Extending the Inhibition Profiles of Coumarin-Based Compounds Against Human Carbonic Anhydrases: Synthesis, Biological, and In Silico Evaluation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Drug Design and Chemistry
2.2. CA Inhibition
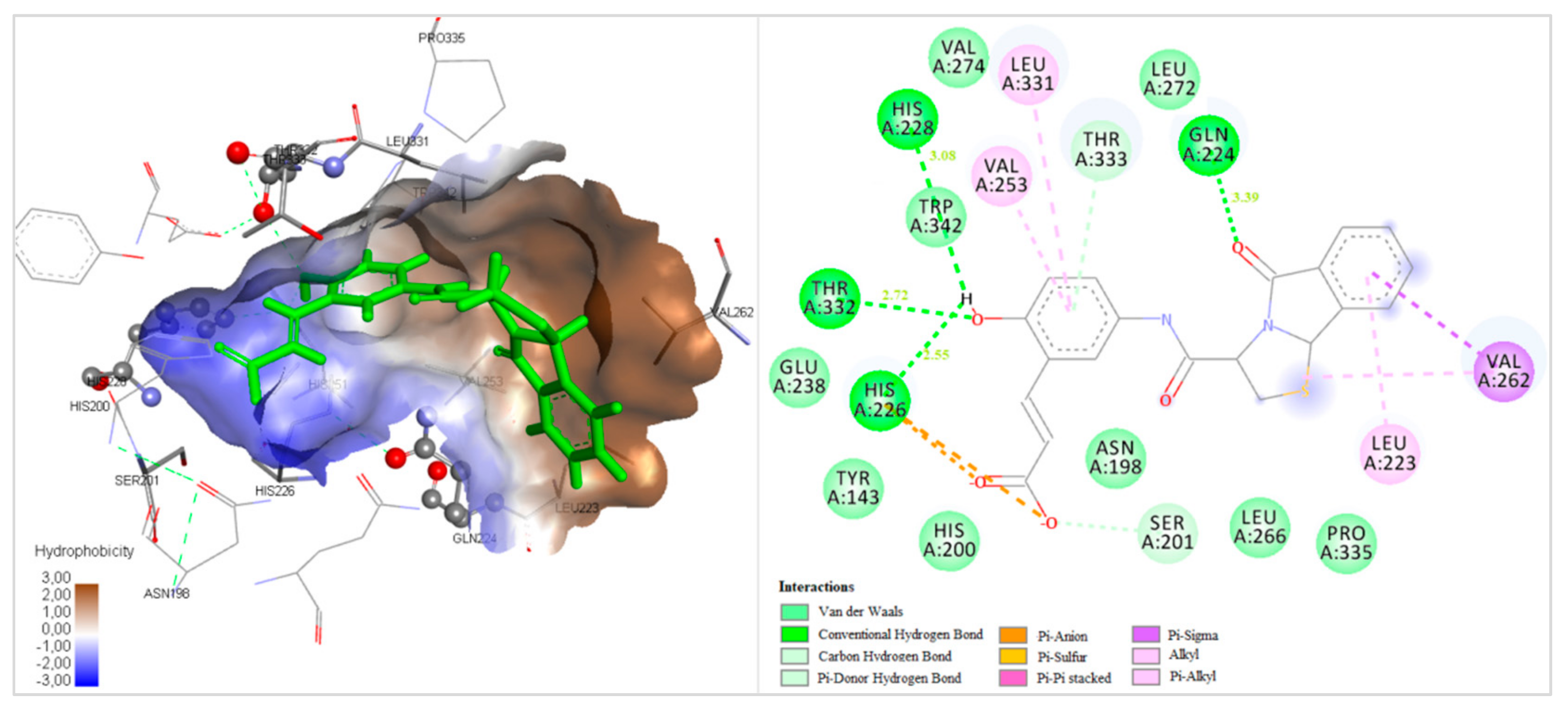
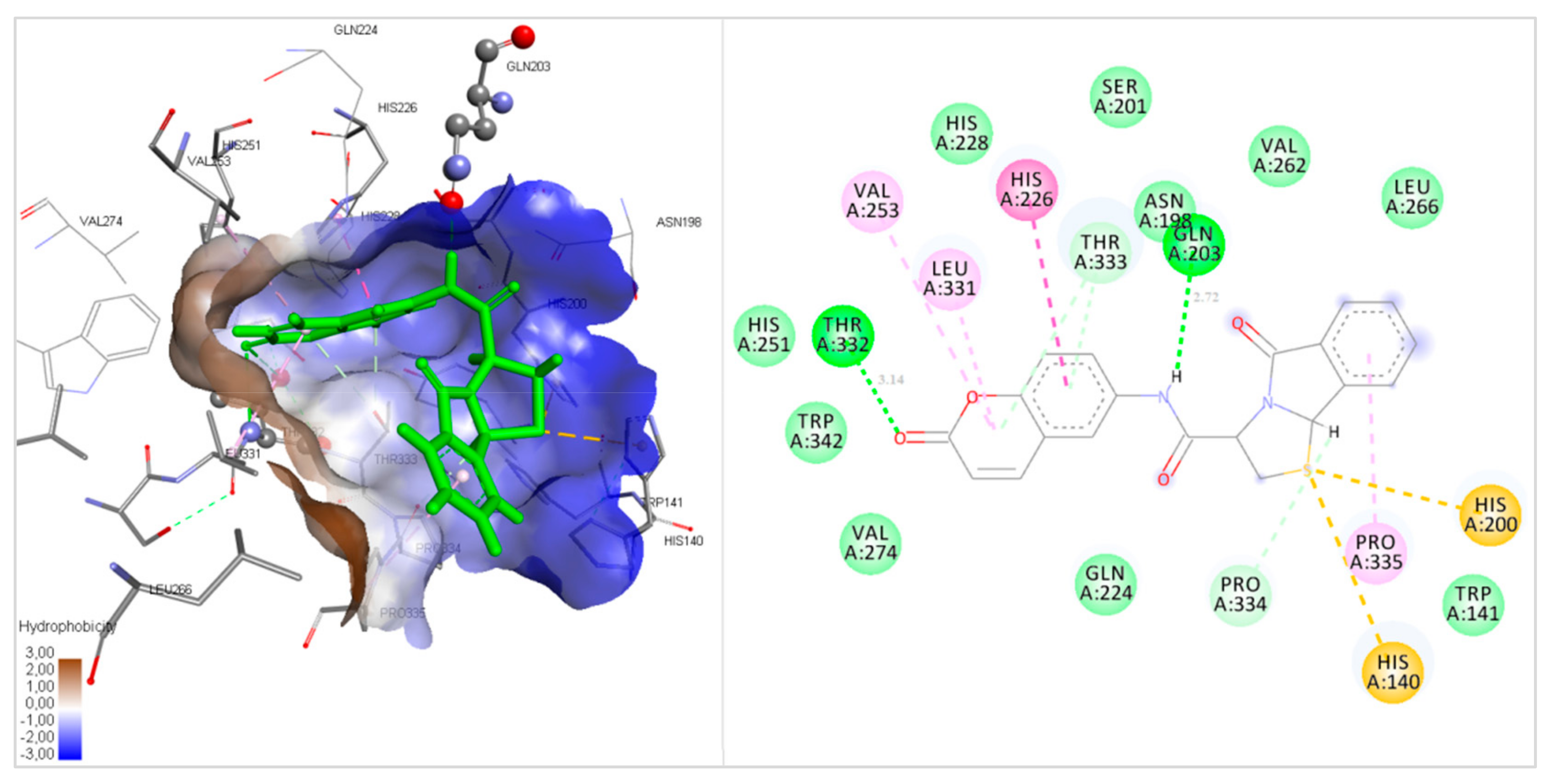
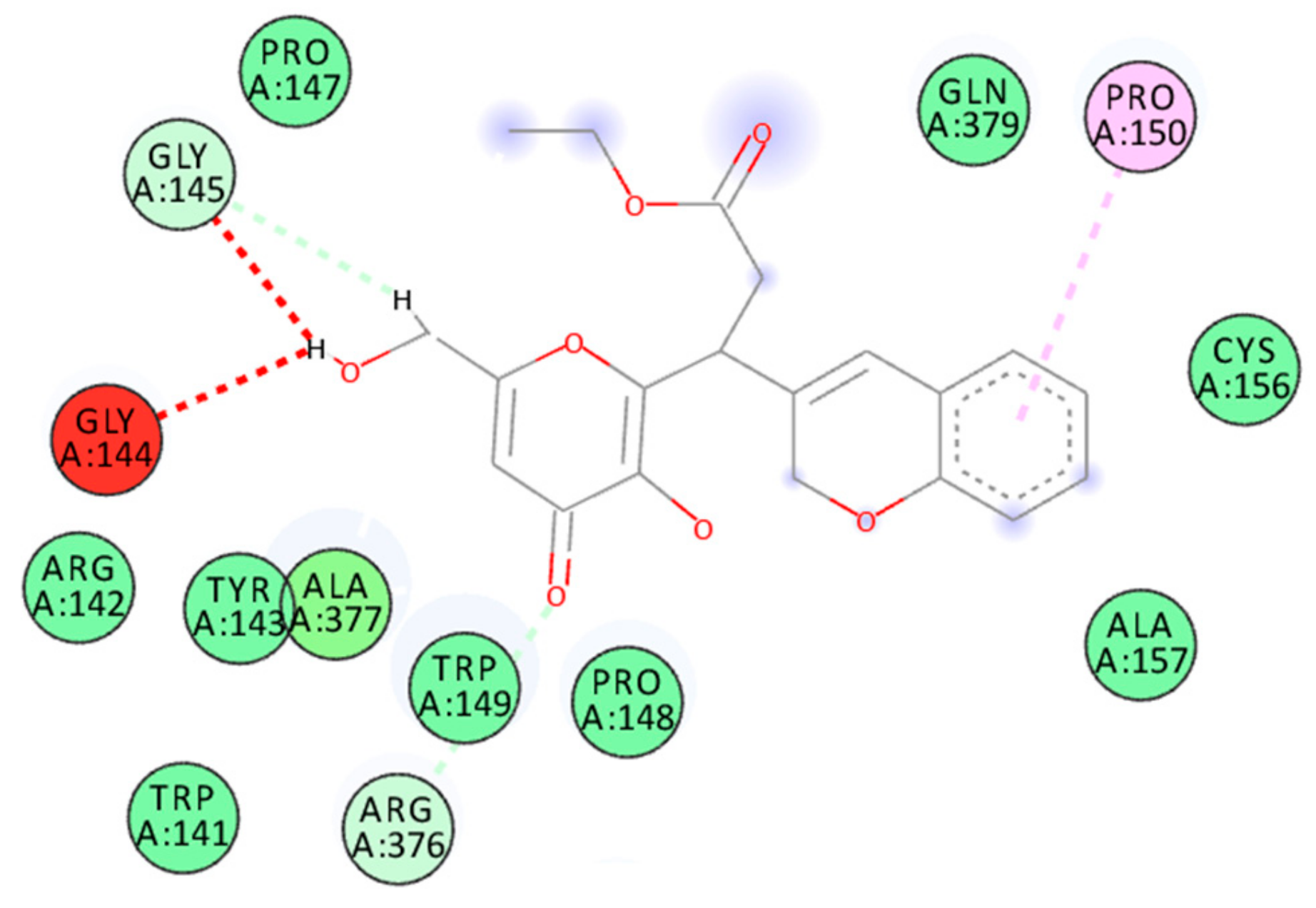
2.3. Docking Studies
3. Materials and Methods
3.1. Chemistry
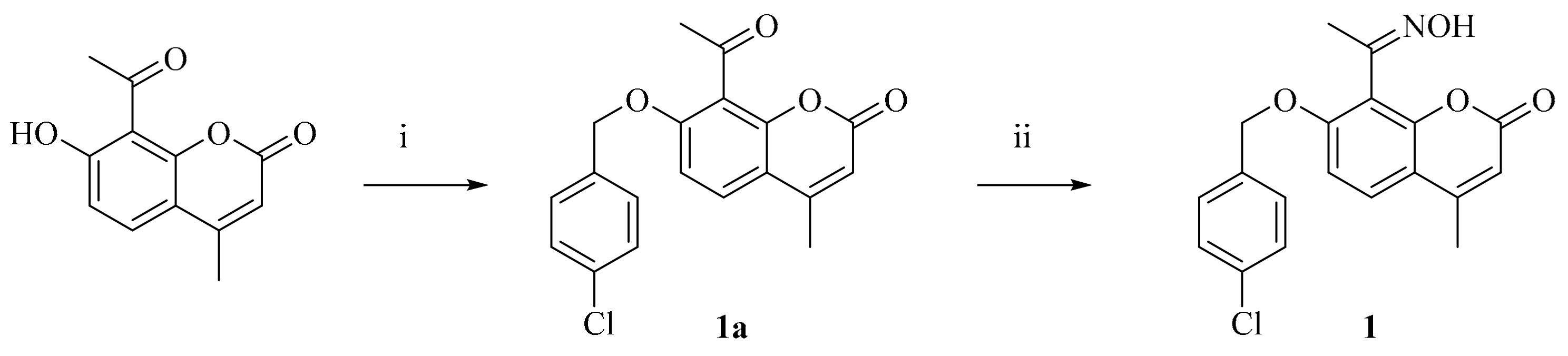
3.1.1. 7-((4-Chlorobenzyl)oxy)-8-(1-(hydroxyimino)ethyl)-4-methyl-2H-chromen-2-one (1)
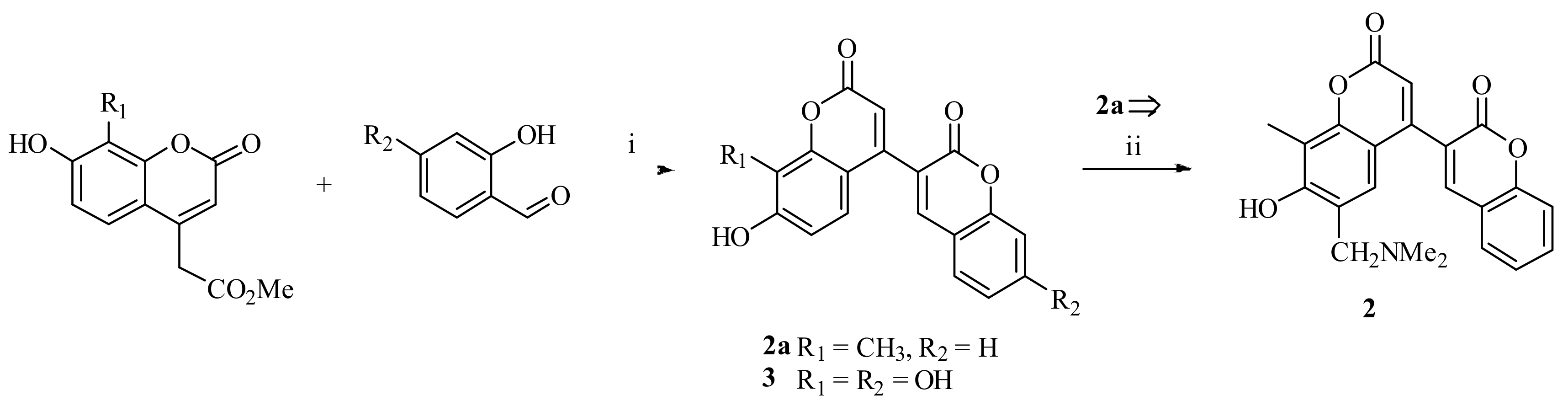
3.1.2. 6’-((Dimethylamino)methyl)-7’-hydroxy-8’-methyl-2H,2’H-[3,4’-bichromene]-2,2’-dione (2)
3.1.3. 7,7’,8’-Trihydroxy-2H,2’H-[3,4’-bichromene]-2,2’-dione (3)
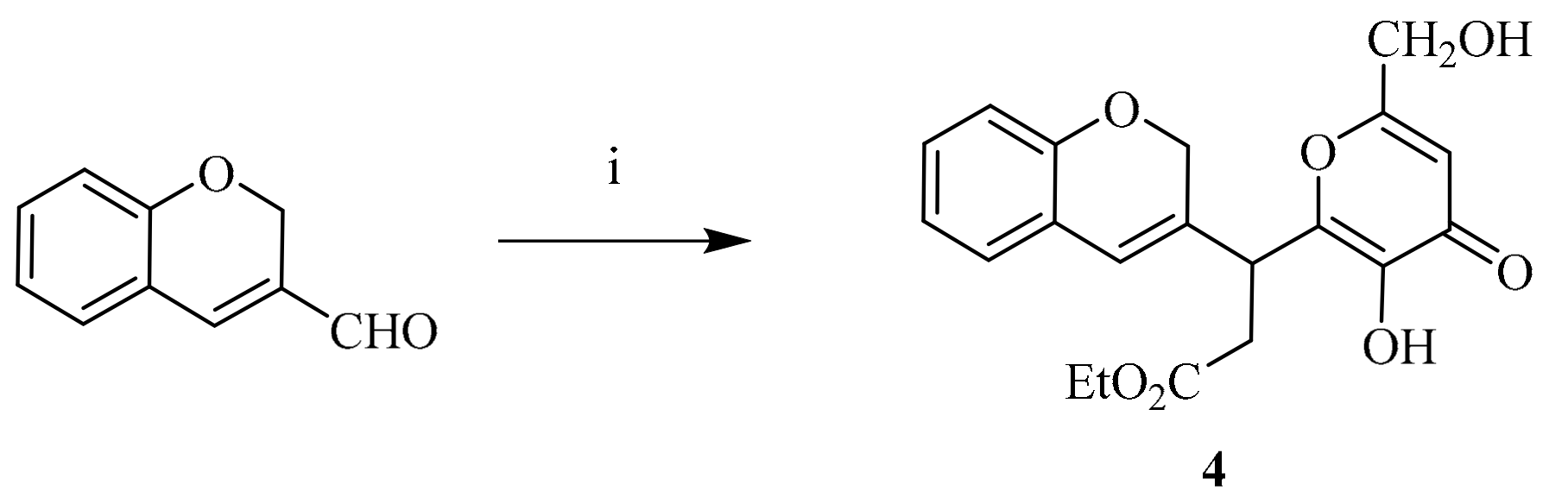
3.1.4. Ethyl 3-(2H-chromen-3-yl)-3-(3-hydroxy-6-(hydroxymethyl)-4-oxo-4H-pyran-2-yl) propanoate (4)
3.1.5. General Procedure for The Synthesis of Compounds 5–8
3.1.6. 6-Acetyl-4-(3-(2-(2,3-dihydrobenzofuran-5-yl)ethoxy)-4-methoxyphenyl)-5-hydroxychroman-2-one (5)
3.1.7. 6-Acetyl-5-hydroxy-4-(4-hydroxy-3-methoxyphenyl)chroman-2-one (6)
3.1.8. 6-Acetyl-5-hydroxy-4-(2-(pyridin-2-ylmethoxy)phenyl)chroman-2-one (7)
3.1.9. 3-(6-Acetyl-5-hydroxy-2-oxochroman-4-yl)-6-methoxy-4H-chromen-4-one (8)
3.2. CA Inhibition
3.3. Molecular Modeling
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jain, P.K.; Joshi, H. Coumarin: Chemical and Pharmacological Profile. J. Appl. Pharm. Sci. 2012, 2, 236–240. [Google Scholar]
- Abdelhafez, O.M.; Amin, K.M.; Batran, R.Z.; Maher, T.J.; Nada, S.A.; Sethumadhavan, S. Synthesis, anticoagulant and PIVKA-II induced by new 4-hydroxycoumarin derivatives. Bioorg. Med. Chem. 2010, 18, 3371–3378. [Google Scholar] [CrossRef] [PubMed]
