecting efforts have resulted in over 20,000 compounds of marine origin. The highest share of marine metabolites (up to 70%) are obtained from marine sponges, corals, and microorganisms, while mollusks, ascidians, and algae metabolites form only a minor part [24]. Oceans are, indeed, still a rather underexploited habitat, and biodiversity appears to be higher in the oceans than on land, which might be relevant when focusing on the marine environment as an untapped reservoir of novel antiretroviral candidates. In the discovery of new antiviral marine-derived drugs, researchers usually implement two strategies. They either screen the extracts from different strains (e.g., cyanobacteria, microalgae) or search directly for bioactive molecules in organisms—extract and purify them for evaluation within the drug development pipeline. It is thought that the marine environment might yield more potent anti-HIV candidates characterized by a higher efficiency (lower effective dose) and a better selectivity and which do not induce resistance development. This could, of course, only be speculation based on some of the previous success stories in the discovery of drugs from natural sources such as, e.g., lovastatin and paclitaxel. However, nature generally does create more sophisticated and perfected systems with a complex mode of action.
An excellent example is protein lectin, derived from marine red algae Griffithsia sp. named Griffithsin with mid-picomolar activities, which groups it among the most potent HIV entry inhibitors reported so far [25]. It inhibits the HIV infection by binding itself to high mannose glycan structures on the surface of gp120, altering the gp120 structure or its oligomeric state [26]. This interaction relies on the specific trimeric “sugar tower,” including N295 and N448 [27]. Griffitshin can also prevent infections caused by other glycoprotein-enveloped viruses such as the Ebola virus, hepatitis C virus, and the severe acute respiratory syndrome coronavirus. It has been shown that the dimerization of Griffithsin is necessary for a high potency inhibition of HIV-1 [28]. However, the discrepancy between the HIV gp120 binding activity and the HIV inhibitory activity points to the presence of mechanism unrelated to a merely simple HIV gp120 binding [26]. The most promising application of Griffithsin would be its incorporation into vaginal and rectal gels, creams, or suppositories acting as an antiviral microbicide to prevent the transmission of HIV.
Despite the vast number of structurally diverse and unique bioactive molecules from the marine environment, the global marine pharmaceutical pipeline includes only eight approved drugs: Adcetris®, Cytosar-U®, Halaven®, Yondelis®, Carragelose®, Vira-A®, Lovaza®, and Prialt® [29]. Overall, it has taken 20 to 30 years from their discovery to their entry into the market. A sustainable supply, structural complexity, optimization of formulation, and ADMET properties, and a scale-up issue have prevented further development of several highly promising marine compounds. It is by no means an easy task to identify a marine candidate that may be considered as a potential drug. Initial high costs of developing a natural product into a drug could be balanced out with careful long-term considerations (biodiversity, supply, and technical, market) [30].
This paper provides an overview of natural marine metabolites that were first identified in the period between 2013 and 2018 or the previously identified marine constituents with a recently confirmed anti-HIV activity that could be potentially used or further optimized as novel ant-HIV agents. We also comprehensively summarize anti-HIV activities for several categories of marine structures including chitosan and its derivatives, sulfated polysaccharides, lectins, bromotyrosine derivatives, peptides, alkaloids, diterpenes, phlorotannins, and xanthones as well as fish oil as an auxiliary to HAART therapy.
2. Marine Compounds in the Treatment of HIV/AIDS
2.1. Chitosan and Its Derivatives
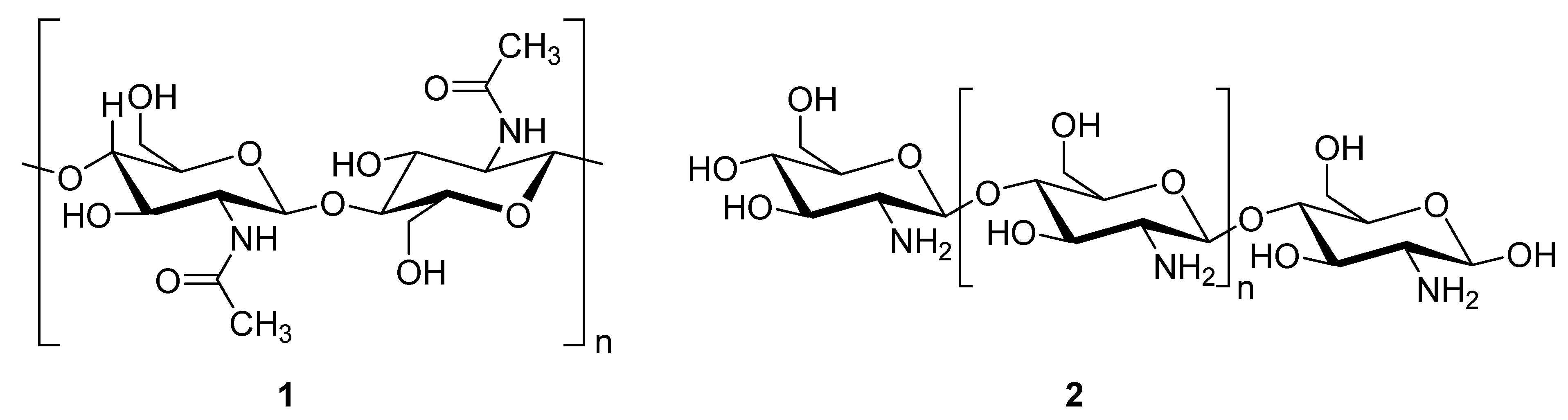
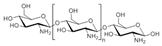
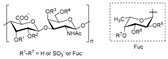
Chitosan (2, Figure 2), a natural marine byproduct, is a poly-cationic linear polysaccharide derived from chitin (1, Figure 2) after partial deacetylation. Chitin is a structural element in the exoskeleton of mainly shrimps and crabs and is mainly composed of the randomly distributed β-(1-4)-linked D-glucosamine and N-acetyl-D-glucosamine. It has been previously shown that this compound can exhibit a large scale of different bioactivities and can also be used as a carrier for anti-HIV drugs [31]. Chitosan is loaded with saquinavir, an anti-HIV drug with a protease inhibitory activity, which showed better cell targeting efficiency than saquinavir alone [32]. Furthermore, trimethyl chitosan has improved Atripla, an anti-HIV drug consisting of efavirenz, emtricitabine, and tenofovir disoproxil fumarate, anti-HIV 1 activity, and has allowed it to be used in lower concentrations [33]. The antiretroviral activity is manifested in the chitosan-specific cationic nature that allows the formation of electrostatic complexes or multilayer structures with other negatively charged polymers [34]. Karagozlu et al. reported about new QMW-COS and WMQ-COS oligomers with anti-HIV activities. These oligomers are conjugates of chitosan and the Gln-Met-Trp peptide, which were constructed as a continuation of the authors’ previous research, in which a high potency of synthetically constructed chitosan oligomers was confirmed in anti-HIV therapy. More specifically, it was shown that these oligomers suppress syncytium formation, which occurs as a fusion of infected cells with neighboring cells, induced by HIV in a dose-dependent manner. However, the authors also noticed that after a certain period, the number of syncytia once again increased, suggesting that the cells should be re-treated with QMW-COS and WMQ-COS oligomers to maintain the primary therapeutically-relevant effect. The inhibition of the HIV-1 induced lytic effect, determined by the cell viability assay, showed that IC50 for QMW-COS was 48.14 µg/mL and was almost identical for WMQ-COS, 48.01 µg/mL. These oligomers effectively reduced the HIV load but showed no effects on HIV-1 RT and protease in vitro. Higher dosages were also required for the reduction in the HIV-1IIIB p24 antigen production assessed by the ELISA assay and the HIV-1RTMDR p24 antigen production. The highest difference between the compounds was reflected in IC50 values obtained from studies on the virus-induced luciferase activity in infected cells, where QMW-COS had a higher potency in comparison with WMQ-COS. Lastly, the authors determined the effects of oligomers on the interaction between gp41 and CD4 by using the CD4-gp41 ELISA assay, whereby both oligomers showed high potency. The effect of these oligomers was highest when they were applied immediately upon the HIV-1 infection of cells, indicating that they should be used as a potential treatment in the early stages of HIV infection, probably at the entry stage [31].
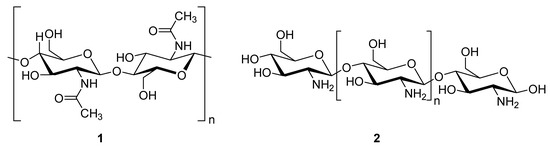
Figure 2.
Chemical structures of chitin (1) and chitosan (2).
2.2. Sulfated Polysaccharides
Sulfated polysaccharides (SP) are the most studied class of antiviral polysaccharides that are structural components of the alga cell wall where they play both the storage and structural role. They are an important source of galactans, commercially known as agar and carrageenan in red alga (Rhodophyta), fucans (fucoidan, sargassan, ascophyllan, and glucuronoxylofucan) in brown alga (Phaeophyta), and ulvans-sulfated heteropolysaccharides that contain galactose, xylose, arabinose, mannose, glucuronic acid, or glucose [35,36,37]. Many studies indicate that, in marine algae, sulfated polysaccharides facilitate water and ion retention in extracellular matrices, which is an important mechanism for coping with desiccation and osmotic stress in a highly salted environment [38,39,40]. The antiviral activity of this group of compounds is mainly connected to the degree of sulfation, constituent sugars, molecular weight, conformation, and dynamic stereochemistry [41,42]. The effect of counter cation should also be considered as an important factor in observed biological activity.
The antagonizing effect of the negatively charged sulfated polysaccharides on the HIV-1 entry into cells may be due to 1) their binding onto the positively charged V3 domain of gp120, thereby preventing the virus attachment to the cell surface [43,44,45] or 2) the masking of the docking sites of gp120 for sCD4 on the surface of T lymphocytes, thereby disrupting the CD4-gp120 interaction [46,47,48] and subsequently inhibiting the expression of the viral antigen and the activity of the viral reverse transcriptase [49,50].
2.2.1. Heparan Sulfate
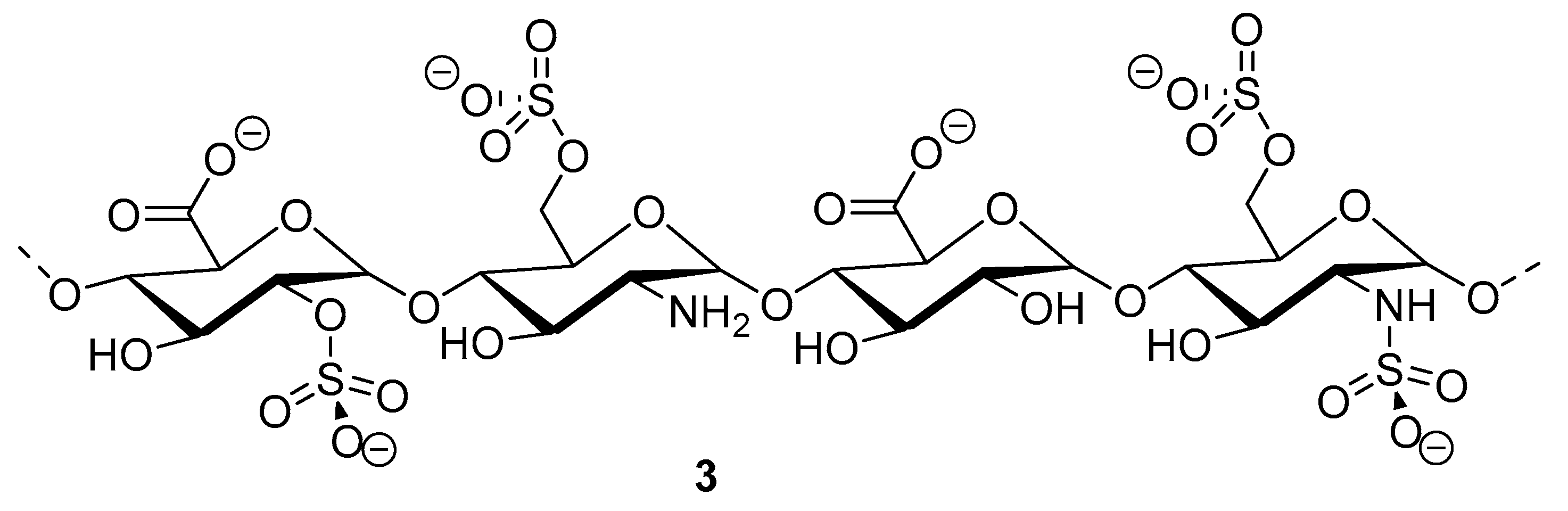
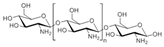
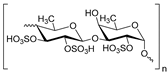
Heparinoid polysaccharides can interact with the positive-charge regions of cell-surface glycoproteins, leading to a shielding effect on these regions, which prevents the binding of viruses to the cell surface [51]. The sulfated polysaccharides content in marine mollusks is high in comparison with the bovine mucosal heparin (73.5%) and the porcine mucosal heparin (72.8%) [52]. The acidic sulfate groups on heparin (3, Figure 3), or heparin-like compounds, can inhibit HIV through electrostatic interactions with basic amino-acid residues of the transcriptional activator Tat protein [53].
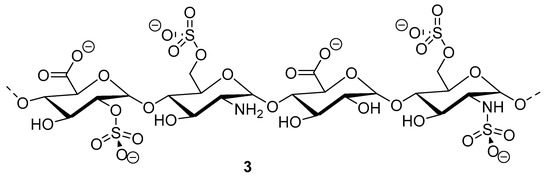
Figure 3.
Structure of heparan sulfate (3).
2.2.2. Fucose Containing SP
So far, the main anti-infectious activities documented for the fucose-containing SP are those against viruses [54]. More importantly, these polysaccharides are selective inhibitors of various enveloped viruses, including HIV [54,55,56]. FCSP acts during the early phase of infection by blocking the virus attachment and entry into the host cells, but may also inhibit subsequent replication stages in vitro [57].
2.2.3. Fucoidans
Three fucoidans extracted from three brown seaweeds (Sargassum mcclurei, Sargassum polycystum, Turbinara ornata) inhibit the early stages of HIV-1 entry into target cells, with IC50 ranging from 0.33 to 0.7 µM. Neither the sulfate content nor the position of sulfate groups are related to the anti-HIV activity of fucoidans, suggesting the involvement of other structural parameters such as the molecular weight, the type of glycosidic linkage, or even a unique fucoidan sequence [56]. Although the presence of sulfo-groups seems to be necessary for anti-HIV activity [58], these data do not support random sulfation as the main antiviral factor.
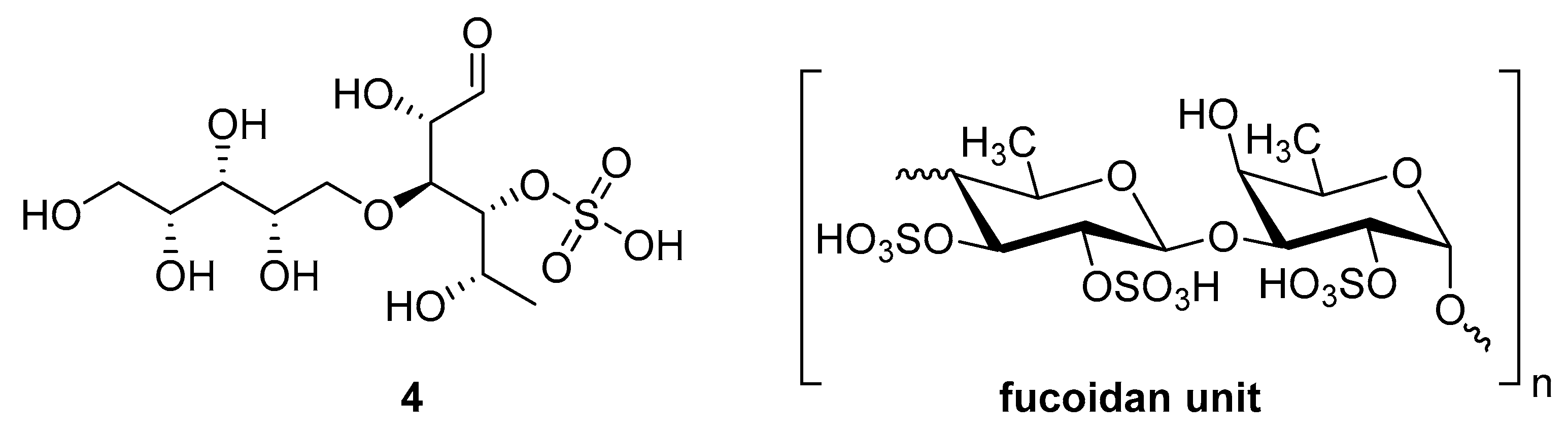
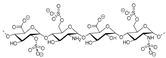
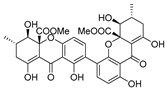
Sulfated fucan polysaccharides, ascophyllan (4, Figure 4), and two fucoidans (S and A) (5 and 6, Table 2), derived from different sources, significantly inhibit (IC50 1.3; 0.3; 0.6 µg/mL) the early step of HIV-1 (R9 and JR-Fl) infection. They also inhibit the VSV-G-pseudotype HIV-1 infection in HeLa cells [59].
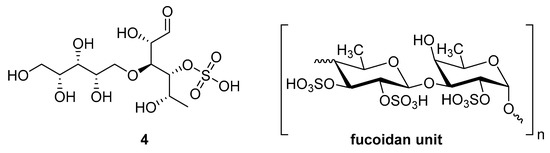
Figure 4.
Structure of ascophyllan (4) and fucoidan unit.

Table 2.
Chemical composition of polysccharides (Fuc, Fucose; Xyl, Xylose; Glu, Glucose; Man, Mannose; Gal, Galactose) in ascophyllan, S- and A-fucoidan.