- Saidu, N.E.; Valente, S.; Bana, E.; Kirsch, G.; Bagrel, D.; Montenarh, M. Coumarin polysulfides inhibit cell growth and induce apoptosis in HCT116 colon cancer cells. Bioorg. Med. Chem. 2012, 20, 1584–1593. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.N.; Kim, N.H.; Park, Y.S.; Kim, H.; Lee, S.; Wang, Q.; Kim, Y.K. 7-Diethylamino-3(2’-benzoxazolyl)-coumarin is a novel microtube inhibitor with antimitotic activity in multidrug resistant cancer cells. Biochem. Pharmacol. 2009, 77, 1773–1779. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.H.; Cheng, C.H.; Chen, K.C.; Lee, W.T.; Wang, Y.F.; Xiao, C.Q.; Lin, C.W. Inhibition of ros-independent jink-activation-mediated apoptosis by a novel coumarin derivative, DMAK in human colon cancer cells. Chem-Biol. Interac. 2014, 218, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hu, J.; Sun, M.; Ji, H.; Chu, S.; Liu, G.; Chen, N. Anti-inflammatory effect of IMMLG5521, a coumarin derivative, on Sephades-induced lung inflammation in rats. Int. Immunopharmacol. 2012, 14, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Togna, A.R.; Firuzi, O.; Latina, V.; Parmar, V.S.; Prasad, A.K.; Salemme, A. 4-Methylcoumarin derivatives with anti-inflammatory effects on activated microglial cells. Biol. Pharm. Bull. 2014, 37, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Advances in structure-based drug discovery of carbonic anhydrase inhibitors. Expert Opin. Drug Discov. 2017, 12, 61–88. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. How many carbonic anhydrase inhibition mechanisms exist? J. Enzyme Inhib. Med. Chem. 2016, 31, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Batra, N.; Batra, S.; Pareek, A.; Nagori, B.P. Diverse harmacological activities of 3-substituted coumarins. Int. Res. J. Pharm. 2012, 3, 24–28. [Google Scholar]
- Khan, K.M.; Saify, Z.S.; Khan, M.Z.; Choudhary, I.M.; Perveen, S.; Chohan, Z.H.; Supuran, C.T. Synthesis ofcoumarin derivatives with cytotoxic, antibacterial and antifungal activity. J. Enzym. Inhib. Med. Chem. 2004, 19, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Brooker, N.L.; Kuzimichev, Y.; Laas, J.; Pavlis, R. Evaluation of coumarin derivatives as anti-fungal agents against soil-borne fungal pathogens. Commun. Agric. Appl. Biol. Sci. 2007, 72, 785–793. [Google Scholar] [PubMed]
- Han, J.; Sun, L.; Huang, X.; Li, Z.; Zhang, C.; Qian, H.; Huang, W. Novel coumarin modified GLP-1derivatives with enhanced plasma stability and prolonged in vivo glucose -lowering ability. Br. J. Pharmacol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, G.Y.; Dai, F.; Cao, X.Y.; Kang, Y.F.; Hu, L.M.; Tang, J.J.; Li, X.Z.; Li, Y.; Jin, X.L.; et al. Synthesis and biological evaluation of hydroxylated 3-phenylcoumarins as antioxidants and antiproliferative agents. Bioorg. Med. Chem. Lett. 2011, 21, 6420–6425. [Google Scholar] [CrossRef]
- Anand, P.; Singh, B.; Singh, N. Areview on coumarins as acetlcholinesterase inhibitors for Alzheimer’s disease. Bioorg. Med. Chem. 2012, 20, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Vina, D.; Matos, M.L.; Yanez, M.; Santana, L.; Uriarte, E. 3-Substituted coumarins as dual inhibitors of AChE and MAO for the treatment of Alzheimer’s disease. MedChemCom. 2012, 3, 213–218. [Google Scholar] [CrossRef]