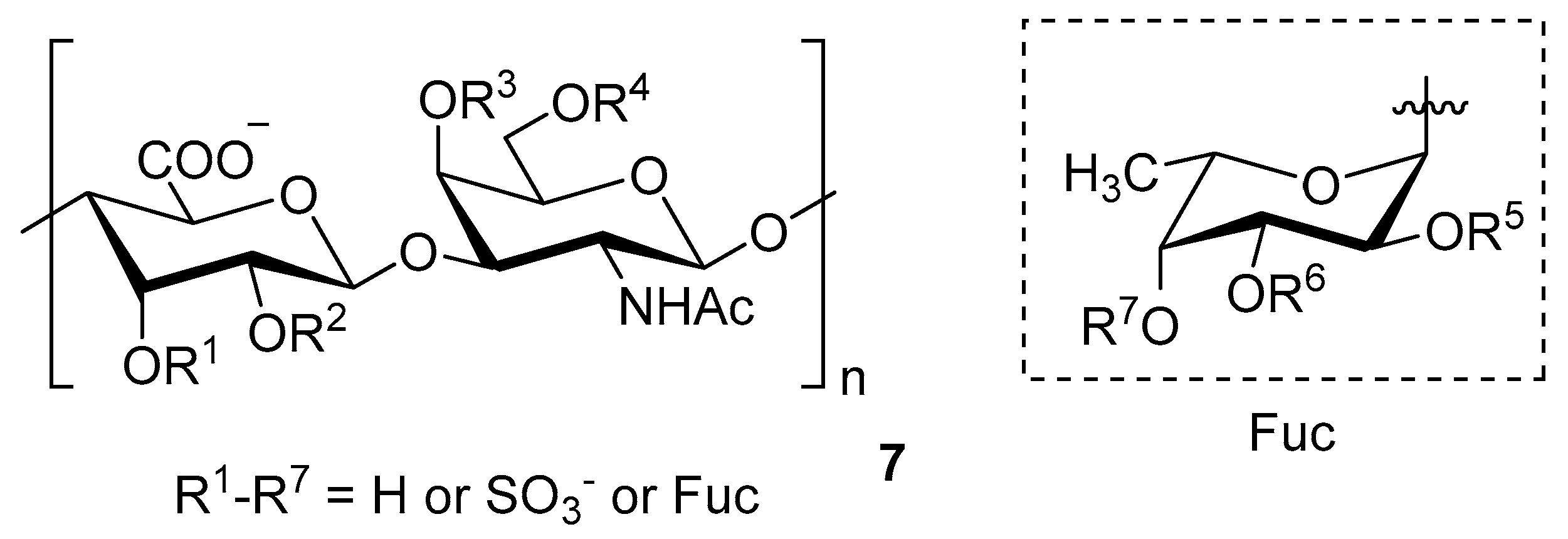
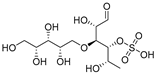
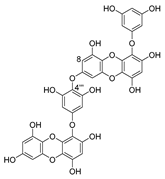
Chondroitin sulfate with fucosylated branches (FuCS) (7, Figure 5) has also attracted attention as an HIV antiviral compound. Depolymerized fucosylated CS, extracted from the sea cucumber, has shown in vitro activity against a range of viral strains, including the resistant ones [60]. FuCS is effective in blocking the laboratory strain HIV-1IIIB entry and replication by inhibiting the p24 antigen production (4.26 and 0.73 μg/mL, respectively) and the infection of the clinic isolate HIV-1KM018 and HIV-1TC-2 (23.75 and 31.86 μg/mL, respectively) as well as suppressing the HIV-1 drug-resistant virus. Additionally, FuCS is also effective in T-20-resistant strains (EC50 values ranging from 0.76 to 1.13 μg/mL). The depolymerized fragments seem to maintain a similar anti-HIV action at the early stages of infection, apparently through interaction with an HIV envelope glycoprotein gp120. The sulfated fucose branches appear necessary for antiviral activity, which is also affected by molecular weight and carboxylation [61]. While the in vitro results of the fucosylated CS against HIV are promising, it is questionable whether the antiviral activity would be maintained in vivo. Other polyanionic HIV entry inhibitors, which advanced into clinical trials, failed to prove effective against the heterosexual HIV-1 transmission. This was related to factors not considered in previous development stages, such as the presence of seminal plasma and the concentration and retention of polyanionic inhibitors [62].
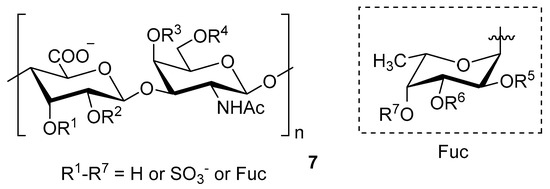
Figure 5.
General chemical structure of fucosylated chondroitin sulfate (7).
The complex chemical architecture and the sulfate patterning of marine polysaccharides depends on numerous factors (species, tidal cycles, environmental variations (e.g., salinity), harvesting season, plant age, geographical location etc.) [39,63,64,65,66,67,68,69], making isolation, purification, and comprehensive chemical characterization a highly challenging task [70]. The development of many polysaccharides into clinical application is hindered by the still limited view of their sophisticated and diverse nature. Despite having good antiviral effects, the use of carbohydrate drugs is still in its infancy, and intensive structure-activity and in vivo studies are needed in the future.
A relatively new strategy in inducing immunity and developing an HIV vaccine is to use carbohydrates. The major difficulty of such an approach lies in mimicking the specific glycan protective epitope. Gp120 of HIV is a highly glycosylated envelope surface glycoprotein responsible for the receptor and co-receptor binding, which, together with gp41, comprises the heterodimeric envelope trimer spikes of HIV. N-linked glycans, mainly mannose and complex-type, cover much of the gp120 surface-accessible face of the HIV envelope spike forming the glycan shield. Inadequate mimicry of the glycan shield, tolerance mechanisms, and/or the inability to induce a domain-exchange are reflecting difficulties in creating the proper specificity of Abs [71]. Most of the vaccines for HIV-1 in preclinical trials are based on a Manα1-2Man oligomannosyl epitope (various conjugates, engineered yeast strains, and modified glycoproteins) [72,73,74,75,76,77,78,79]. Better specificity could potentially be gained using carbohydrates of marine origin.
2.3. Lectins
Lectins are a group of proteins that specifically, but reversibly, bind glycosylated molecules on the cell surface. Precisely, this group of molecules can affect cell-cell interactions, protect cells from pathogens, influence cell adhesion, and affect the intracellular glycoprotein translocation [80]. Recently, lectins have become promising agents for antiretroviral therapy, and different researches have confirmed their anti-HIV properties. Their antiretroviral activity is manifested through an alteration of the interaction between HIV gp120 or gp41 and the corresponding receptors [81], which, in the end, inhibit the HIV cell function, HIV infectivity, and the formation of the syncytium, multi-nucleated cells [82,83,84].
Several published review papers describe the previously found marine lectins with antiretroviral action [85,86]. For example, Gogineni et al. reported about some new, unusual lectins, such as the β-galactose specific lectin (CVL), CGL, DTL, DTL-A, SVL-1, and SVL-2 [86]. Additionally, Akkouh et al. reported about some new algal lectins, such as Boodlea Coacta Lectin, Griffithsin and Oscillatoria Agardhii Agglutinin (OAA), and some cyanobacterial lectins, such as Cyanovirin-N, Scytovirin, Microcystis Viridis Lectin, and Microvirin.
However, in the last few years, there has not been as much research focused on anti-HIV lectins from marine sources. Only Hirayama et al. (2016) reported about the new high-mannose specific lectin and its recombinants that possess anti-HIV activity [87]. In their research performed on the red alga Kappaphycus alvarezii, authors confirmed KAA-1 and KAA-2, two KAA mannose-binding lectin isomers, as potent anti-HIV agents. The anti-HIV role of action of these two compounds includes a strong binding to the virus envelope glycoprotein gp120 and, consequently, the inhibition of HIV entry into the host cells. These KAA recombinants, as well as the native one, inhibited the HIV-1 entry at IC50s (neutralization assay in Jurkat cells) of 7.3–12.9 nM. Authors concluded in the end that KAAs, besides their strong inhibitory effect on HIV entry into the cells, have a potential as agents in treatments against other viruses possessing high mannose glycans on their envelope as well.
2.4. Peptides
It has been shown that the majority of marine peptides have strong anti-HIV activity. They are usually isolated from marine organisms through the process of enzymatic hydrolysis [88]. The most common source of such constituents is marine sponges that are known for their unique metabolome [89] and are a source of more than 36% of all marine bioactive compounds [90]. Their bioactive peptides can be found in cyclic or linear forms and contain unusual amino acids that form unique structures rarely found in other species. Antiretroviral activity of such structures works on several different levels: blocking of virus entry, inhibition of the cytopathic viral activity, neutralization of viral particles, or inhibition of viral fusion and entry [89,91].
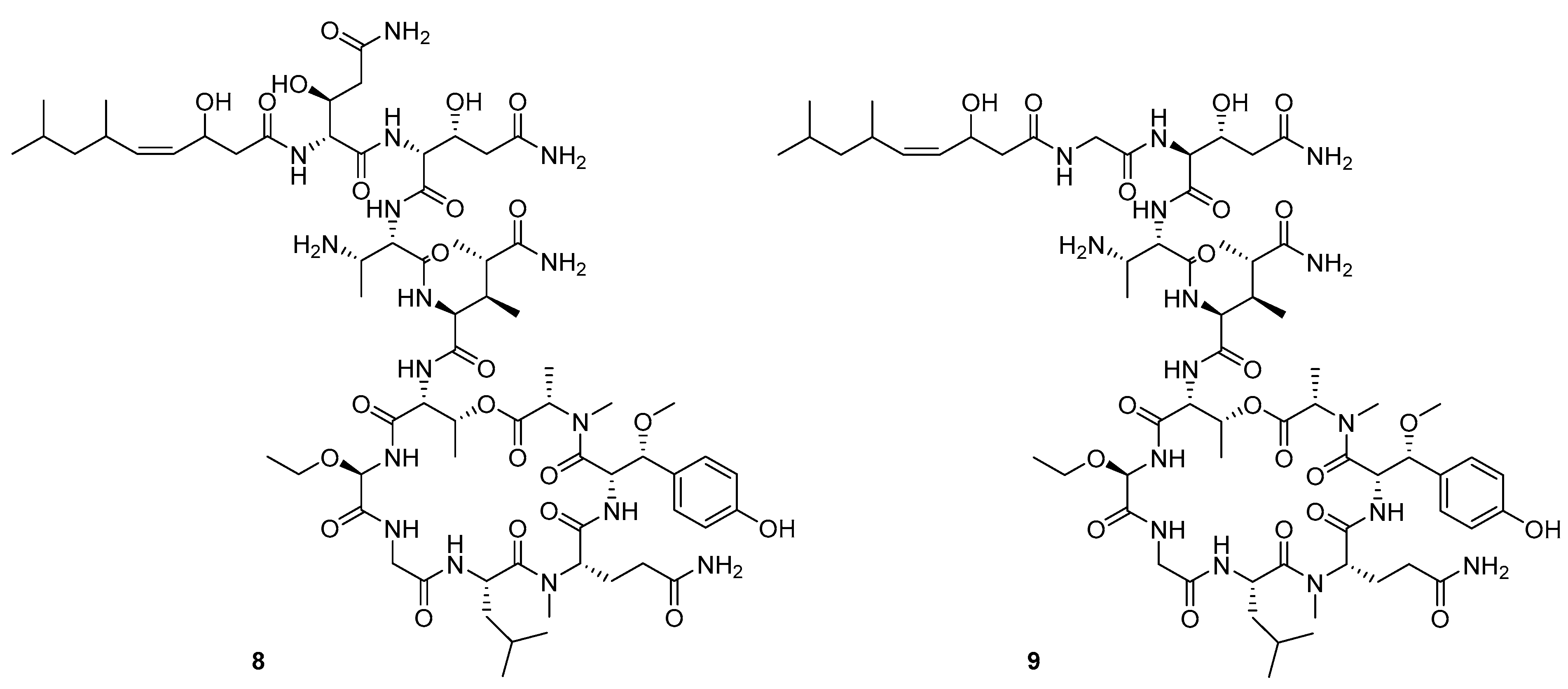
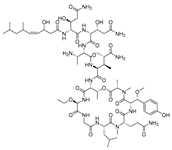
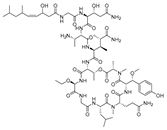
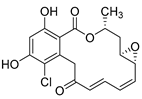
Recently, Shin et al. discovered two new depsipeptides from marine sponges Stelletta sp., stellettapeptin A (8, Figure 6), and stellettapeptin B (9, Figure 6), with the inhibition of the cytopathic effect of HIV-1 infection [92]. Confirming the mentioned theory about the unique metabolome of marine sponges, the authors revealed that these two compounds have previously undescribed nonproteinogenic amino-acid parts on peptides that are rarely found in nature. Namely, stellettapeptin A and stellettapeptin B have an unexpected polyketide subunit, 3-hydroxy-6,8-dimethylnon-4-enoic acid, 3-OHGln, and 3-OHAsn residues. Their high potency is witnessed through low EC50 values (inhibition of the cytotoxic effect upon HIV infection)—values of 23 nM for stellettapeptin A and 27 nM for stellettapeptin B.
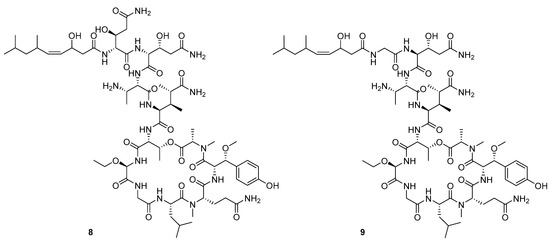
Figure 6.
Structures of stelletapeptin (8) A and stelletapeptin B (9).
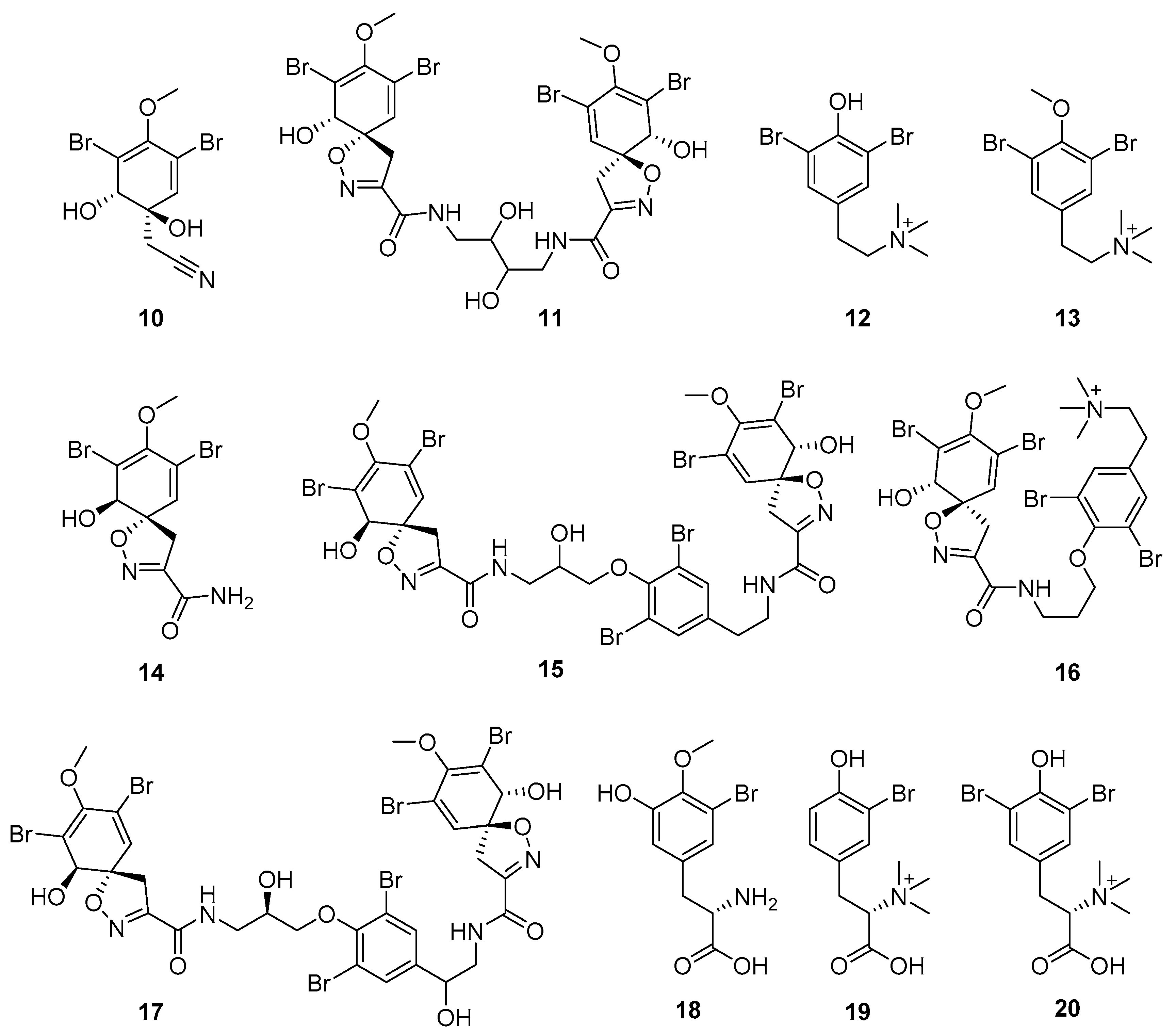
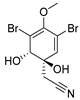
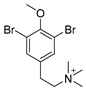
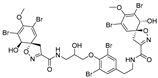
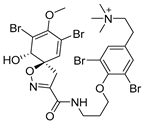
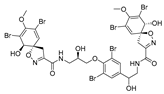
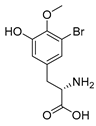
Furthermore, newly discovered anti-HIV constituents derived from marine sponges Verongula rigida and Aiolochoria crassa with amino-acid structure were published by Gomez-Archila et al. (2014) [93]. In their paper, they evaluated and confirmed the anti-HIV effect of 11 bromotyrosine derivatives (Table 3), whereby aeroplysinin-1 (10), 19-deoxyfistularin 3 (15), purealidin B (16), fistularin 3 (17) and 3-bromo-5-hydroxy-O-methyltyrosine (18, Figure 7) were the most potent in their anti-HIV activity. Aeroplysinin 1 (15) and purealidin B (16), compounds found in V. rigida species inhibited the HIV-1 replication in a dose-dependent manner by more than 50%. Specifically, for aeroplysinin 1, HIV-a replication was inhibited by 74% at a concentration of 20 µM, whereas purealidin was less potent with inhibitory power of 57% at a concentration of 80 µM. These two compounds had been previously isolated; however, their anti-HIV activity was proven in this research. The same was with 3-bromo-5-hydroxy-O-methyltyrosine (18) that has a relatively high percentage of inhibition of HIV activity (47%) in a dose-dependent manner. However, the exact mechanism of action remains unclear. In the same study, additional tests with these compounds on the HIV RT inhibition (qPCR of the early and late transcripts), nuclear import (qPCR analysis of 2-LTR transcript), and HIV entry inhibition (viral infectivity assay) were performed. The results showed that aeroplysinin-1 (10), 19-deoxyfistularin 3 (15), purealidin B (16), fistularin 3 (17), and 3-bromo-5-hydroxy-O-methyltyrosine (18) influenced the nuclear import of the HIV virus with around or more than 50% of inhibition: aeroplysinin-1 (10) showed 67% of inhibition at 10 µM, 19-deoxyfistularin 3 62% inhibition at 20 µM, purealidin B 66% of inhibition at 20 µM, fistularin 3 47% of inhibition at 10 µM, and 3-bromo-5-hydroxy-O-methyltyrosine 73% of inhibition at 80 µM. Viral RT inhibition was not high for all compounds, whereby the highest results were around 50% of inhibition. For example, purealidin B had 58% of inhibition at 20 µM in the qPCR analysis of early transcripts. As for the HIV entry inhibition, all compounds were active in a dose-depended manner, with the highest results of inhibition obtained for 3,5-dibromo-N,N,N,O-tetramethyltyraminium (13), from 14% to 30%. Finally, the authors stressed the structural similarity of these compounds with the HIV integrase and protease inhibitors, suggesting that these compounds can have a broader mode of antiviral action.

Table 3.
Summary of anti-HIV compounds from marine organisms.
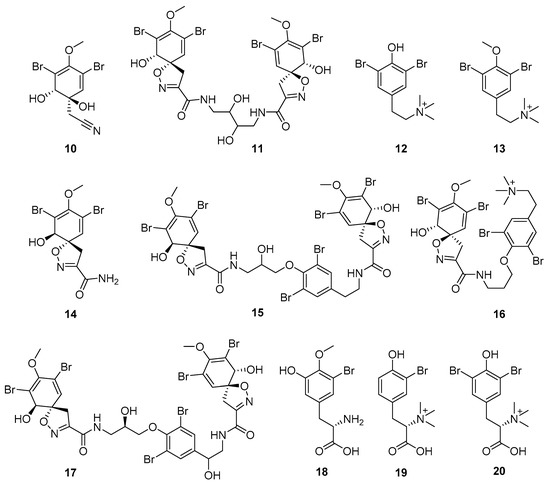
Figure 7.
Structures of aeroplysinin-1 (10), dihydroxyaerothionin (11), 3,4-dibromo-N,N,N-trimethyltyraminium (12), 3,5-dibromo-N,N,N,O-tetramethyltyraminium (13), purealidin R (14), 19-deohxyfistularin 3 (15), purealidin B (16), fistularin-3 (17), 3-bromo-5-hydroxy-O-methyltyrosine (18), 3-bromo-N,N,N-trimethyltyrosinium (19), and 3,5-dibromo-N,N,N-trimethyltyrosinium (20).
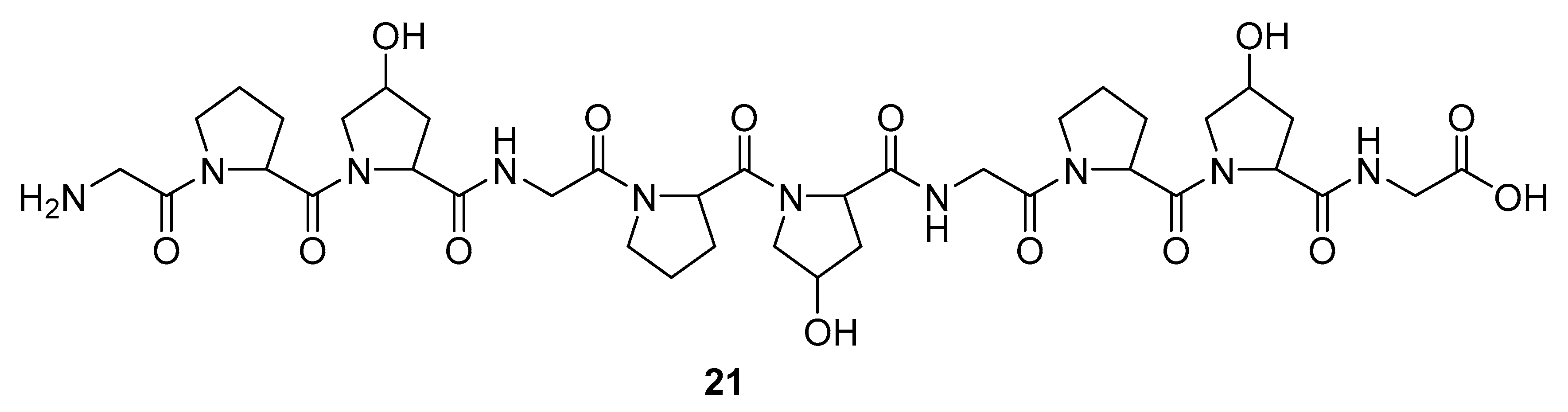
Marine sponges are not the sole source of bioactive proteins. For example, Jang et al. reported about a new small hydroxyproline-rich peptide from Alaska Pollack collagen (APHCP, 21, Figure 8) that exhibits a unique antiviral activity [94]. This peptide is a Gly-Pro-Hyp-Gly-Pro-Hyp-Gly-Pro-Hyp-Gly peptide, and the authors showed that the most important part of a peptide for anti-HIV activity is the hydroxyl group at hydroxyproline, whereas a peptide with only prolines does not exhibit antiviral activity. Its anti-HIV 1 mode of action is manifested through the inhibition of the induced syncytia formation by the interference of an HIV fusion, inhibition of cell lysis, RT activity, and the production of the p24 antigen. It was shown that APHCP can decrease the HIV-1 induced cell lysis at a potency around EC50 of 459 µM (EC50 against anti-HIV-1 induced cell lysis—MTT assay). Additionally, through the inhibition of the viral RT at EC50 at 374 µM, this peptide’s crucial role in the inhibition of the conversion of viral RNA to DNA was also confirmed. With EC50 of 405 µM, this compound effectively suppressed the p24 production in viral cells, as determined by the Western blot analysis.
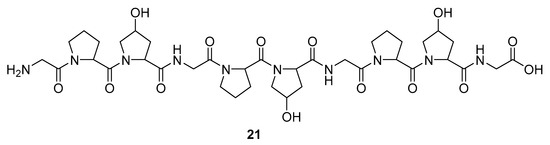
Figure 8.
Structure of the Alaska Pollack collagen hydroxyl proline (APCHP) peptide (21).
Similarly, one new anti-HIV peptide was isolated from Spirulina maxima (SM-peptide) [95]–the Leu-Asp-Ala-Val-Asn-Arg peptide, and the authors showed its HIV-1 infection inhibition in a human T cell line MT4. The peptide inhibited cell lysis, p24 antigen production, and HIV-1 RT. Specifically, IC50 (obtained by a cell viability assay) against an anti-HIV 1 infection was determined as 0.691 mM, the inhibition of the HIV-1-induced RT activation (RT assay kit) in MT4 cells was at a high 90% at a concentration of 1.093 mM, and the p24 production (p24 antigen production assay) was inhibited at 95% at a concentration of 1.093 mM.
2.5. Alkaloids
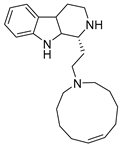
Marine organisms are well-established sources of natural alkaloids. Although the term ‘alkaloid’ seems puzzling and is prone to scientific controversy, alkaloids are generally defined as nitrogen-containing compounds derived from plants and animals. Relatively few alkaloids from marine sources have been found to possess antiretroviral properties and, so far, none have found their clinical use.
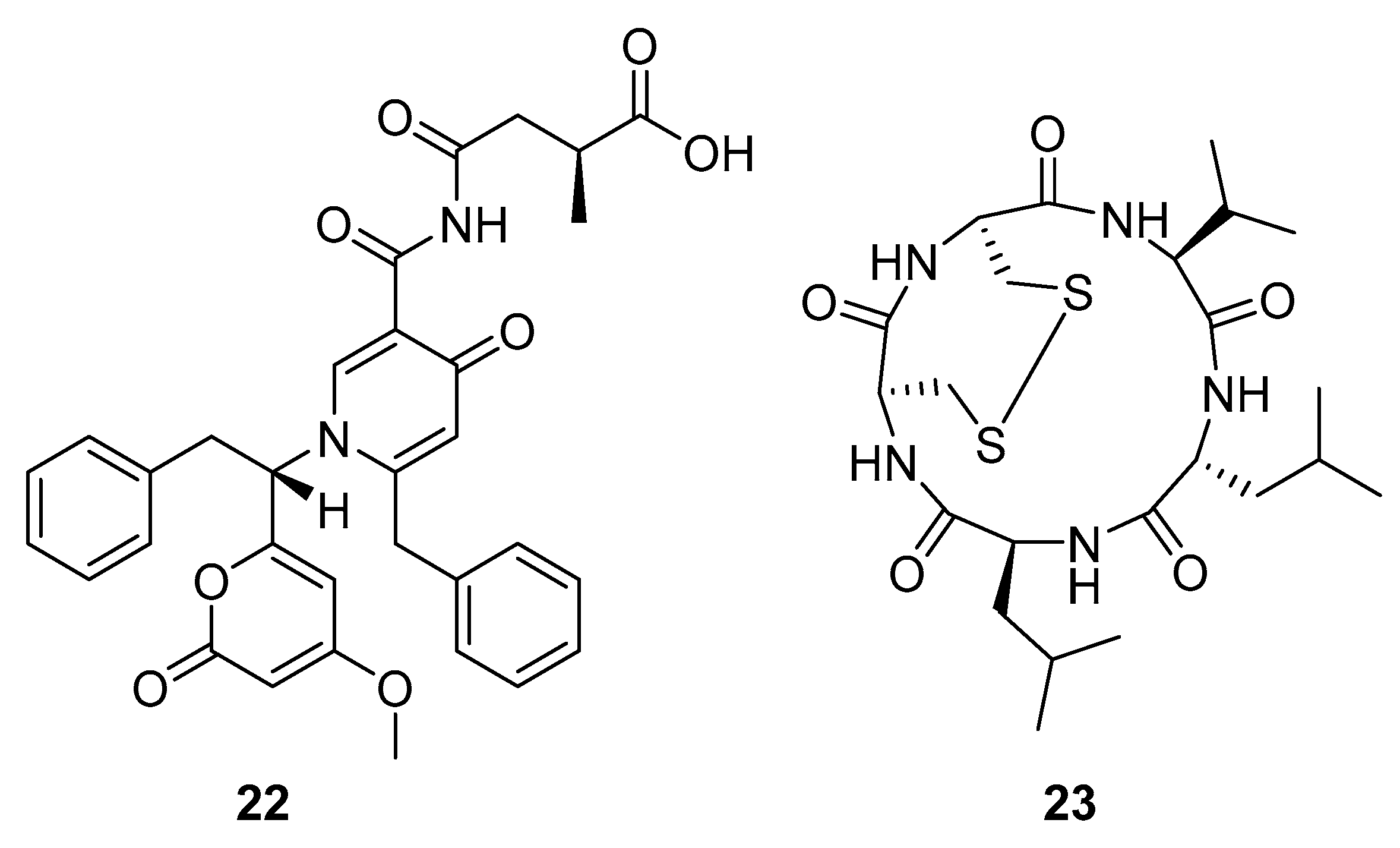
Aspernigrin C (22, Figure 9) and malformin C (23, Figure 9) have been isolated from marine-derived black aspergili, Aspergillus niger SCSIO Jcw6F30, and their inhibitory activity against the chemokine receptor subtype 5 (CCR5) tropic HIV-1 SF162 has been evaluated. They show potent inhibition of infection with IC50 values 4.7 ± 0.4 µM and 1.4 ± 0.06 µM, which is comparable to the nucleoside reverse transcriptase inhibitor—abacavir (IC50 = 0.8 ± 0.1 µM) and the HIV-1 entry inhibitor ADS-J1 (IC50 = 0.8 ± 0.1 µM). In comparison to other aspernigrins, it has been suggested that the 2-methylsuccinic moiety is responsible for the potency of aspernigrin C [96].
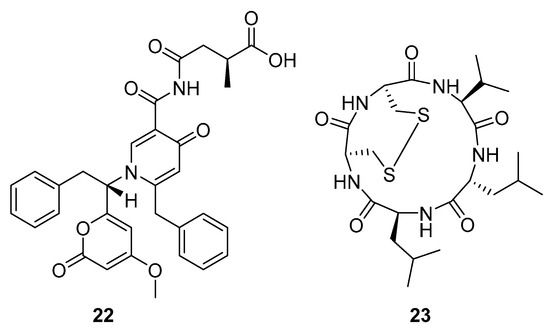
Figure 9.
Structures of aspernigrin C (22) and malformin C (23).
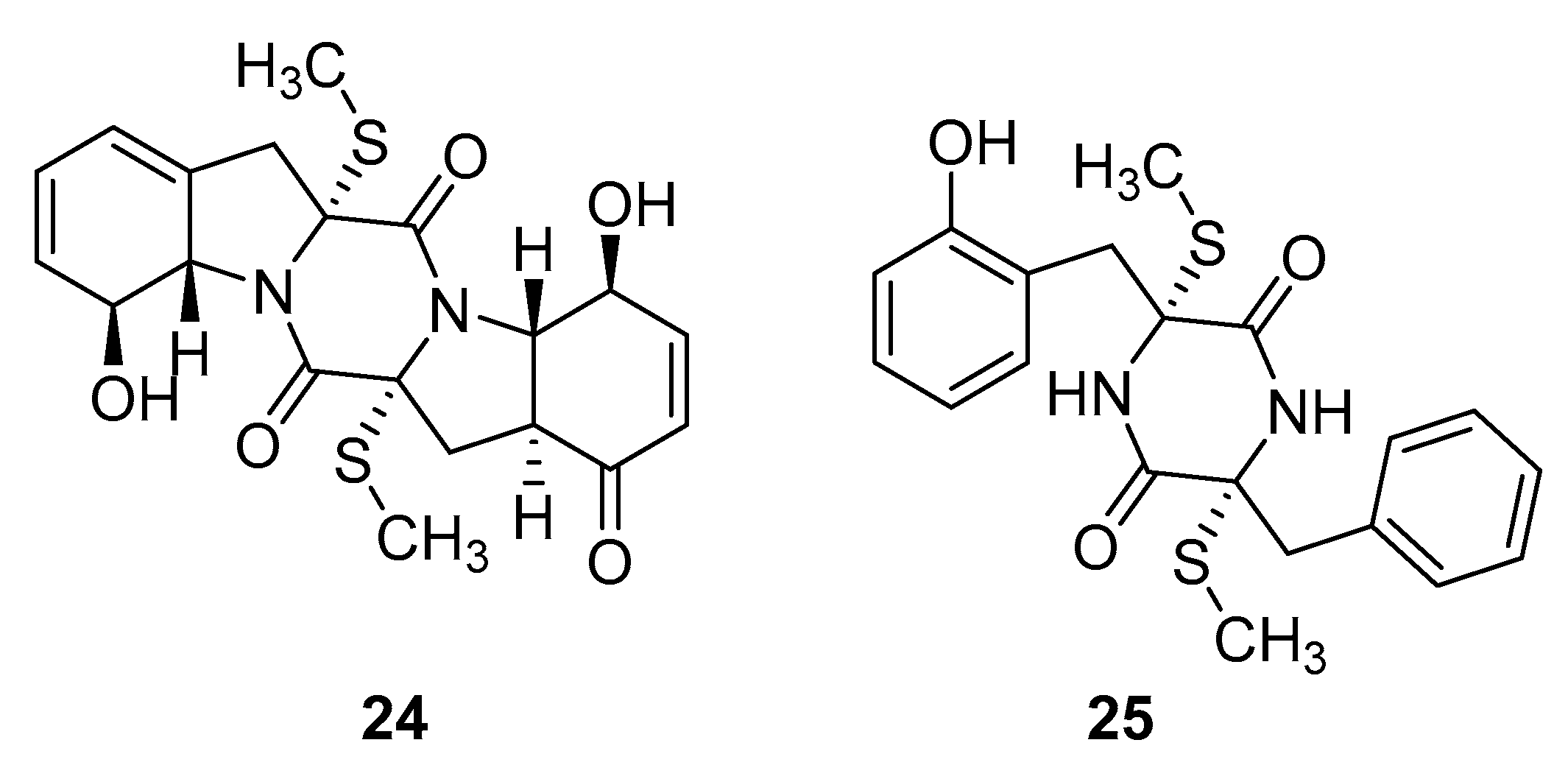
Thiodiketopiperazine-type alkaloids, eutypellazines A-M, isolated from the EtOAc extract of the fermentation broth of deep-sea sediment fungus Eutypella sp. shows potent inhibitory effects against pNL4.3.Env-.Luc co-transfected 293T HIV model cells. Eutypellazine E (24, Figure 10) exerts activity in a low micromolar range (IC50 = 3.2 ± 0.4 µM), while eutypellazine J (25, Figure 10) shows a reactivating effect toward latent HIV-1 in J-Lat A2 cells. This could be used as a promising strategy to expunge the HIV-1 infection by activating latent virus cellular reservoirs in combination with HAART [97].
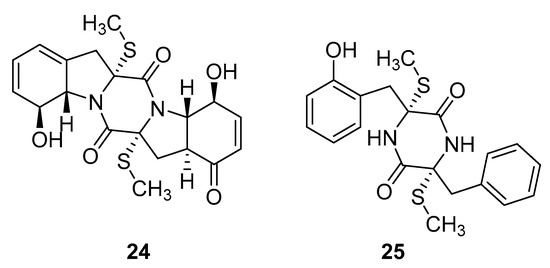
Figure 10.
Structures of eutypellazine E (24) and eutypellazine J (25).
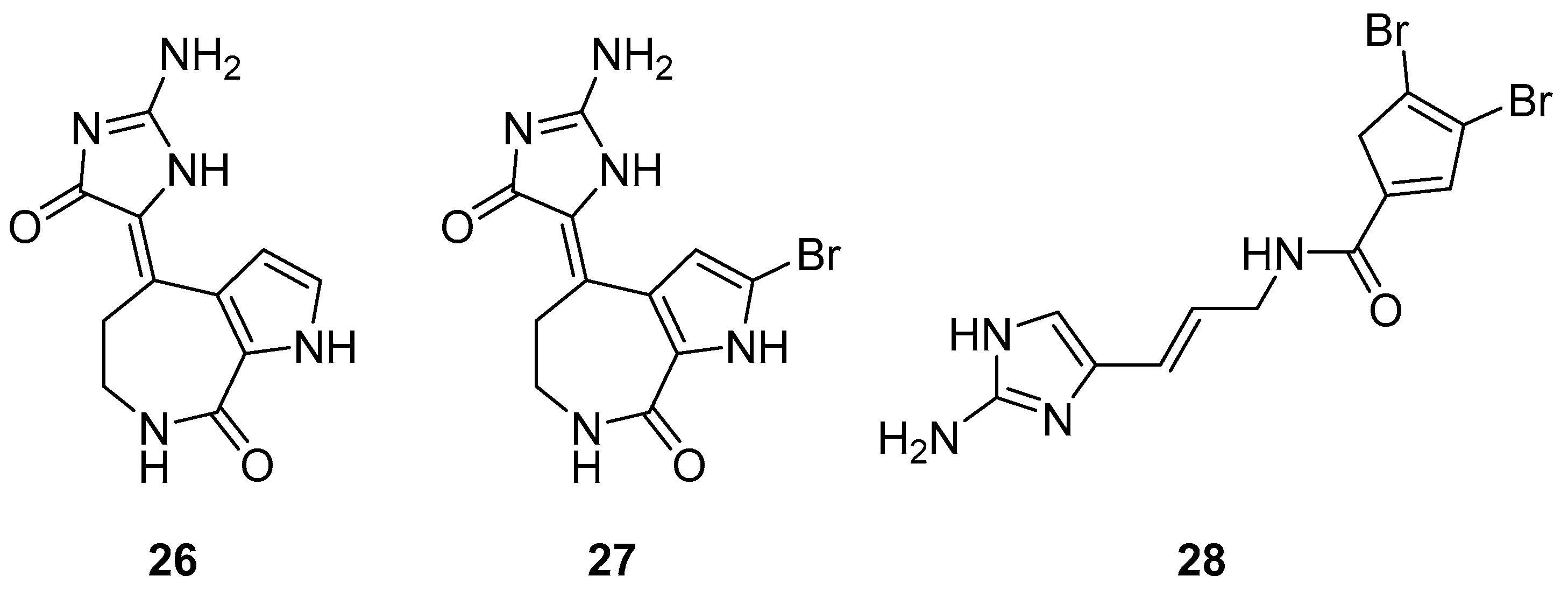
The S. carteri Red Sea sponge extract yields three previously characterized compounds: debromohymenialdisine (DBH) (26, Figure 11), hymenialdisine (HD) (27, Figure 11), and oroidin (28, Figure 11). DBH and HD exhibited a 30–40% inhibition of HIV-1 at 3.1 µM and 13 µM but with associated cytotoxicity. Conversely, oroidin displayed a 50% inhibition of viral replication at 50 µM without observed cytotoxicity. Also, it showed inhibition of HIV-1 reverse transcriptase up to 90% at 25 µMc [98].