- Xie, S.-S.; Wang, X.-B.; Li, J.-Y.; Yang, L.; Kong, L.-Y. Design, synthesis and evaluation of novel tacrine-coumarin hubrids as multifunctional cholinesterase inhibitors against Alzheimer’s disease. Eur. J. Med. Chem. 2013, 64, 540–553. [Google Scholar] [CrossRef]
- Huang, M.; Xie, S.-S.; Jiang, N.; Lan, J.-S.; Kong, L.-Y.; Wang, X.-B. Multifunctional coumarin derivatives: Monoamine oxidase B (MAO-B) inhibition, anti-β-amyloid (Aβ) aggregation and metal chelation properties against Alzheimer’s disease. Bioorg. Med. Chem. Lett. 2015, 25, 508–513. [Google Scholar] [CrossRef]
- Al-Majedy, Y.; Al-Amiery, A.; Kadhum, A.A.; Bakar, M.A. Antioxidant activity of coumarins. Sys. Rev. Pharm. 2017, 8, 24–30. [Google Scholar] [CrossRef]
- Kadhum, A.A.H.; Al-Amiery, A.A.; Musa, A.Y.; Mohamad, A.B. The antioxidant activity of new coumarin derivatives. Int. J. Mol. Sci. 2011, 12, 5747–5761. [Google Scholar] [CrossRef]
- Sashidhara, K.V.; Kumar, A.; Kumar, M.; Srivastava, A.; Puri, A. Synthesis and antihyperlipidemic activity of novel coumarin bisindole derivatives. Bioorg. Med. Chem. Lett. 2010, 20, 6504–6507. [Google Scholar] [CrossRef] [PubMed]
- Jalal, S.; Chand, K.; Kathuria, A.; Singh, P.; Priya, N.; Gupta, B.; Raj, H.G.; Sharma, S.K. Calreticulin transacetylase: A novel enzyme-mediated protein acetylation by acetoxy derivativesof 3-alkyl-4-methylcoumarins. Bioorg. Chem. 2012, 40, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, Y.G.; Cho, H.S.; Ryu, S.Y.; Cho, M.H.; Lee, J. Coumarins reduce biofilm