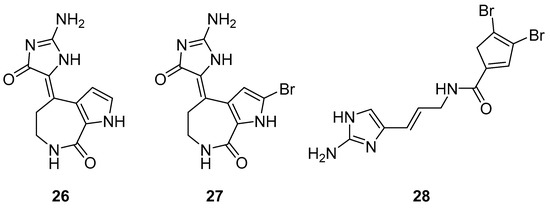
Figure 11.
Structures of debromohymenialdisine (26), 10Z-hymenialdisine (27), and oroidin (28).
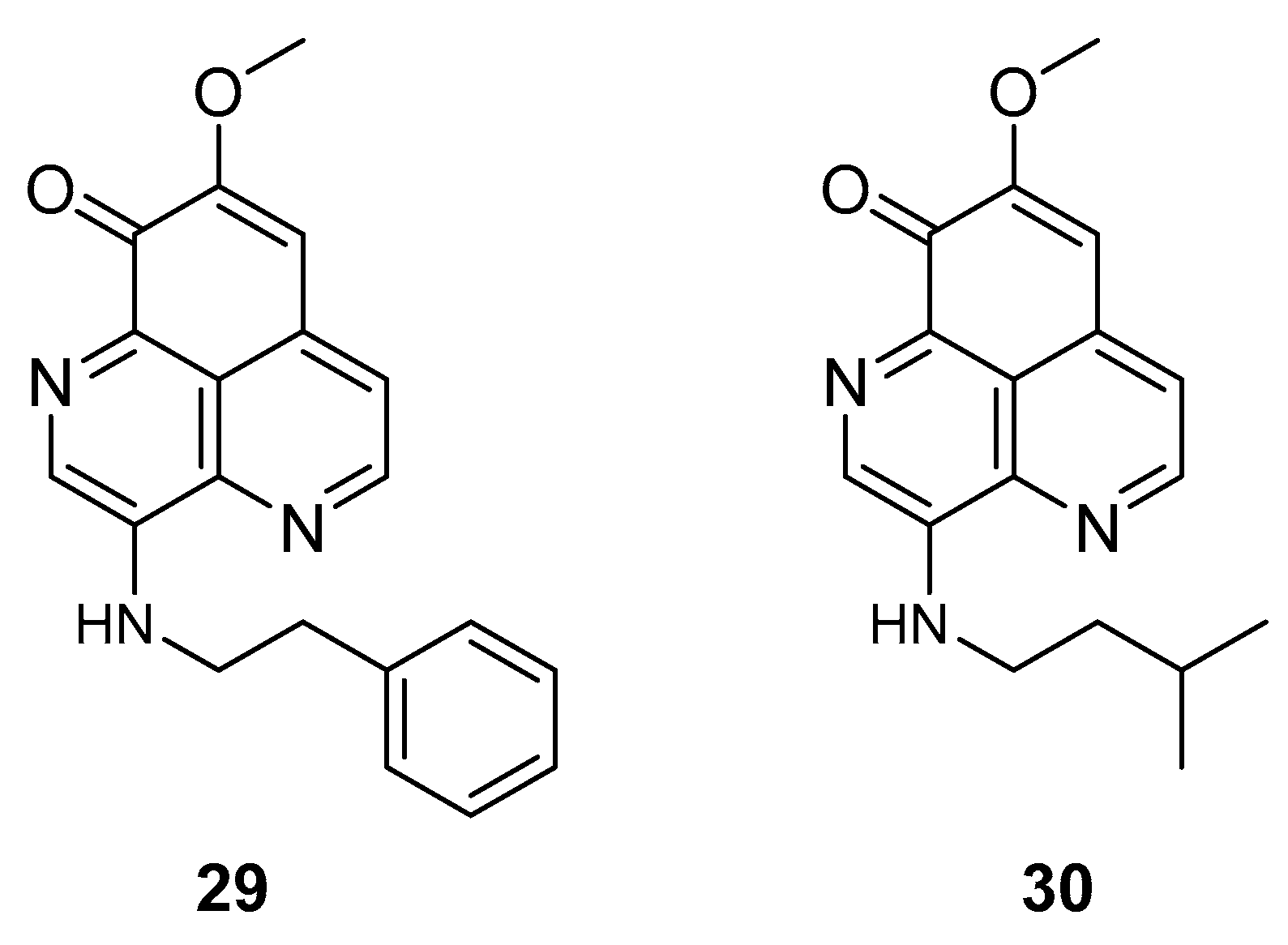
The two known alkaloids of the aaptamine family containing 1H-benz[de]-1,6-naphthyridine skeleton, namely 3-(phenetylamino)demethyl(oxy)aaptamine (29, Figure 12) and 3-(isopentylamino)demethyl(oxy)aaptamine (30, Figure 11), were isolated from the sponge A. aptos. They exhibited anti-HIV activity, with inhibitory rates of 88.0% and 72.3%, respectively, at a concentration of 10 µM [99].
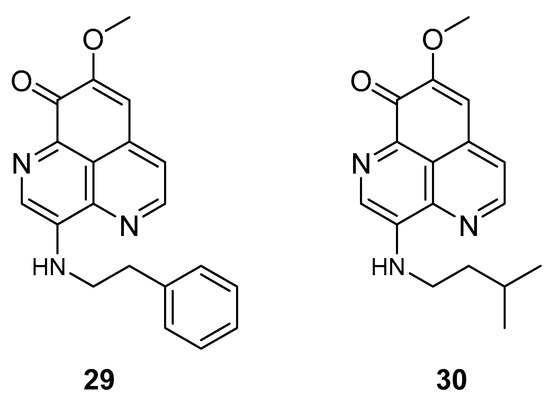
Figure 12.
Structures of 3-(phenetylamino)dimethyl(oxo)aaptamine (29) and 3-(isopentylamino)dimethyl (oxo)aaptamine (30).
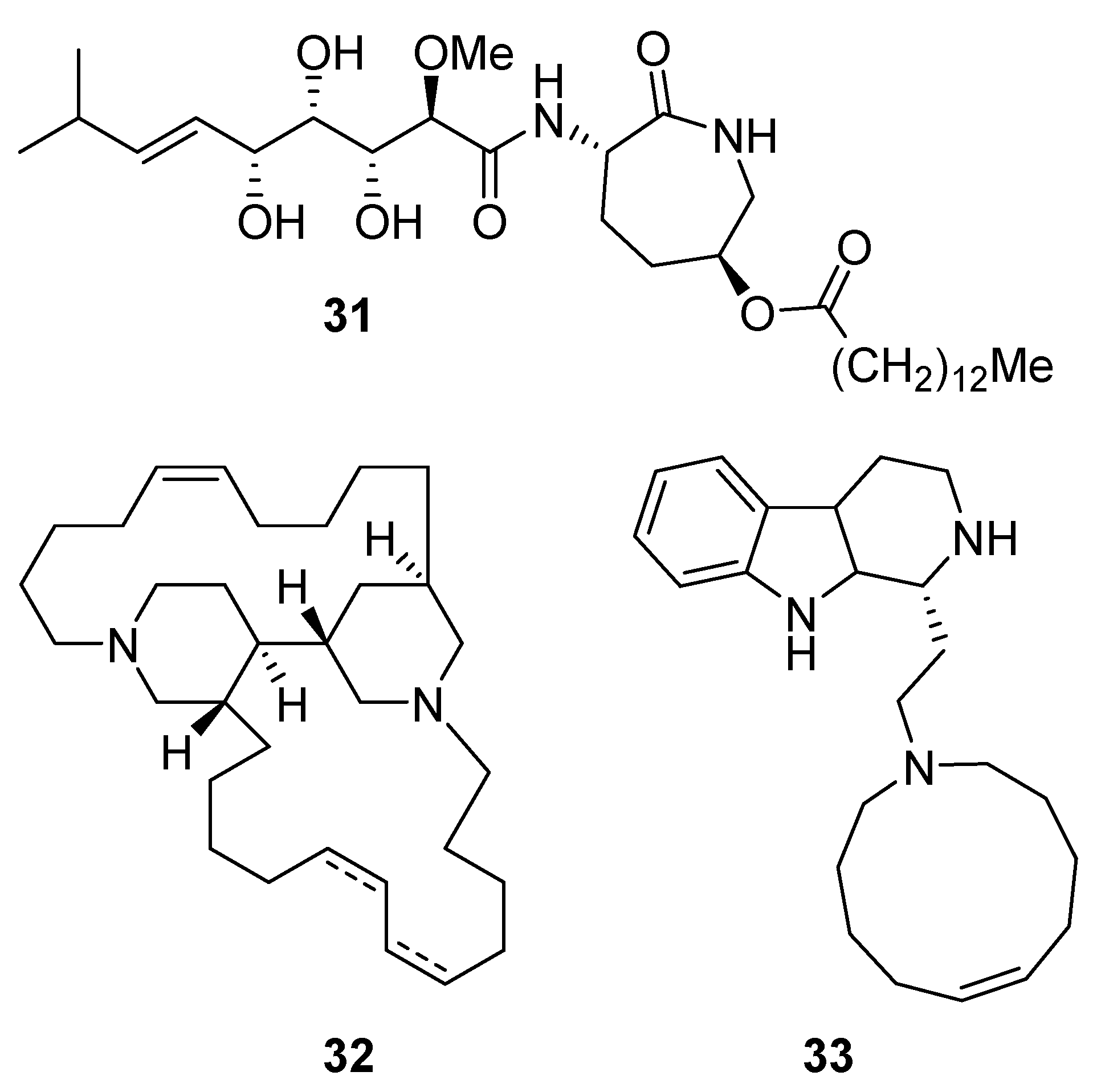
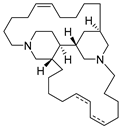
Bengamide A (31, Figure 13), haliclonycyclamine A+B (32, Figure 13) and keramamine C (33, Figure 13) inhibit HIV-1 with a 50% effective concentration of 3.8 µM or less. The most potent among them, bengamide A, blocked HIV-1 in a T cell line with an EC50 of 0.015 µM (which was comparable to control antiretrovirals indinavir 0.029 µM, efavirenz 0.0024 µM, and raltegravir 0.011 µM) and in peripheral blood mononuclear cells with EC50 of 0.032 µM. It was concluded that HIV-1 LTR NF-κB response elements are required for a bengamide A-mediated inhibition of LTR-dependent gene expression [100].
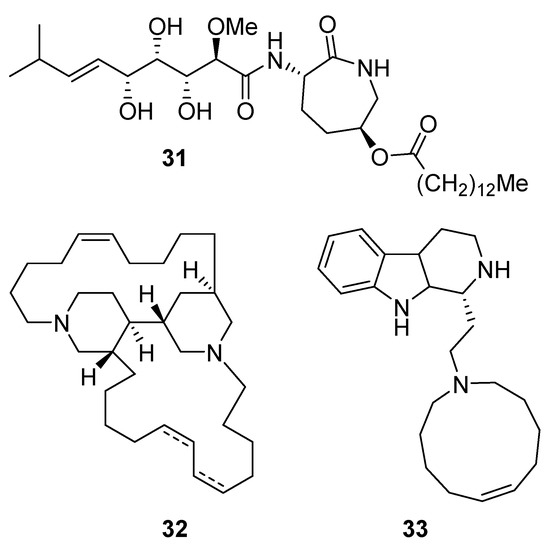
Figure 13.
Structures of bengamide A (31), haliclonacyclamines A + B (32), keramamine C (33).
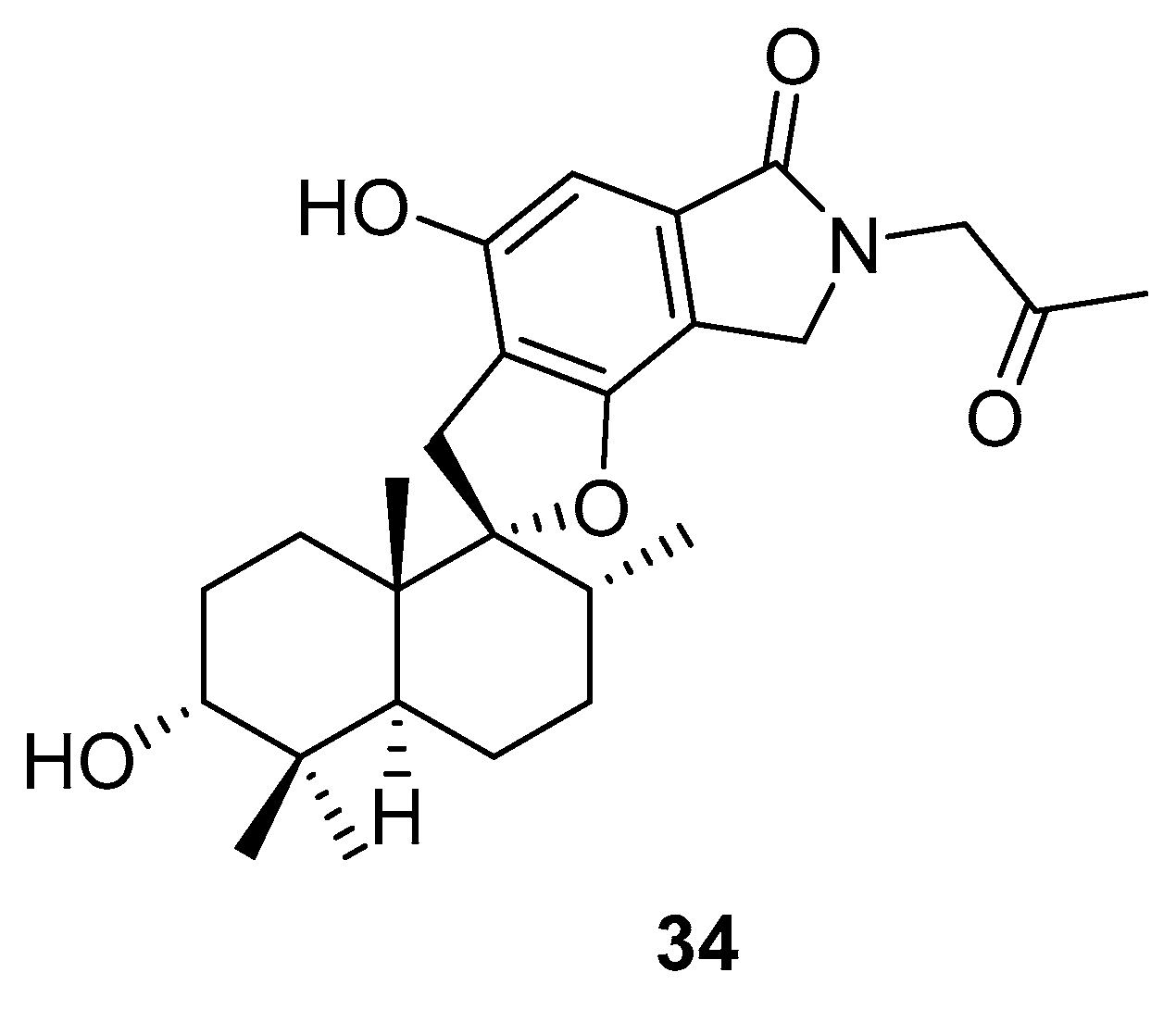
Phenylspirodrimane, stachybotrin D (34, Figure 14) isolated from the sponge-derived fungus Stachybotrys chartarum MXH-X73, was discovered to be a HIV-1 RT inhibitor, which showed inhibitory effects on the wild type (EC50 8.4 µM) and five NNRTI-resistant HIV-1 strains (EC50 7.0; 23.8; 13.3; 14.2; 6.2 µM) [101].
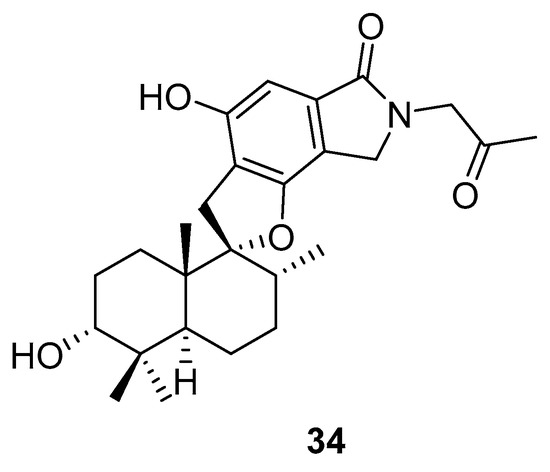
Figure 14.
Structure of stachybotrin D (34).
2.6. Diterpenes
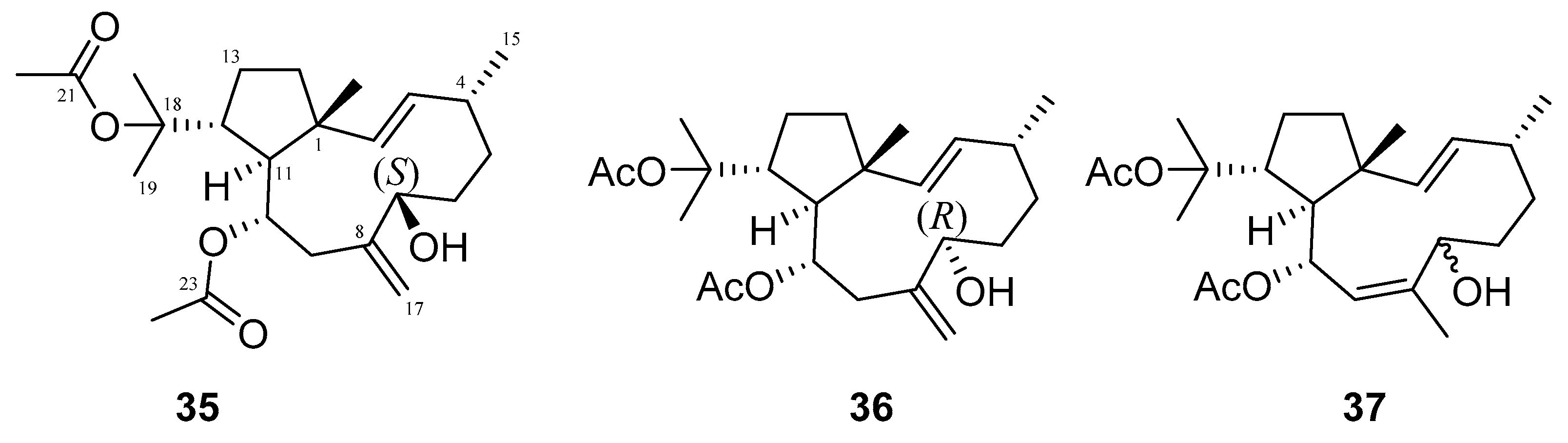
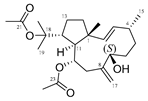
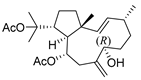
Many terpenes from marine natural products demonstrated anti-HIV properties. Mechanisms of action involve blocking of different steps of the HIV-1 replicative cycle as reverse transcriptase inhibitors, protease inhibitors, or entry inhibitors. Among them, diterpenes from marine algae are nowadays in the spotlight due to their promising anti-HIV activities [102]. Dolabellane diterpenes are compounds from the diterpene group that have recently been extensively studied for their anti-HIV activity. Pardo-Vargas et al. characterized three new dolabellane diterpenes isolated from the marine brown alga Dictyota pfaffii from Northeast Brazil: (1R*,2E,4R*,7S,10S*,11S*,12R*)10,18-diacetoxy-7-hydroxy-2,8(17)-dolabelladiene, (1R*,2E,4R*,7R*,10S*,11S*,12R*)10,18-diacetoxy-7-hydroxy-2,8(17)-dolabelladiene, (1R*,2E,4R*,8E,10S*,11S,12R*)10,18-diacetoxy-7-hydroxy-2,8-dolabelladiene, named dolabelladienols A–C (35–37, Figure 15), respectively [102]. In particular, the new compounds, dolabelladienols A and B, showed potent anti-HIV-1 activities that can be confirmed with their low IC50 values of 2.9 and 4.1 μM and low cytotoxic activity against MT-2 lymphocyte tumor cells. These promising anti-HIV-1 agents were even more active than previously known 2,6-dolabelladienes series.
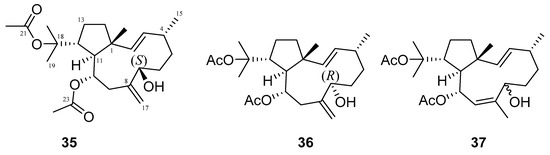
Figure 15.
Structures of the new dolabellane diterpenoids dolabelladienols A–C (35–37).
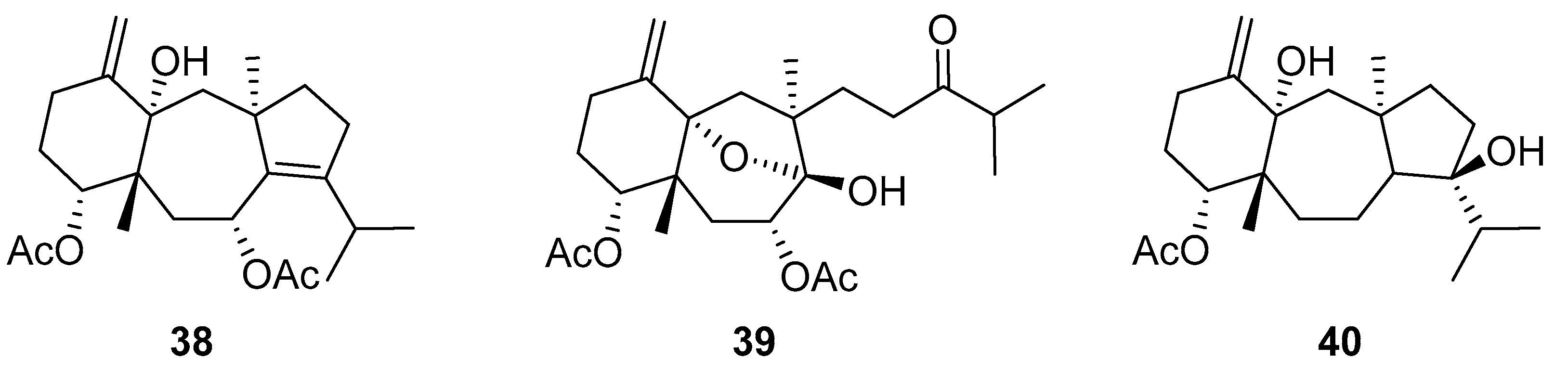
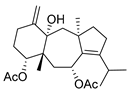
De Souza Barros et al. tested marine dolastanes (38, 40, Figure 16) and secodolastane diterpenes (39, Figure 16) isolated from the brown alga Canistrocarpus cervicornis for anti-HIV-1 activity [103]. They observed that the marine diterpenes 38–40 inhibit the HIV-1 replication in a dose-dependent manner (EC50 values of 0.35, 3.67, and 0.794 μM) without a cytotoxic effect (CC50 values ranging from 935 to 1910 μM). Additionally, they investigated the virucidal effect of these diterpenes and their potential use as microbicides. Dolastane-diterpenes 38 and 40 showed a potent effect on HIV-1 infectivity, whereas no virucidal effect was observed for secodolastane diterpene 39, demonstrating another mechanism of antiretroviral activity. Therefore, the authors suggested a potential use of marine dolastanes 38 and 40 as microbicides that could directly inhibit virus infectivity and possibly act before the virus penetrates the target cells [103].
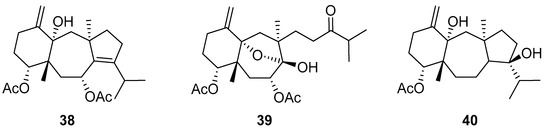
Figure 16.
Structures of marine dolastanes (38 and 40) and secodolastane diterpene (39) derived from Canistrocarpus cervicornis.
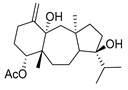
Dolabelladienetriol from brown alga Dictyota spp has also been evaluated as a potential microbicide against HIV-1 in tissue explants. Namely, Stephens et al. examined the 8,10,18-trihydroxy-2,6-dolabelladiene (41, Figure 17) in pretreated peripheral blood cells (PBMC) and macrophages along with their protective effect in the ex vivo explant model of the uterine cervix [104]. Pre-treatment of peripheral PBMC and macrophages with dolabelladienotriol showed inhibitory effects on HIV-1 replication. Furthermore, in the explant model dolabelladienetriol inhibited viral replication in a dose-dependent manner from 20 to 99% in concentrations of 0.15 and 14.4 μM without a loss in the viability of the tissue. The authors concluded that this compound has great potential as a possible microbicide. The same compound was also theoretically analyzed as an inhibitor of the wild-type and mutants’ HIV-1 reverse transcriptase [105]. Firstly, the structure-activity relationship studies revealed that a low dipole moment and high HOMO (highest occupied molecular orbital)-LUMO (lowest unoccupied molecular orbital) gap values are related to the antiviral activity. Secondly, molecular docking studies with RT wild-type and mutants showed a seahorse-like conformation of 8,10,18-trihydroxy-2,6-dolabelladiene, hydrophobic interactions, and hydrogen bonds with important residues of the binding pocket. Finally, the authors suggested a new derivative of the 8,10,18-trihydroxy-2,6-dolabelladiene with an aromatic moiety in the double bond to improve its biological activity.
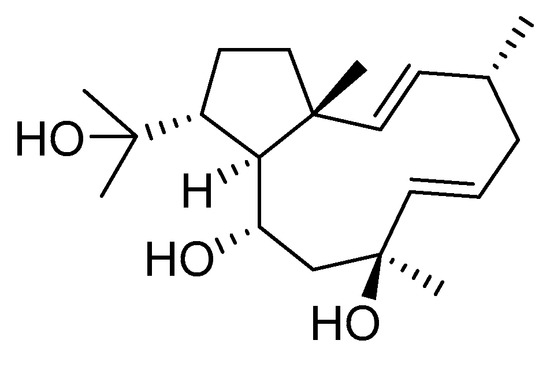
Figure 17.
Structure of (1R,2E,4R,6E,8S,10S,11S,12R)-8,10,18-trihydroxy-2,6-dolabelladiene (41).
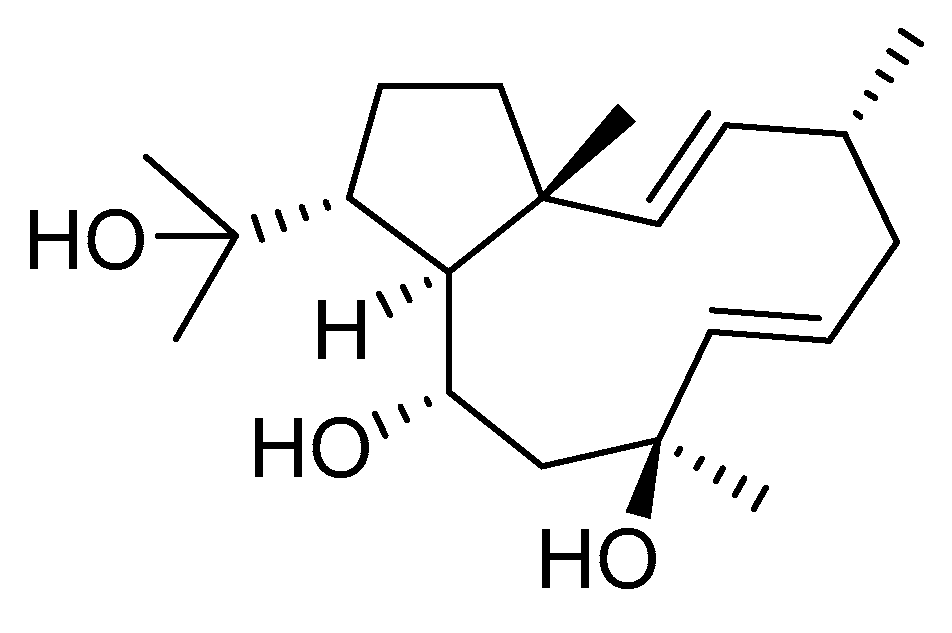
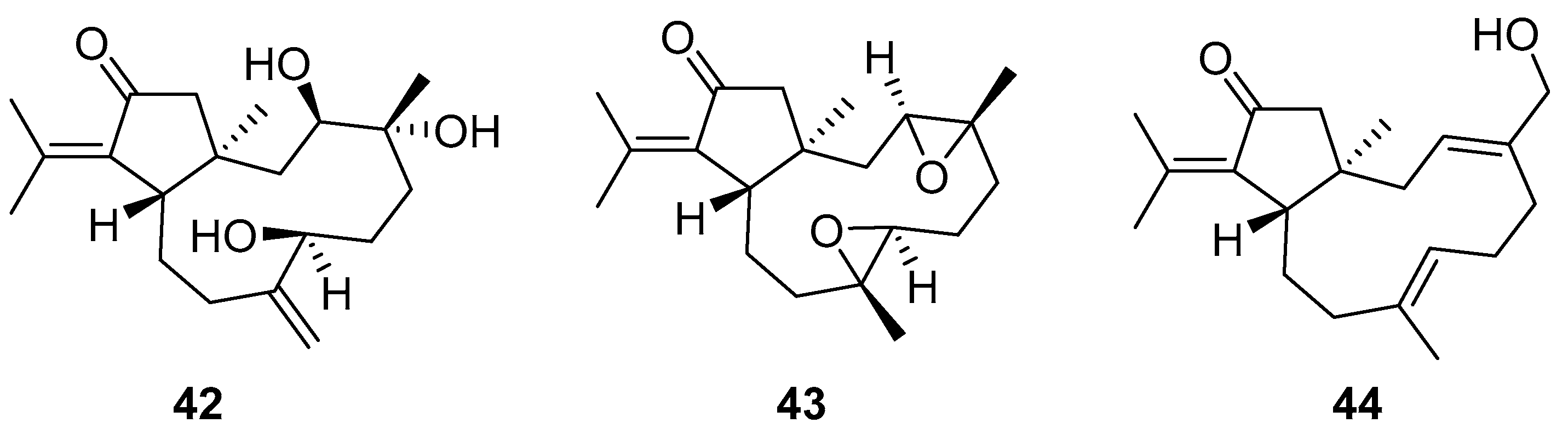
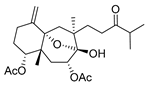
Although dolabellane diterpenes of brown alga Dictyota spp showed a strong anti-HIV-1 activity, this was not confirmed for dolabellane diterpenes isolated from octocorals. Therefore, some chemical transformations have been conducted to improve the anti-HIV-1 potency of the main dolabellane 13-keto-1(R),11(S)-dolabella-3(E),7(E),12(18)-triene from Caribbean octocoral Eunicea laciniata [106]. Oxygenated dolabellanes derivatives (42–44, Figure 18), obtained by epoxidation, epoxide opening, and allylic oxidation of ketodolbellatriene have shown significantly improved antiviral activities and a low cytotoxicity to MT-2 cells, which makes them promising antiviral compounds.
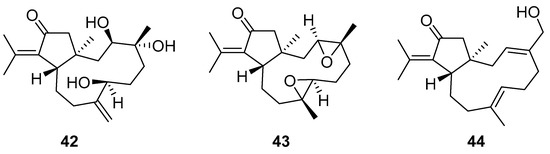
Figure 18.
Structures of semi-synthesized oxygenated dolabellanes (42–44) originally isolated from the Caribbean octocoral Eunicea laciniata.
2.7. Phlorotannins and Xanthones
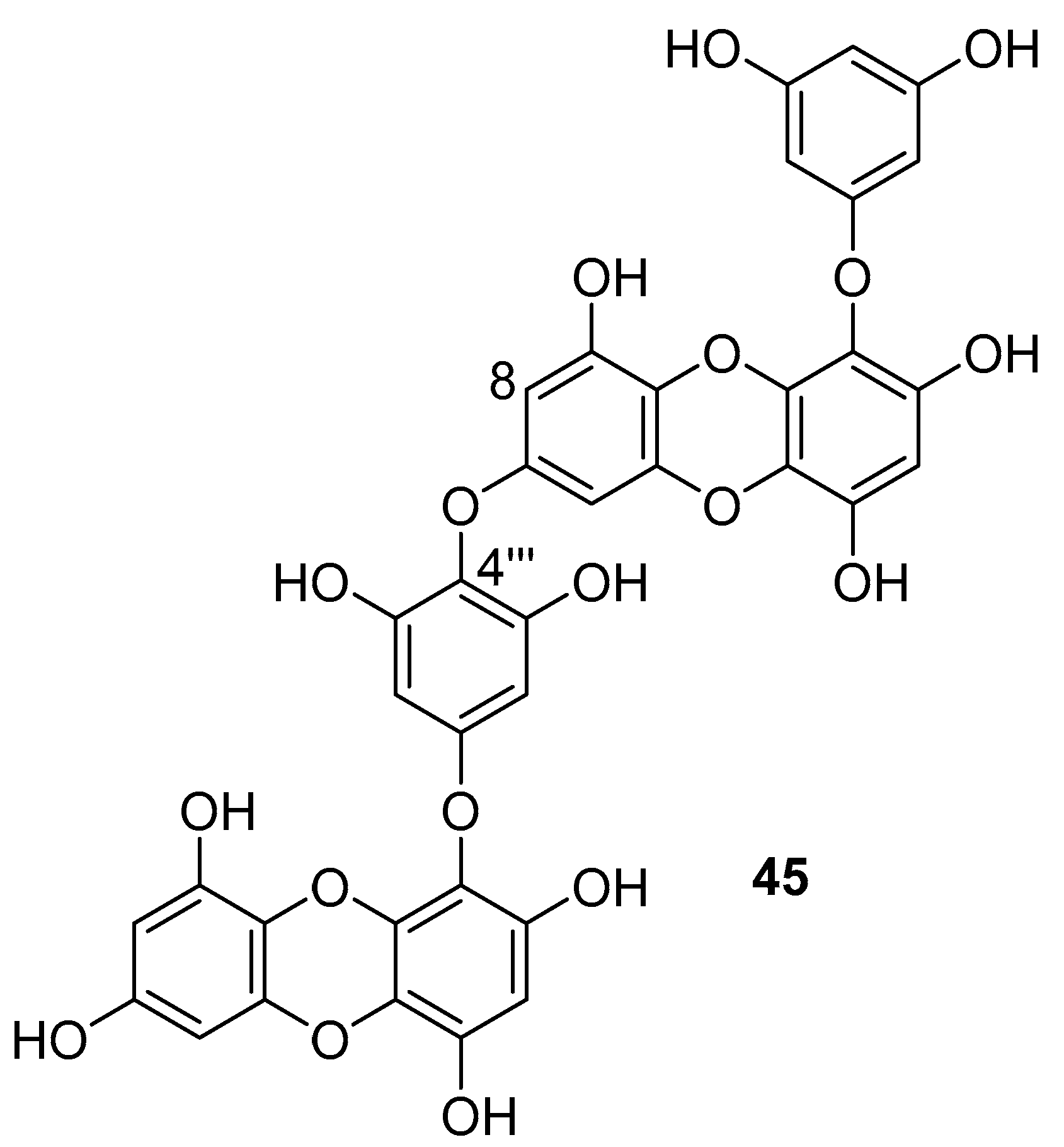
Phlorotannins are tannin derivatives made from several phloroglucinol units linked to each other in different ways. Phlorotannins contain phenyl linkage (fucols), ether linkage (fuhalols and phlorethols), phenyl and ether linkage (fucophloroethols), and dibenzodioxin linkage (eckols) [86,107]. So far, a series of phlorotannins have been identified with potent anti-HIV activity. For example, 8,8′-bieckol and 6,6′-bieckol from marine brown alga Ecklonia cava has shown an enhanced HIV-1 inhibitory effect [112,113]. Karadeniz et al. reported that 8,4′′′-dieckol (45, Figure 19) is another phlorotannin derivative isolated from the same brown alga that could be used as a drug candidate for the development of new generation anti-HIV therapeutic agents [107]. The compound showed HIV-1 inhibitory activity at noncytotoxic concentrations. More precisely, the results indicated that 8,4′′′-dieckol inhibited the cytopathic effects of HIV-1, including HIV-1 induced syncytia formation, lytic effects, and viral p24 antigen production. Furthermore, 8,4′′′-dieckol inhibited an HIV-1 entry and RT enzyme with the inhibition ratio of 91% at a concentration of 50 μM.