formation and the virulence of Escherichia coli O157:H7. Phytomedicine 2014, 21, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Mandlik, V.; Patil, S.; Bopanna, R.; Basu, S.; Singh, S. Biological Activity of Coumarin Derivatives as Anti-Leishmanial Agents. Plos ONE 2016, 11, e0164585. [Google Scholar] [CrossRef] [PubMed]
- Melis, C.; Distinto, S.; Bianco, G.; Meleddu, R.; Cottiglia, F.; Fois, B.; Taverna, D.; Angius, R.; Alcaro, S.; Ortuso, F.; et al. Targeting Tumor Associated Carbonic Anhydrases IX and XII: Highly Isozyme Selective Coumarin and Psoralen Inhibitors. ACS Med. Chem. Lett. 2018, 9, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Narayanaswamy, V.K.; Gleiser, R.M.; Kasumbwe, K.; Aldhubiab, B.E.; Attimarad, M.V.; Odhav, B. Evaluation of Halogenated Coumarins for Antimosquito Properties. Sci. World J. 2014, 189824, 1–6. [Google Scholar] [CrossRef]
- Maresca, A.; Temperini, C.; Vu, H.; Pham, N.B.; Poulsen, S.A.; Scorrafava, A.; Quinn, R.J.; Supuran, C.T. Non-zinc mediated inhibition of carbonic anhydrase; coumarins are a new class of suicide inhibitors. J. Am. Chem. Soc. 2009, 131, 3057–3062. [Google Scholar] [CrossRef]
- Bonneau, A.; Maresca, A.; Winum, J.-V.; Supuran, C.T. Metronidazole –coumain conjugates and 3-cyano-7-hydroxy-coumarin act as isoform-selective carbonic anhydrase inhibitor. J. Enz. Inhib. Med. Chem. 2013, 28, 397–401. [Google Scholar] [CrossRef]
- Küçükbay, F.Z.; Küçükbay, H.; Tanc, M.; Supuran, C.T. Synthesis and carbonic anhydrase inhibitory properties of amino acid-coumarin/quinolinone conjugates incorporating glycine, alanine and phenylalanine moieties. J. Enzyme. Inhib. Med. Chem. 2016, 31, 1198–1202. [Google Scholar] [CrossRef]