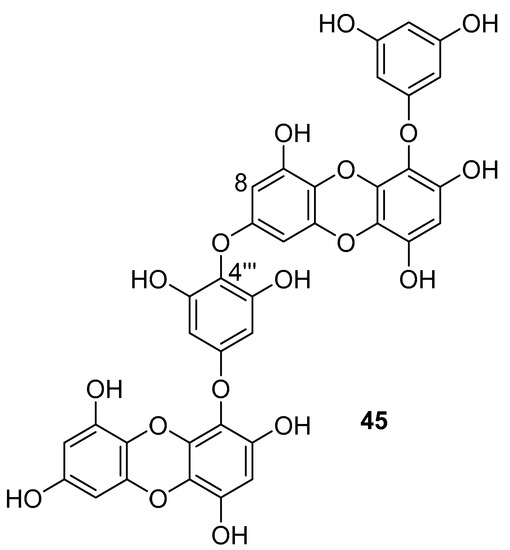
Figure 19.
Chemical structure of 8,4′′′-dieckol (45) from E. cava.
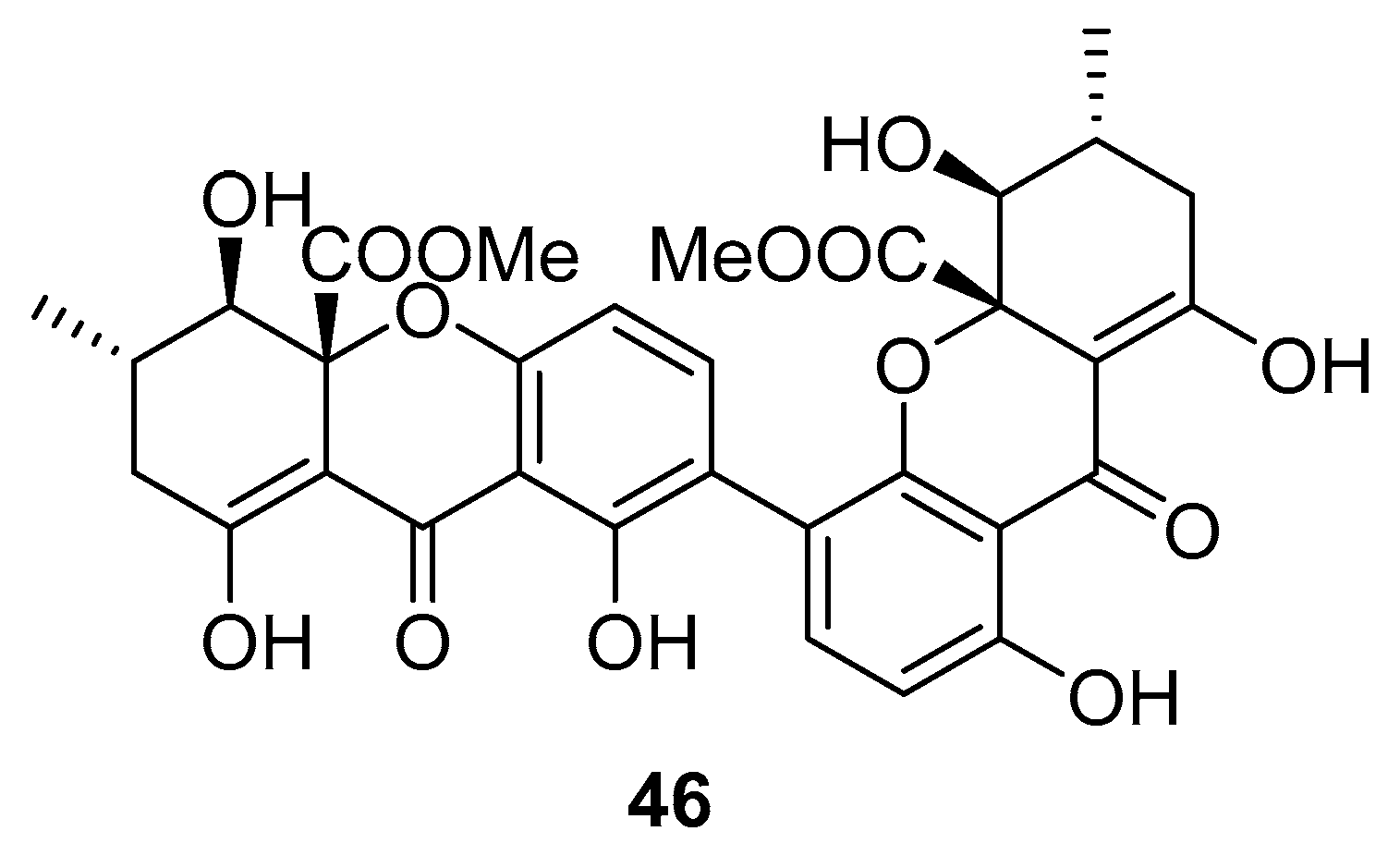
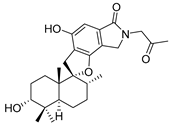
Recently, for the first time, xanthone dimer was identified as a potential anti-HIV-1 agent [108]. Xanthones are secondary metabolites from higher plant families, fungi, and lichen [114,115]. Although structurally related to flavonoids, xanthones are not as frequently encountered in nature [9]. Penicillixanthone A (PXA) (46, Figure 20), a natural xanthone dimer, has been isolated from the jellyfish-derived fungus Aspergillus fumigates with fourteen other natural products [108]. However, only penicillixanthone A showed inhibitory activities in an HIV infection. Marine-derived PXA displayed potent anti-HIV-1 activity against CCR5-tropic HIV-1 SF162 and CXCR4-tropic HIV-1 NL4-3, with IC50 of 0.36 and 0.26 μM, respectively. A molecular docking study confirmed that PXA might bind to either CCR5 or CXCR4 to prevent HIV entry into target cells. Therefore, PXA, as a CCR5/CXCR4 dual-coreceptor antagonist, may be seen as a new potential lead product type for the development of anti-HIV therapeutics.
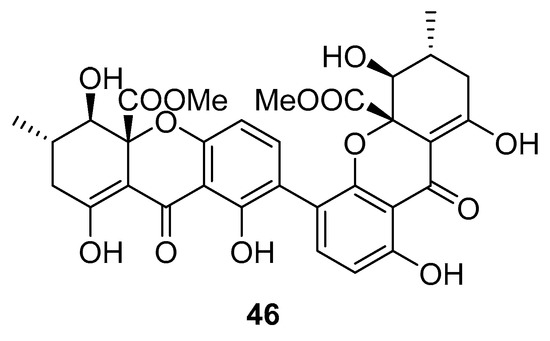
Figure 20.
Structure of penicillixanthone A (46).
2.8. Fish Oil as an Adjuvant to HAART Therapy
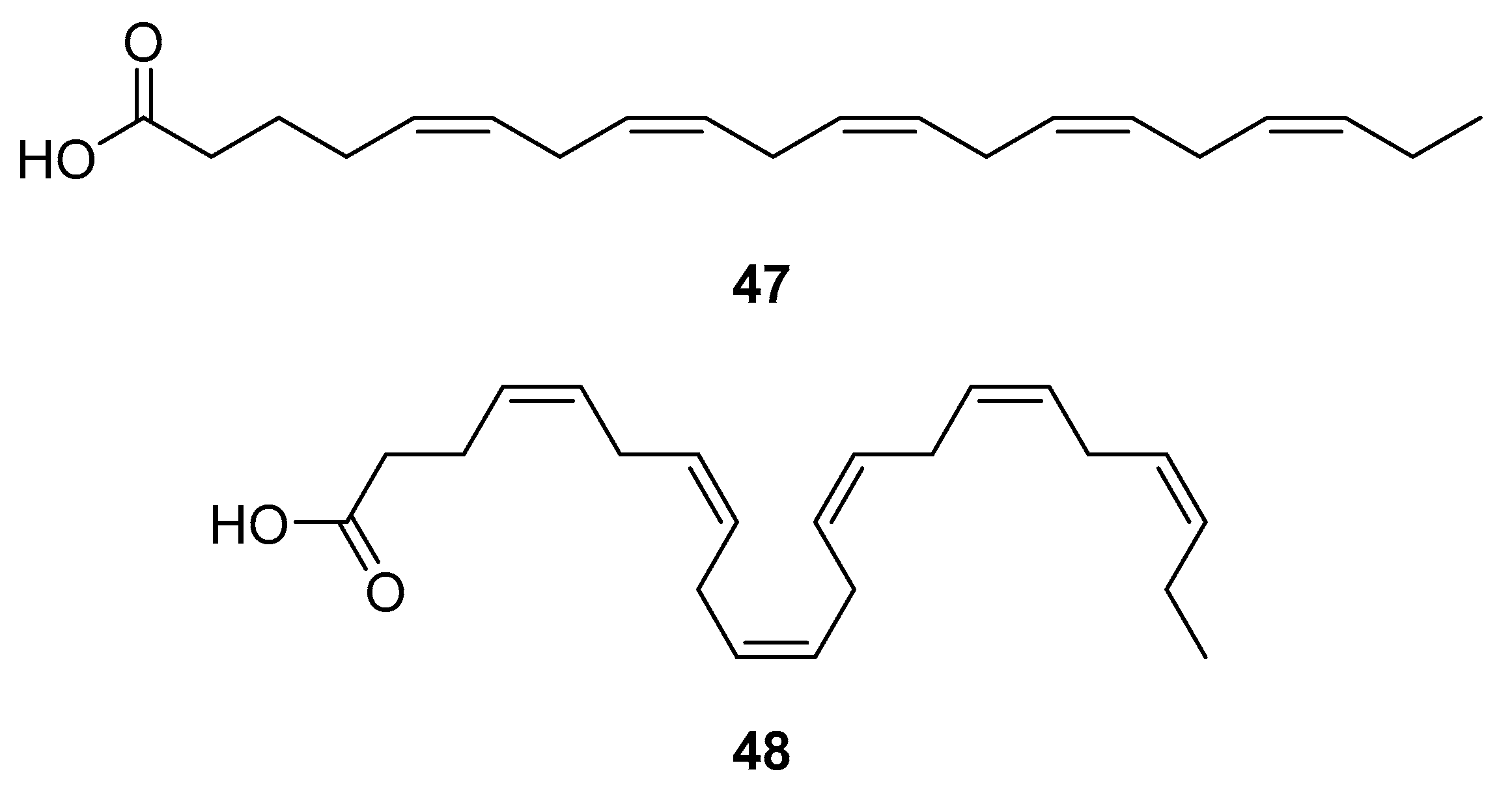
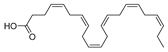
HAART therapy can cause severe side effects, e.g., insulin resistance, lipoatrophy, dyslipidemia, and abnormalities of fat distribution. Therefore, finding an adequate diet and supplementation to lower the negative effects of the HAART combination therapy is desirable [116]. Fish oil contains omega-3 polyunsaturated fatty acids (PUFA), eicosapentaenoic (EPA, 20:5n-3) (47, Figure 21) and docosahexaenoic (DHA, 22:6n-3) (48, Figure 21) acids, which may have beneficial effects for HIV-infected patients. It has been shown that the addition of fish oil to the diet of HIV-infected individuals receiving usual antiretroviral therapy can significantly lower serum triglycerides levels [117], which is highly relevant knowing that HIV dyslipidemia is a serious problem related to an increased frequency of cardiovascular disease.
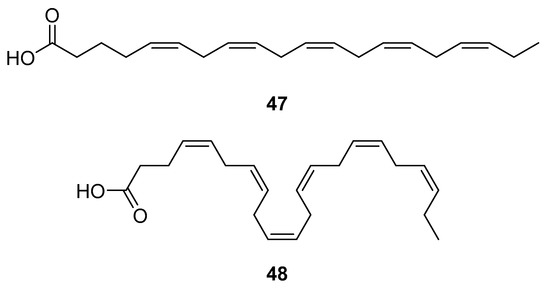
Figure 21.
Structures of eicosapentanoic (47) and docosahexanoic acid (48).
Recently, He et al. analyzed the influence of DHA on the locomotor activity in ethanol-treated HIV-1 transgenic rats [109]. The prevalence of alcohol use and alcohol abuse in infected individuals is much higher, and numerous ethanol and HIV-1 viral proteins have synergistic effects on inflammation in the central nervous system [118,119,120]. HIV remains in the body in its latent form after HAART therapy and, as such, can induce neuroinflammation. DHA depletion has been found to be associated with various neurological abnormalities, and its administration can have a neuroprotective effect. DHA taken daily could reverse the effects of the ethanol negative effect on the locomotor activity in the presence of HIV viral proteins. An in vivo study, using real-time quantitative PCR, showed that the addition of DHA can reduce elevated levels of IL-6, IL-18, and increase the expression of NF-κB in the striatum. This proved the potential of this fish oil constituent as an adjuvant in HIV patients’ treatment that can help in lowering the interactive effects of ethanol consumption during HIV infection.
2.9. Others
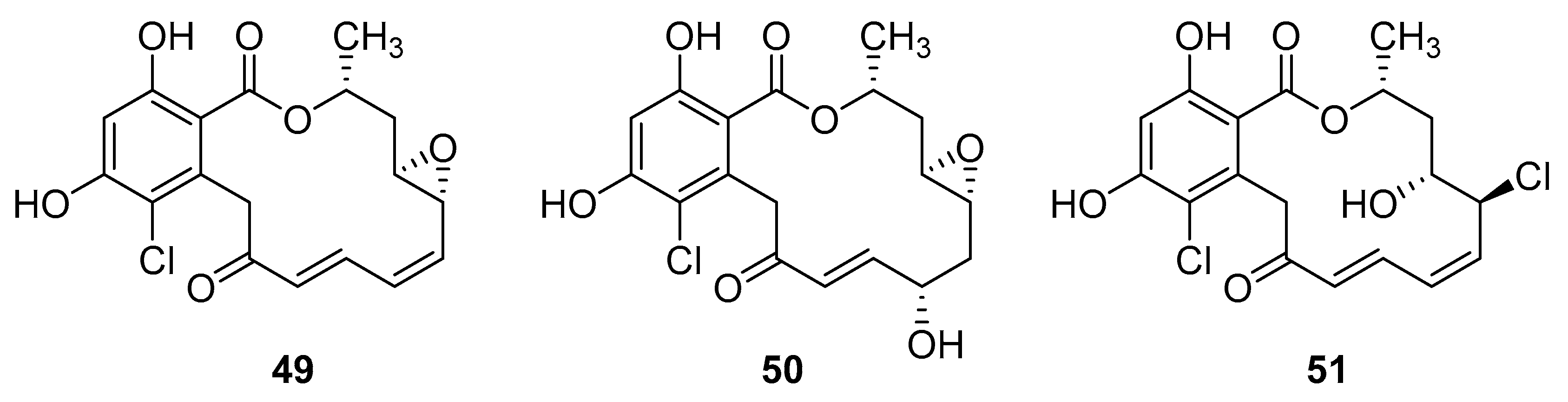
Resorcyclic acid lactones, namely radicicol (49, Figure 22) pochonin B (50, Figure 22) and C (51, Figure 22) isolated from H. fuscoatra exhibited a 92–98% reactivation efficiency of the latent HIV-1 relative to SAHA (subeoylanilide hydroxamic acid, vorinostat, HDAC inhibitor) and EC50 of 9.1, 39.6 and 6.3 µM [110]. The reactivation strategy is, indeed, a promising strategy to expunge the HIV-1 infection by reactivating latent viral loads, mainly in CD4 + T-cells, which quickly rebound when antiviral treatment is interrupted. It was noted that all active compounds contain Michael acceptor functionality. The PKC-independent mechanism of reactivation of the latent HIV-1 remains to be elucidated.
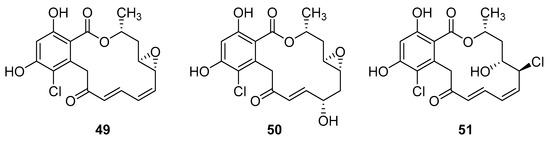
Figure 22.
Structures of radicicol (49), pochonin B (50), pochonin C (51).
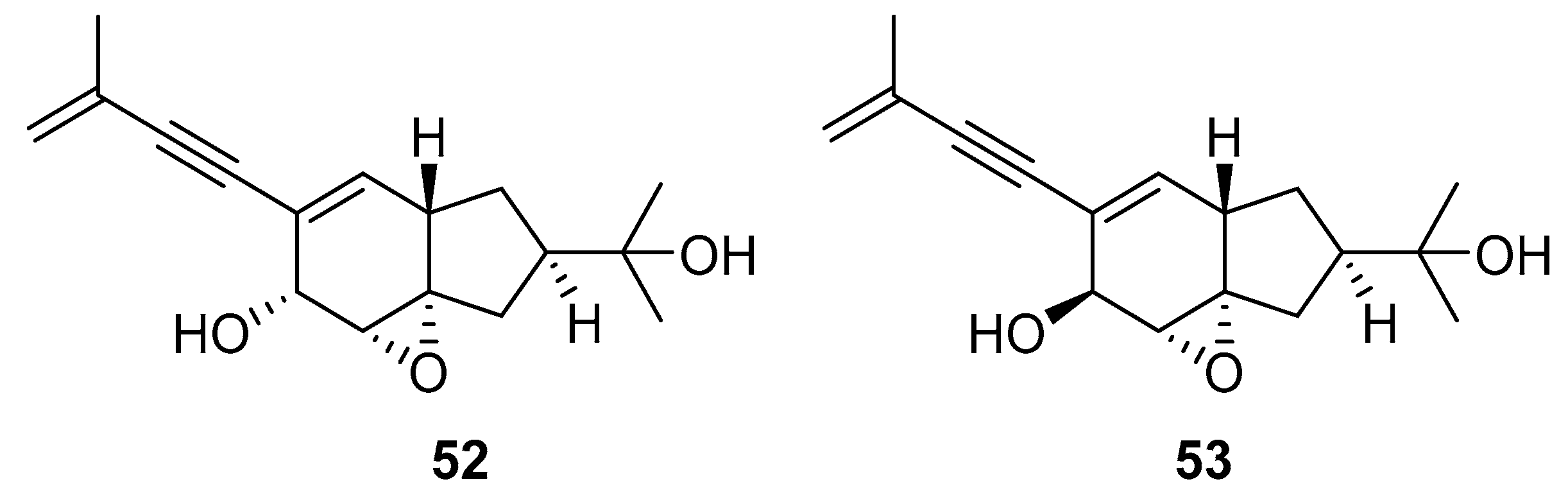
A team of researchers led by Zhao isolated new isoprenylated cyclohexanols from the sponge-associated fungus Truncatella angustata named truncateols O-V [111]. In vitro testing showed that truncateols O and P (52 and 53, Figure 23), analogues bearing the alkynyl group in the side chain, exhibit a significant inhibition toward the HIV-1 virus with IC50 values of 39.0 μM and 16.1 μM, respectively. These compounds could be considered as new anti-HIV lead compounds due to lower cytotoxicity (CC50 > 100 μM) in comparison with the positive control efavirenz (CC50 = 40.6 μM).
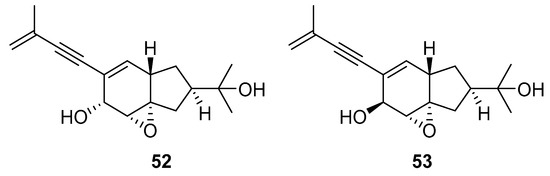
Figure 23.
Structures of truncateols O (52) and P (53).
3. Future Directions in the Anti-HIV Marine Drug Development
Marine organisms have been acknowledged as a precious source of bioactive compounds that may provide novel anti-HIV structures or lead structures for structural optimization. A large amount of evidence from scientific research confirmed a high biological potential of these compounds to treat serious diseases, including infective ones. Some of the marine-derived bioactive compounds discovered much earlier have emerged with novel properties and potential applications after a decade or two. Isolation and structural elucidation of compounds from marine organisms is not an easy task and still carries challenges. Identification of all the compounds is a daunting task, especially with regards to complex structural motifs that may be present in a single marine extract. Taxonomic knowledge is still insufficient to enable unambiguous species classification that can result in the false prediction of chemical constituents and hamper structural analysis. Furthermore, a temporal lag between the discovery, chemical characterization, and associated pharmacological activities is quite common, and the majority of marine metabolites are usually tested for anticancer activity, whereas anti-HIV and other possible biological effects are neglected or mostly not performed due to a lack of funding. Targeted assays and in vivo analyses are similarly performed only for some of the potential candidates, while the translation into clinical trials remains very limited. Thus, the financial gap is certainly a relevant factor contributing to the slow drug development process in this area. In particular, the development of anti-HIV compounds, which act by mechanisms that differ from existing antivirals, requires a well-designed and focused approach to studying the mode of action. Libraries should be created for specifically defined crude extracts, their corresponding simplified fractions as well as for pure compounds for a well-balanced natural product discovery program. Additionally, there exist but few publications in which scientists have tried to modify known compounds of marine origin to improve their bioactivity. We are, however, continuously witnessing advancements in the deep-sea exploration technology, sampling strategies, genome sequencing, genome mining, genetic engineering, chemo-enzymatic synthesis, nanoscale NMR structure determination, and development and optimization of suitable fermentation strategies to ensure a continued supply of unique bioactive compounds from the oceans. Therefore, the grounds have been met for a broad, international effort based on scientific collaboration that would rely on well-equipped infrastructure and human resources as a prerequisite for a full advancement in the field and development of new drug candidates for the pharmaceutical market in the future.
Author Contributions
K.W. devised the main conceptual idea and, together with L.S. and Ž.P., wrote the manuscript parts related to medicinal chemistry. K.W. and Ž.P. performed literature searches and L.S. prepared the table. S.K.P. participated in the manuscript writing and wrote and discussed parts relevant for clinical applications, shaped the paper concept, and performed the final revision.
Funding
We want to thank the Croatian Government and the European Union (European Regional Development Fund—the Competitiveness and Cohesion Operational Programme—KK.01.1.1.01) for funding this research through project Bioprospecting of the Adriatic Sea (KK.01.1.1.01.0002) granted to The Scientific Centre of Excellence for Marine Bioprospecting—BioProCro. We also acknowledge the project “Research Infrastructure for Campus-based Laboratories at the University of Rijeka,” co-financed by European Regional Development Fund (ERDF) and the University of Rijeka research grant uniri-biomed-18-133 (1277).
Conflicts of Interest
The authors declare no conflict of interests.
References
- AVERT. Symptoms and Stages of HIV Infection. Available online: https://www.avert.org/about-hiv-aids/symptoms-stages (accessed on 12 April 2019).
- HIV/AIDS. Available online: https://www.who.int/news-room/fact-sheets/detail/hiv-aids (accessed on 12 April 2019).
- Li, G.; Piampongsant, S.; Faria, N.R.; Voet, A.; Pineda-Peña, A.-C.; Khouri, R.; Lemey, P.; Vandamme, A.-M.; Theys, K. An integrated map of HIV genome-wide variation from a population perspective. Retrovirology 2015, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- Saphire, A.C.S.; Bobardt, M.D.; Zhang, Z.; David, G.; Gallay, P.A. Syndecans Serve as Attachment Receptors for Human Immunodeficiency Virus Type 1 on Macrophages. J. Virol. 2001, 75, 9187–9200. [Google Scholar] [CrossRef] [PubMed]
- Arthos, J.; Cicala, C.; Martinelli, E.; Macleod, K.; Van Ryk, D.; Wei, D.; Xiao, Z.; Veenstra, T.D.; Conrad, T.P.; Lempicki, R.A.; et al. HIV-1 envelope protein binds to and signals through integrin α4β7, the gut mucosal homing receptor for peripheral T cells. Nat. Immunol. 2008, 9, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Cicala, C.; Martinelli, E.; McNally, J.P.; Goode, D.J.; Gopaul, R.; Hiatt, J.; Jelicic, K.; Kottilil, S.; Macleod, K.; O’Shea, A.; et al. The integrin α4β7 forms a complex with cell-surface CD4 and defines a T-cell subset that is highly susceptible to infection by HIV-1. PNAS 2009, 106, 20877–20882. [Google Scholar] [CrossRef] [PubMed]
- Geijtenbeek, T.B.; Kwon, D.S.; Torensma, R.; van Vliet, S.J.; van Duijnhoven, G.C.; Middel, J.; Cornelissen, I.L.; Nottet, H.S.; KewalRamani, V.N.; Littman, D.R.; et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 2000, 100, 587–597. [Google Scholar] [CrossRef]
- Orloff, G.M.; Orloff, S.L.; Kennedy, M.S.; Maddon, P.J.; McDougal, J.S. Penetration of CD4 T cells by HIV-1. The CD4 receptor does not internalize with HIV, and CD4-related signal transduction events are not required for entry. J. Immunol. 1991, 146, 2578–2587. [Google Scholar] [PubMed]
- Maddon, P.J.; Dalgleish, A.G.; McDougal, J.S.; Clapham, P.R.; Weiss, R.A.; Axel, R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell 1986, 47, 333–348. [Google Scholar] [CrossRef]
- McDougal, J.; Kennedy, M.; Sligh, J.; Cort, S.; Mawle, A.; Nicholson, J. Binding of HTLV-III/LAV to T4+ T cells by a complex of the 110K viral protein and the T4 molecule. Science 1986, 231, 382–385. [Google Scholar] [CrossRef]
- Alkhatib, G. The biology of CCR5 and CXCR4. Curr. Opin. HIV AIDS 2009, 4, 96–103. [Google Scholar] [CrossRef]
- Okoye, A.A.; Picker, L.J. CD4(+) T-cell depletion in HIV infection: Mechanisms of immunological failure. Immunol. Rev. 2013, 254, 54–64. [Google Scholar] [CrossRef]
- Schrager, L.K.; D’Souza, M.P. Cellular and anatomical reservoirs of HIV-1 in patients receiving potent antiretroviral combination therapy. JAMA 1998, 280, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Berkhout, B.; Eggink, D.; Sanders, R.W. Is there a future for antiviral fusion inhibitors? Curr. Opin. Virol. 2012, 2, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, M.L.; Cammack, N. Resistance to enfuvirtide, the first HIV fusion inhibitor. J. Antimicrob. Chemother. 2004, 54, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, C.E.; Sanders, R.W.; Deng, Y.; Jurriaans, S.; Lange, J.M.; Lu, M.; Berkhout, B. Emergence of a drug-dependent human immunodeficiency virus type 1 variant during therapy with the T20 fusion inhibitor. J. Virol. 2004, 78, 12428–12437. [Google Scholar] [CrossRef] [PubMed]
- Rimsky, L.T.; Shugars, D.C.; Matthews, T.J. Determinants of human immunodeficiency virus type 1 resistance to gp41-derived inhibitory peptides. J. Virol. 1998, 72, 986–993. [Google Scholar] [PubMed]
- Abner, E.; Jordan, A. HIV “shock and kill” therapy: In need of revision. Antiviral Res. 2019, 166, 19–34. [Google Scholar] [CrossRef]
- Hütter, G.; Nowak, D.; Mossner, M.; Ganepola, S.; Müßig, A.; Allers, K.; Schneider, T.; Hofmann, J.; Kücherer, C.; Blau, O.; et al. Long-Term Control of HIV by CCR5 Delta32/Delta32 Stem-Cell Transplantation. N. Engl. J. Med. 2009, 360, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Ananworanich, J.; Robb, M.L. The transient HIV remission in the Mississippi baby: Why is this good news? J. Int. AIDS Soc. 2014, 17, 19859. [Google Scholar] [CrossRef]
- The Lancet HIV. Like London buses, two putative cure cases arrive at once. Lancet HIV 2019, 6, e205. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Erakovic Haber, V.; Spaventi, R. Discovery and Development of Novel Drugs. In Progress in Molecular and Subcellular Biology; Springer: Cham, Switzerland, 2017; Volume 55, pp. 91–104. [Google Scholar]
- Blunt, J.W.; Copp, B.R.; Hu, W.-P.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2009, 26, 170. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; O’Keefe, B.R.; Sowder, R.C.; Bringans, S.; Gardella, R.; Berg, S.; Cochran, P.; Turpin, J.A.; Buckheit, R.W.; McMahon, J.B.; et al. Isolation and Characterization of Griffithsin, a Novel HIV-inactivating Protein, from the Red Alga Griffithsia sp. J. Biol. Chem. 2005, 280, 9345–9353. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Gao, Y.; Hoorelbeke, B.; Kagiampakis, I.; Zhao, B.; Demeler, B.; Balzarini, J.; Liwang, P.J. The role of individual carbohydrate-binding sites in the function of the potent anti-HIV lectin griffithsin. Mol. Pharm. 2012, 9, 2613–2625. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Jin, W.; Griffin, G.E.; Shattock, R.J.; Hu, Q. Removal of two high-mannose N-linked glycans on gp120 renders human immunodeficiency virus 1 largely resistant to the carbohydrate-binding agent griffithsin. J. Gen. Virol. 2011, 92, 2367–2373. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Hoorelbeke, B.; Kagiampakis, I.; Demeler, B.; Balzarini, J.; Liwang, P.J. The griffithsin dimer is required for high-potency inhibition of HIV-1: Evidence for manipulation of the structure of gp120 as part of the griffithsin dimer mechanism. Antimicrob. Agents Chemother. 2013, 57, 3976–3989. [Google Scholar] [CrossRef] [PubMed]
- Gerwick, W.H.; Moore, B.S. Lessons from the Past and Charting the Future of Marine Natural Products Drug Discovery and Chemical Biology. Chem. Biol. 2012, 19, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.; Vieira, H.; Gaspar, H.; Santos, S.; Martins, A.; Vieira, H.; Gaspar, H.; Santos, S. Marketed Marine Natural Products in the Pharmaceutical and Cosmeceutical Industries: Tips for Success. Mar. Drugs 2014, 12, 1066–1101. [Google Scholar] [CrossRef] [PubMed]
- Karagozlu, M.Z.; Karadeniz, F.; Kim, S.-K. Anti-HIV activities of novel synthetic peptide conjugated chitosan oligomers. Int. J. Biol. Macromol. 2014, 66, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Ramana, L.N.; Sharma, S.; Sethuraman, S.; Ranga, U.; Krishnan, U.M. Evaluation of chitosan nanoformulations as potent anti-HIV therapeutic systems. Biochim. Biophys. Acta 2014, 1840, 476–484. [Google Scholar] [CrossRef]
- Shohani, S.; Mondanizadeh, M.; Abdoli, A.; Khansarinejad, B.; Salimi-Asl, M.; Ardestani, M.; Ghanbari, M.; Haj, M.; Zabihollahi, R. Trimethyl Chitosan Improves Anti-HIV Effects of Atripla as a New Nanoformulated Drug. Curr. HIV Res. 2017, 15, 56–65. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef] [PubMed]
- Wijesekara, I.; Pangestuti, R.; Kim, S.-K. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr. Polym. 2011, 84, 14–21. [Google Scholar] [CrossRef]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Abu-Ghannam, N. Recent developments in the application of seaweeds or seaweed extracts as a means for enhancing the safety and quality attributes of foods. Innov. Food Sci. Emerg. Technol. 2011, 12, 600–609. [Google Scholar] [CrossRef]
- Aquino, R.S.; Grativol, C.; Mourão, P.A.S. Rising from the Sea: Correlations between Sulfated Polysaccharides and Salinity in Plants. PLoS ONE 2011, 6, e18862. [Google Scholar] [CrossRef]
- Torode, T.A.; Marcus, S.E.; Jam, M.; Tonon, T.; Blackburn, R.S.; Hervé, C.; Knox, J.P. Monoclonal Antibodies Directed to Fucoidan Preparations from Brown Algae. PLoS One 2015, 10, e0118366. [Google Scholar] [CrossRef] [PubMed]
- Deniaud-Bouët, E.; Kervarec, N.; Michel, G.; Tonon, T.; Kloareg, B.; Hervé, C. Chemical and enzymatic fractionation of cell walls from Fucales: Insights into the structure of the extracellular matrix of brown algae. Ann. Bot. 2014, 114, 1203–1216. [Google Scholar] [CrossRef]
- Adhikari, U.; Mateu, C.G.; Chattopadhyay, K.; Pujol, C.A.; Damonte, E.B.; Ray, B. Structure and antiviral activity of sulfated fucans from Stoechospermum marginatum. Phytochemistry 2006, 67, 2474–2482. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.-Q.; Nie, S.-P.; Wang, Y.-X.; Cui, S.W.; Xie, M.-Y. Sulfated modification, characterization and property of a water-insoluble polysaccharide from Ganoderma atrum. Int. J. Biol. Macromol. 2015, 79, 248–255. [Google Scholar] [CrossRef]
- Witvrouw, M.; De Clercq, E. Sulfated polysaccharides extracted from sea algae as potential antiviral drugs. Gen. Pharmacol. 1997, 29, 497–511. [Google Scholar] [CrossRef]
- Meiyu, G.; Fuchuan, L.; Xianliang, X.; Jing, L.; Zuowei, Y.; Huashi, G. The potential molecular targets of marine sulfated polymannuroguluronate interfering with HIV-1 entry. Interaction between SPMG and HIV-1 rgp120 and CD4 molecule. Antiviral Res. 2003, 59, 127–135. [Google Scholar] [CrossRef]
- Damonte, E.B.; Matulewicz, M.C.; Cerezo, A.S. Sulfated seaweed polysaccharides as antiviral agents. Curr. Med. Chem. 2004, 11, 2399–2419. [Google Scholar] [CrossRef] [PubMed]
- Witvrouw, M.; Schols, D.; Andrei, G.; Snoeck, R.; Ikeda, S.; Pauwels, R.; Van Schepdael, A.; Arnout, J.; Claes, P.; Desmyter, J.; et al. New Polyacetal Polysulphate Active against Human Immunodeficiency Virus and other Enveloped Viruses. Antivir. Chem. Chemother. 1992, 3, 351–360. [Google Scholar] [CrossRef]
- Parish, C.R.; Jakobsen, K.B.; Coombe, D.R.; Bacic, A. Isolation and characterization of cell adhesion molecules from the marine sponge, Ophlitaspongia tenuis. Biochim. Biophys. Acta 1991, 1073, 56–64. [Google Scholar] [CrossRef]
- Lynch, G.; Low, L.; Li, S.; Sloane, A.; Adams, S.; Parish, C.; Kemp, B.; Cunningham, A.L. Sulfated polyanions prevent HIV infection of lymphocytes by disruption of the CD4-gp120 interaction, but do not inhibit monocyte infection. J. Leukoc. Biol. 1994, 56, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Talyshinsky, M.M.; Souprun, Y.Y.; Huleihel, M.M. Anti-viral activity of red microalgal polysaccharides against retroviruses. Cancer Cell Int. 2002, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Gerenčer, M.; Turecek, P.L.; Kistner, O.; Mitterer, A.; Savidis-Dacho, H.; Barrett, N.P. In vitro and in vivo anti-retroviral activity of the substance purified from the aqueous extract of Chelidonium majus L. Antiviral Res. 2006, 72, 153–156. [Google Scholar] [CrossRef]
- Neyts, J.; Snoeck, R.; Schols, D.; Balzarini, J.; Esko, J.D.; Van Schepdael, A.; De Clercq, E. Sulfated polymers inhibit the interaction of human cytomegalovirus with cell surface heparan sulfate. Virology 1992, 189, 48–58. [Google Scholar] [CrossRef]
- Vidhyanandhini, R.; Saravanan, R.; Vairamani, S.; Shanmugam, A. The anticoagulant activity and structural characterization of fractionated and purified glycosaminoglycans from venerid clam Meretrix casta (Chemnitz). J. Liq. Chromatogr. Relat. Technol. 2014, 37, 917–929. [Google Scholar] [CrossRef]
- Li, P.; Sheng, J.; Liu, Y.; Li, J.; Liu, J.; Wang, F. Heparosan-Derived Heparan Sulfate/Heparin-Like Compounds: One Kind of Potential Therapeutic Agents. Med. Res. Rev. 2013, 33, 665–692. [Google Scholar] [CrossRef]