- Maresca, A.; Supuran, C.T. Coumarins incorporating hydroxy- and chloro-moieties selectively inhibit the transmembrane, tumor-associated carbonic anhydrase isoforms IX and XII over the cytosolic ones I and II. Bioorg. Med. Chem. Lett. 2010, 20, 4511–4514. [Google Scholar] [CrossRef]
- Maresca, A.; Scozzafava, A.; Supuran, C.T. 7,8-disubstituted- but not 6,7-disubstituted coumarins selectively inhibit the transmembrane, tumor-associated carbonic anhydrase isoforms IX and XII over the cytosolic ones I and II in the low nanomolar/subnanomolar range. Bioorg. Med. Chem. Lett. 2010, 20, 7255–7258. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Structure-based drug discovery of carbonic anhydrase inhibitors. J. Enzym. Inhib. Med. Chem. 2012, 27, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Nocentini, A.; Supuran, C.T. Advances in the structural annotation of human carbonic anhydrases and impact on future drug discovery. Expert Opin. Drug. Discov. 2019. [Google Scholar] [CrossRef] [PubMed]
- Khilya, V.P.; Kovalev, S.V.; Miroshnichenko, N.S.; Turov, A.V. Synthesis and spectral properties of 3-furyl-4-hydroxycoumarins. Chem. Nat. Compd. 1998, 34, 32–37. [Google Scholar] [CrossRef]
- Szabo, V.; Grishko, L.G.; Borbei, S.; Khilya, V.P. Chemistry of heteroanalogs of isoflavones. Chem. Heterocycl. Compd. 1975, 11, 147–151. [Google Scholar] [CrossRef]
- Khalifah, R.G. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J. Biol. Chem. 1971, 246, 2561–2573. [Google Scholar] [PubMed]