- Ahmadi, A.; Zorofchian Moghadamtousi, S.; Abubakar, S.; Zandi, K. Antiviral Potential of Algae Polysaccharides Isolated from Marine Sources: A Review. Biomed. Res. Int. 2015, 2015, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rabanal, M.; Ponce, N.M.A.; Navarro, D.A.; Gómez, R.M.; Stortz, C.A. The system of fucoidans from the brown seaweed Dictyota dichotoma: Chemical analysis and antiviral activity. Carbohydr. Polym. 2014, 101, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Thuy, T.T.T.; Ly, B.M.; Van, T.T.T.; Van Quang, N.; Tu, H.C.; Zheng, Y.; Seguin-Devaux, C.; Mi, B.; Ai, U. Anti-HIV activity of fucoidans from three brown seaweed species. Carbohydr. Polym. 2015, 115, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, T.; Hayashi, T.; Hayashi, K.; Hamada, J.; Lee, J.B.; Sankawa, U. An antivirally active sulfated polysaccharide from Sargassum horneri (TURNER) C. AGARDH. Biol. Pharm. Bull. 1998, 21, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, D.J.; Krylov, V.S. Anti-HIV Activity of Extracts and Compounds from Algae and Cyanobacteria. Ecotoxicol. Environ. Saf. 2000, 45, 208–227. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Nogawa, M.; Siddiqui, R.; Watashi, K.; Wakita, T.; Kato, N.; Ikeda, M.; Okimura, T.; Isaka, S.; Oda, T.; et al. Acidic polysaccharides isolated from marine algae inhibit the early step of viral infection. Int. J. Biol. Macromol. 2019, 124, 282–290. [Google Scholar] [CrossRef]
- Huang, N.; Wu, M.-Y.; Zheng, C.-B.; Zhu, L.; Zhao, J.-H.; Zheng, Y.-T. The depolymerized fucosylated chondroitin sulfate from sea cucumber potently inhibits HIV replication via interfering with virus entry. Carbohydr. Res. 2013, 380, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Lian, W.; Wu, M.; Huang, N.; Gao, N.; Xiao, C.; Li, Z.; Zhang, Z.; Zheng, Y.; Peng, W.; Zhao, J. Anti-HIV-1 activity and structure–activity-relationship study of a fucosylated glycosaminoglycan from an echinoderm by targeting the conserved CD4 induced epitope. Biochim. Biophys. Acta 2013, 1830, 4681–4691. [Google Scholar] [CrossRef]
- Pirrone, V.; Wigdahl, B.; Krebs, F.C. The rise and fall of polyanionic inhibitors of the human immunodeficiency virus type 1. Antiviral Res. 2011, 90, 168–182. [Google Scholar] [CrossRef]
- Torode, T.A.; Siméon, A.; Marcus, S.E.; Jam, M.; Le Moigne, M.-A.; Duffieux, D.; Knox, J.P.; Hervé, C. Dynamics of cell wall assembly during early embryogenesis in the brown alga Fucus. J. Exp. Bot. 2016, 67, 6089–6100. [Google Scholar] [CrossRef]
- Raimundo, S.C.; Avci, U.; Hopper, C.; Pattathil, S.; Hahn, M.G.; Popper, Z.A. Immunolocalization of cell wall carbohydrate epitopes in seaweeds: Presence of land plant epitopes in Fucus vesiculosus L. (Phaeophyceae). Planta 2016, 243, 337–354. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.R.; Leal, R.N.; Noseda, M.; Duarte, M.E.R.; Pereira, M.S.; Mourão, P.A.S.; Farina, M.; Amado Filho, G.M. Brown algae overproduce cell wall polysaccharides as a protection mechanism against the heavy metal toxicity. Mar. Pollut. Bull. 2010, 60, 1482–1488. [Google Scholar] [CrossRef]
- Zvyagintseva, T.N.; Shevchenko, N.M.; Chizhov, A.O.; Krupnova, T.N.; Sundukova, E.V.; Isakov, V.V. Water-soluble polysaccharides of some far-eastern brown seaweeds. Distribution, structure, and their dependence on the developmental conditions. J. Exp. Mar. Bio. Ecol. 2003, 294, 1–13. [Google Scholar] [CrossRef]
- Mak, W.; Hamid, N.; Liu, T.; Lu, J.; White, W.L. Fucoidan from New Zealand Undaria pinnatifida: Monthly variations and determination of antioxidant activities. Carbohydr. Polym. 2013, 95, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, H.R.; Biller, P.; Ross, A.B.; Adams, J.M.M. The seasonal variation of fucoidan within three species of brown macroalgae. Algal Res. 2017, 22, 79–86. [Google Scholar] [CrossRef]
- Stengel, D.B.; Connan, S.; Popper, Z.A. Algal chemodiversity and bioactivity: Sources of natural variability and implications for commercial application. Biotechnol. Adv. 2011, 29, 483–501. [Google Scholar] [CrossRef]
- Fitton, J.H.; Stringer, D.N.; Karpiniec, S.S. Therapies from Fucoidan: An Update. Mar. Drugs 2015, 13, 5920–5946. [Google Scholar] [CrossRef]
- Astronomo, R.D.; Burton, D.R. Carbohydrate vaccines: Developing sweet solutions to sticky situations? Nat. Rev. Drug Discov. 2010, 9, 308–324. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, X.-S.; Zhang, L.-H. Oligosaccharide assembly by one-pot multi-step strategy. Org. Biomol. Chem. 2007, 5, 2189. [Google Scholar] [CrossRef]
- Astronomo, R.D.; Lee, H.-K.; Scanlan, C.N.; Pantophlet, R.; Huang, C.-Y.; Wilson, I.A.; Blixt, O.; Dwek, R.A.; Wong, C.-H.; Burton, D.R. A Glycoconjugate Antigen Based on the Recognition Motif of a Broadly Neutralizing Human Immunodeficiency Virus Antibody, 2G12, Is Immunogenic but Elicits Antibodies Unable To Bind to the Self Glycans of gp120. J. Virol. 2008, 82, 6359–6368. [Google Scholar] [CrossRef]
- Luallen, R.J.; Lin, J.; Fu, H.; Cai, K.K.; Agrawal, C.; Mboudjeka, I.; Lee, F.-H.; Montefiori, D.; Smith, D.F.; Doms, R.W.; et al. An Engineered Saccharomyces cerevisiae Strain Binds the Broadly Neutralizing Human Immunodeficiency Virus Type 1 Antibody 2G12 and Elicits Mannose-Specific gp120-Binding Antibodies. J. Virol. 2008, 82, 6447–6457. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dunlop, D.C.; Ulrich, A.; Appelmelk, B.J.; Burton, D.R.; Dwek, R.A.; Zitzmann, N.; Scanlan, C.N. Antigenic mimicry of the HIV envelope by AIDS-associated pathogens. AIDS 2008, 22, 2214–2217. [Google Scholar] [CrossRef] [PubMed]
- Joyce, J.G.; Krauss, I.J.; Song, H.C.; Opalka, D.W.; Grimm, K.M.; Nahas, D.D.; Esser, M.T.; Hrin, R.; Feng, M.; Dudkin, V.Y.; et al. An oligosaccharide-based HIV-1 2G12 mimotope vaccine induces carbohydrate-specific antibodies that fail to neutralize HIV-1 virions. PNAS 2008, 105, 15684–15689. [Google Scholar] [CrossRef] [PubMed]
- Astronomo, R.D.; Kaltgrad, E.; Udit, A.K.; Wang, S.-K.; Doores, K.J.; Huang, C.-Y.; Pantophlet, R.; Paulson, J.C.; Wong, C.-H.; Finn, M.G.; et al. Defining Criteria for Oligomannose Immunogens for HIV Using Icosahedral Virus Capsid Scaffolds. Chem. Biol. 2010, 17, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-K.; Liang, P.-H.; Astronomo, R.D.; Hsu, T.-L.; Hsieh, S.-L.; Burton, D.R.; Wong, C.-H. Targeting the carbohydrates on HIV-1: Interaction of oligomannose dendrons with human monoclonal antibody 2G12 and DC-SIGN. PNAS 2008, 105, 3690–3695. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-X. Toward oligosaccharide- and glycopeptide-based HIV vaccines. Curr. Opin. Drug Discov. Devel. 2006, 9, 194–206. [Google Scholar] [PubMed]
- Kumar, K.K.; Reddy, G.S.; Reddy, B.; Shekar, P.C.; Sumanthi, J.; Chandra, K.I.P. Biological role of lectins: A review. J. Orofac. Sci. 2012, 4, 20. [Google Scholar] [CrossRef]
- Balzarini, J. Inhibition of HIV entry by carbohydrate-binding proteins. Antiviral Res. 2006, 71, 237–247. [Google Scholar] [CrossRef]
- Balzarini, J.; Neyts, J.; Schols, D.; Hosoya, M.; Van Damme, E.; Peumans, W.; De Clercq, E. The mannose-specific plant lectins from Cymbidium hybrid and Epipactis helleborine and the (N-acetylglucosamine)n-specific plant lectin from Urtica dioica are potent and selective inhibitors of human immunodeficiency virus and cytomegalovirus replication. Antiviral Res. 1992, 18, 191–207. [Google Scholar] [CrossRef]
- Hansen, J.E.; Nielsen, C.M.; Nielsen, C.; Heegaard, P.; Mathiesen, L.R.; Nielsen, J.O. Correlation between carbohydrate structures on the envelope glycoprotein gp120 of HIV-1 and HIV-2 and syncytium inhibition with lectins. AIDS 1989, 3, 635–641. [Google Scholar] [CrossRef]
- Sato, T.; Hori, K. Cloning, expression, and characterization of a novel anti-HIV lectin from the cultured cyanobacterium, Oscillatoria agardhii. Fish. Sci. 2009, 75, 743–753. [Google Scholar] [CrossRef]
- Akkouh, O.; Ng, T.B.; Singh, S.S.; Yin, C.; Dan, X.; Chan, Y.S.; Pan, W.; Cheung, R.C.F. Lectins with anti-HIV activity: A review. Molecules 2015, 20, 648–668. [Google Scholar] [CrossRef] [PubMed]
- Gogineni, V.; Schinazi, R.F.; Hamann, M.T. Role of Marine Natural Products in the Genesis of Antiviral Agents. Chem. Rev. 2015, 115, 9655–9706. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, M.; Shibata, H.; Imamura, K.; Sakaguchi, T.; Hori, K. High-Mannose Specific Lectin and Its Recombinants from a Carrageenophyta Kappaphycus alvarezii Represent a Potent Anti-HIV Activity Through High-Affinity Binding to the Viral Envelope Glycoprotein gp120. Mar. Biotechnol. 2016, 18, 144–160. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.-S.; Kim, S.-K. Potential Anti-HIV Agents from Marine Resources: An Overview. Mar. Drugs 2010, 8, 2871–2892. [Google Scholar] [CrossRef] [PubMed]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H. Marine Peptides: Bioactivities and Applications. Mar. Drugs 2015, 13, 4006–4043. [Google Scholar] [CrossRef] [PubMed]
- Qaralleh, H. Chemical and bioactive diversities of marine sponge Neopetrosia. Bangladesh J. Pharmacol. 2016, 11, 433. [Google Scholar] [CrossRef]
- ANEIROS, A.; GARATEIX, A. Bioactive peptides from marine sources: Pharmacological properties and isolation procedures. J. Chromatogr. B 2004, 803, 41–53. [Google Scholar] [CrossRef]
- Shin, H.J.; Rashid, M.A.; Cartner, L.K.; Bokesch, H.R.; Wilson, J.A.; McMahon, J.B.; Gustafson, K.R. Stellettapeptins A and B, HIV-inhibitory cyclic depsipeptides from the marine sponge Stelletta sp. Tetrahedron Lett. 2015, 56, 4215–4219. [Google Scholar] [CrossRef]
- Wildeman;Gomez-Archila, L.G.; Galeano, E.; Martínez, A.; Castrillón, F.J.D.; Rugeles, M.T. Bromotyrosine Derivatives from Marine Sponges Inhibit the HIV-1 Replication in Vitro. Vitae 2014, 21, 114–125. [Google Scholar]
- Jang, I.S.; Park, S.J. Hydroxyproline-containing collagen peptide derived from the skin of the Alaska pollack inhibits HIV-1 infection. Mol. Med. Rep. 2016, 14, 5489–5494. [Google Scholar] [CrossRef] [PubMed]
- Jang, I.-S.; Park, S.J. A Spirulina maxima-derived peptide inhibits HIV-1 infection in a human T cell line MT4. Fish. Aquat. Sci. 2016, 19, 37. [Google Scholar] [CrossRef]
- Zhou, X.; Fang, W.; Tan, S.; Lin, X.; Xun, T.; Yang, B.; Liu, S.; Liu, Y. Aspernigrins with anti-HIV-1 activities from the marine-derived fungus Aspergillus niger SCSIO Jcsw6F30. Bioorg. Med. Chem. Lett. 2016, 26, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Liu, D.; Shao, Z.; Proksch, P.; Lin, W. Eutypellazines A–M, thiodiketopiperazine-type alkaloids from deep sea derived fungus Eutypella sp. MCCC 3A00281. RSC Adv. 2017, 7, 33580–33590. [Google Scholar] [CrossRef]
- O’Rourke, A.; Kremb, S.; Bader, T.; Helfer, M.; Schmitt-Kopplin, P.; Gerwick, W.; Brack-Werner, R.; Voolstra, C. Alkaloids from the Sponge Stylissa carteri Present Prospective Scaffolds for the Inhibition of Human Immunodeficiency Virus 1 (HIV-1). Mar. Drugs 2016, 14, 28. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-B.; Yang, F.; Sun, F.; Li, J.; Jiao, W.-H.; Gan, J.-H.; Hu, W.-Z.; Lin, H.-W. Aaptamine derivatives with antifungal and anti-HIV-1 activities from the South China Sea sponge Aaptos aaptos. Mar. Drugs 2014, 12, 6003–6013. [Google Scholar] [CrossRef] [PubMed]
- Tietjen, I.; Williams, D.E.; Read, S.; Kuang, X.T.; Mwimanzi, P.; Wilhelm, E.; Markle, T.; Kinloch, N.N.; Naphen, C.N.; Tenney, K.; et al. Inhibition of NF-κB-dependent HIV-1 replication by the marine natural product bengamide A. Antiviral Res. 2018, 152, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, L.; Zhu, T.; Ba, M.; Li, G.; Gu, Q.; Guo, Y.; Li, D. Phenylspirodrimanes with Anti-HIV Activity from the Sponge-Derived Fungus Stachybotrys chartarum MXH-X73. J. Nat. Prod. 2013, 76, 2298–2306. [Google Scholar] [CrossRef] [PubMed]
- Pardo-Vargas, A.; de Barcelos Oliveira, I.; Stephens, P.; Cirne-Santos, C.; de Palmer Paixão, I.; Ramos, F.; Jiménez, C.; Rodríguez, J.; Resende, J.; Teixeira, V.; et al. Dolabelladienols A–C, New Diterpenes Isolated from Brazilian Brown Alga Dictyota pfaffii. Mar. Drugs 2014, 12, 4247–4259. [Google Scholar] [CrossRef]
- de Souza Barros, C.; Cirne-Santos, C.C.; Garrido, V.; Barcelos, I.; Stephens, P.R.S.; Giongo, V.; Teixeira, V.L.; de Palmer Paixão, I.C.N. Anti-HIV-1 activity of compounds derived from marine alga Canistrocarpus cervicornis. J. Appl. Phycol. 2016, 28, 2523–2527. [Google Scholar] [CrossRef]
- Stephens, P.R.S.; Cirne-Santos, C.C.; de Souza Barros, C.; Teixeira, V.L.; Carneiro, L.A.D.; Amorim, L.; dos, S.C.; Ocampo, J.S.P.; Castello-Branco, L.R.R.; de Palmer Paixão, I.C.N. Diterpene from marine brown alga Dictyota friabilis as a potential microbicide against HIV-1 in tissue explants. J. Appl. Phycol. 2017, 29, 775–780. [Google Scholar] [CrossRef]
- Miceli, L.; Teixeira, V.; Castro, H.; Rodrigues, C.; Mello, J.; Albuquerque, M.; Cabral, L.; de Brito, M.; de Souza, A.; Miceli, L.A.; et al. Molecular Docking Studies of Marine Diterpenes as Inhibitors of Wild-Type and Mutants HIV-1 Reverse Transcriptase. Mar. Drugs 2013, 11, 4127–4143. [Google Scholar] [CrossRef] [PubMed]
- Pardo-Vargas, A.; Ramos, F.A.; Cirne-Santos, C.C.; Stephens, P.R.; Paixão, I.C.P.; Teixeira, V.L.; Castellanos, L. Semi-synthesis of oxygenated dolabellane diterpenes with highly in vitro anti-HIV-1 activity. Bioorg. Med. Chem. Lett. 2014, 24, 4381–4383. [Google Scholar] [CrossRef] [PubMed]
- Karadeniz, F.; Kang, K.-H.; Park, J.W.; Park, S.-J.; Kim, S.-K. Anti-HIV-1 activity of phlorotannin derivative 8,4‴-dieckol from Korean brown alga Ecklonia cava. Biosci. Biotechnol. Biochem. 2014, 78, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Yang, B.; Liu, J.; Xun, T.; Liu, Y.; Zhou, X. Penicillixanthone A, a marine-derived dual-coreceptor antagonist as anti-HIV-1 agent. Nat. Prod. Res. 2017, 1–5. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Huang, W.; Zheng, S.; Vigorito, M.; Chang, S.L. Effects of docosahexaenoic acid on locomotor activity in ethanol-treated HIV-1 transgenic rats. J. Neurovirol. 2018, 24, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Mejia, E.J.; Loveridge, S.T.; Stepan, G.; Tsai, A.; Jones, G.S.; Barnes, T.; White, K.N.; Drašković, M.; Tenney, K.; Tsiang, M.; et al. Study of Marine Natural Products Including Resorcyclic Acid Lactones from Humicola fuscoatra That Reactivate Latent HIV-1 Expression in an in Vitro Model of Central Memory CD4+ T Cells. J. Nat. Prod. 2014, 77, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, D.; Proksch, P.; Zhou, D.; Lin, W. Truncateols O-V, further isoprenylated cyclohexanols from the sponge-associated fungus Truncatella angustata with antiviral activities. Phytochemistry 2018, 155, 61–68. [Google Scholar] [CrossRef]
- Ahn, M.-J.; Yoon, K.-D.; Min, S.-Y.; Lee, J.S.; Kim, J.H.; Kim, T.G.; Kim, S.H.; Kim, N.-G.; Huh, H.; Kim, J. Inhibition of HIV-1 reverse transcriptase and protease by phlorotannins from the brown alga Ecklonia cava. Biol. Pharm. Bull. 2004, 27, 544–547. [Google Scholar] [CrossRef]
- Artan, M.; Li, Y.; Karadeniz, F.; Lee, S.-H.; Kim, M.-M.; Kim, S.-K. Anti-HIV-1 activity of phloroglucinol derivative, 6,6′-bieckol, from Ecklonia cava. Bioorg. Med. Chem. 2008, 16, 7921–7926. [Google Scholar] [CrossRef]
- Cardona, M.L.; Fernández, I.; Pedro, J.R.; Serrano, A. Xanthones from Hypericum reflexum. Phytochemistry 1990, 29, 3003–3006. [Google Scholar] [CrossRef]
- Negi, J.S.; Bisht, V.K.; Singh, P.; Rawat, M.S.M.; Joshi, G.P. Naturally Occurring Xanthones: Chemistry and Biology. J. Appl. Chem. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Ahmed, M.; Husain, N.E. Managing dyslipidemia in HIV/AIDS patients: Challenges and solutions. HIV/AIDS 2014, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.D.S.; Silveira, G.R.M. da Effectiveness of n-3 fatty acids in the treatment of hypertriglyceridemia in HIV/AIDS patients: A meta-analysis. Cien. Saude Colet. 2017, 22, 2659–2669. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maria Jose MB, M.N.; MB, M.J.; Agudelo, M.; Yndart, A.; Vargas-Rivera, M.E. Platelets Contribute to BBB Disruption Induced by HIV and Alcohol. J. Alcohol. Drug Depend. 2015, 3, 1–6. [Google Scholar] [CrossRef]
- Flora, G.; Pu, H.; Lee, Y.W.; Ravikumar, R.; Nath, A.; Hennig, B.; Toborek, M. Proinflammatory synergism of ethanol and HIV-1 Tat protein in brain tissue. Exp. Neurol. 2005, 191, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Acheampong, E.; Mukhtar, M.; Parveen, Z.; Ngoubilly, N.; Ahmad, N.; Patel, C.; Pomerantz, R.J. Ethanol strongly potentiates apoptosis induced by HIV-1 proteins in primary human brain microvascular endothelial cells. Virology 2002, 304, 222–234. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).