- Bondarenko, S.P.; Frasinyuk, M.S.; Khilya, V.P. Synthesis of Pseudobaptigenin Analogs. Chem. Nat. Compd. 2003, 39, 265–270. [Google Scholar] [CrossRef]
- Galayev, O.; Garazd, Y.; Garazd, M.; Lesyk, R. Synthesis and anticancer activity of 6-heteroarylcoumarins. Synthesis and anticancer activity of 6-heteroarylcoumarins. Eur. J. Med. Chem. 2015, 105, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Entezari Heravi, Y.; Bua, S.; Nocentini, A.; Del Prete, S.; Saboury, A.A.; Sereshti, H.; Capasso, C.; Gratteri, P.; Supuran, C.T. Inhibition of Malassezia globosa carbonic anhydrase with phenols. Bioorg. Med. Chem. 2017, 25, 2577–2582. [Google Scholar] [CrossRef]
- Nocentini, A.; Carta, F.; Tanc, M.; Selleri, S.; Supuran, C.T.; Bazzicalupi, C.; Gratteri, P. Deciphering the Mechanism of Human Carbonic Anhydrases Inhibition with Sulfocoumarins: Computational and Experimental Studies. Chemistry 2018, 24, 7840–7844. [Google Scholar] [CrossRef]
- Nocentini, A.; Gratteri, P.; Supuran, C.T. Phosphorus versus Sulfur: Discovery of Benzenephosphonamidates as Versatile Sulfonamide-Mimic Chemotypes Acting as Carbonic Anhydrase Inhibitors. Chemistry 2019, 25, 1188–1192. [Google Scholar] [CrossRef] [PubMed]
- Nocentini, A.; Trallori, E.; Singh, S.; Lomelino, C.L.; Bartolucci, G.; Di Cesare Mannelli, L.; Ghelardini, C.; McKenna, R.; Gratteri, P.; Supuran, C.T. 4-Hydroxy-3-nitro-5-ureido-benzenesulfonamides Selectively Target the Tumor-Associated Carbonic Anhydrase Isoforms IX and XII Showing Hypoxia-Enhanced Antiproliferative Profiles. J. Med. Chem. 2018, 61, 10860–10874. [Google Scholar] [CrossRef] [PubMed]
- Mahon, B.P.; Bhatt, A.; Socorro, L.; Driscoll, J.M.; Okoh, C.; Lomelino, C.L.; Mboge, M.Y.; Kurian, J.J.; Tu, C.; Agbandje-McKenna, M.; et al. The Structure of Carbonic Anhydrase IX Is Adapted for Low-pH Catalysis. Biochemistry 2016, 55, 4642–4653. [Google Scholar] [CrossRef] [PubMed]
- Whittington, D.A.; Waheed, A.; Ulmasov, B.; Shah, G.N.; Grubb, J.H.; Sly, W.S.; Christianson, D.W. Crystal structure of the dimeric extracellular domain of human carbonic anhydrase XII, a bitopic membrane protein overexpressed in certain cancer tumor cells. Proc. Natl. Acad. Sci. USA 2001, 98, 9545–9550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of the compounds are available from the authors in small amounts. |

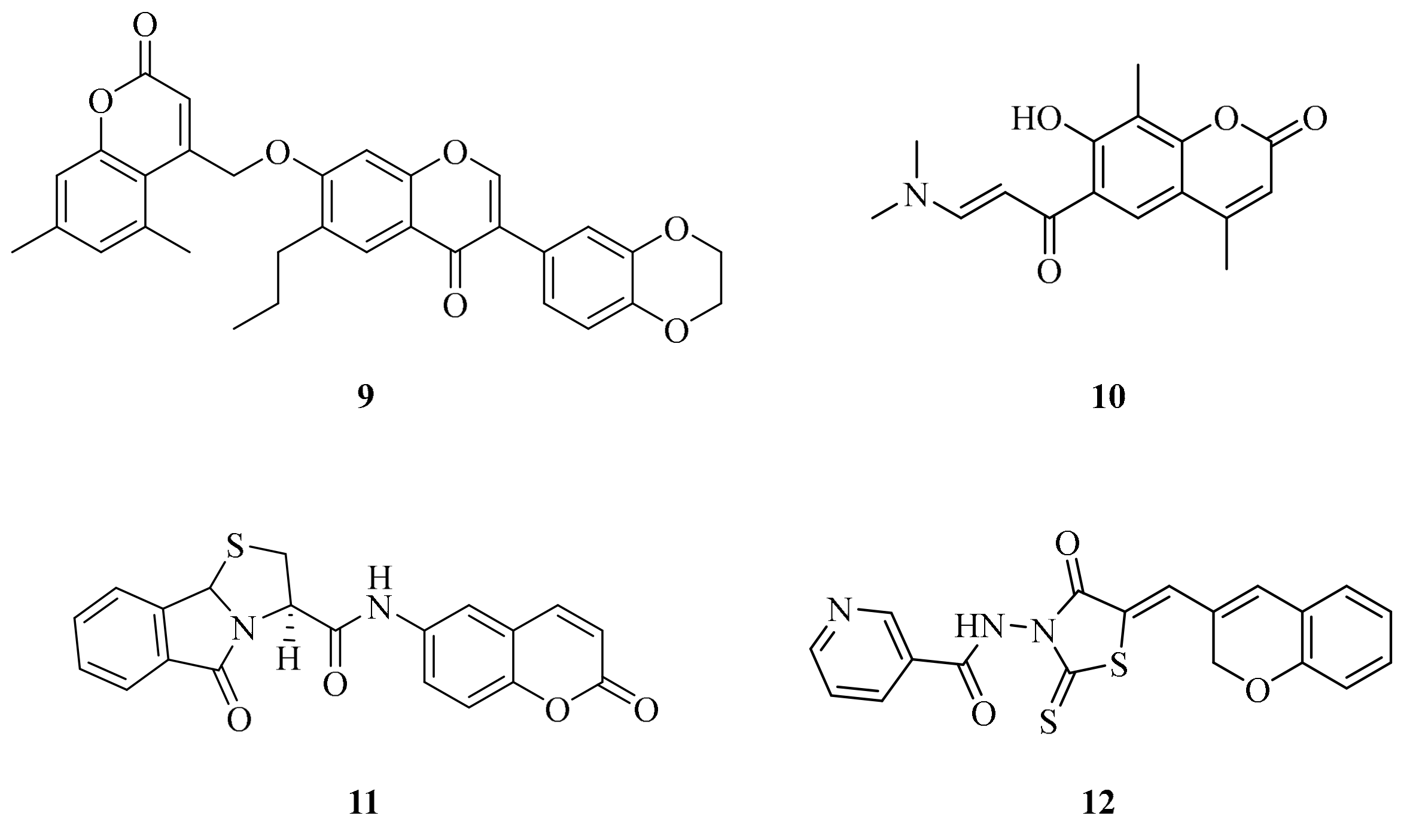

| Cmpd | KI* (nM) | |||
|---|---|---|---|---|
| hCA II | hCA II | hCA IX | hCAXII | |
| 1 | >10,000 | >10,000 | 49.3 | 558.1 |
| 2 | >10,000 | >10,000 | 85.6 | >10,000 |
| 3 | >10,000 | >10,000 | 132.6 | >10,000 |
| 4 | >10,000 | >10,000 | 243.1 | 466.7 |
| 5 | >10,000 | >10,000 | 171.6 | >10,000 |
| 6 | >10,000 | >10,000 | 188.6 | >10,000 |
| 7 | >10,000 | >10,000 | 138.6 | >10,000 |
| 8 | >10,000 | >10,000 | 174.8 | >10,000 |
| 9 | >10,000 | >10,000 | 171.2 | >10,000 |
| 10 | >10,000 | >10,000 | 25.7 | 603.8 |
| 11 | >10,000 | >10,000 | 9.4 | 590.9 |
| 12 | >10,000 | >10,000 | 240.9 | 432.1 |
| AAZ | 250 | 12 | 25 | 5.7 |
| Estimated Binding Energy (kcal/mol) | Binding Affinity Score | I-H | Residues CA IX | ||
|---|---|---|---|---|---|
| CA-XII | CA-IX | ||||
| 1 | −5.13 | −7.03 | −26.49 | 1 | Thr332 |
| H1 | −6.89 | −8.16 | −27.25 | 2 | Gln224, Thr333 |
| 2 | −1.24 | −6.80 | −23.37 | 1 | Gln203 |
| H2 | −3.85 | −7.55 | −25.12 | 2 | Gln224, Thr332 |
| 3 | - | −6.33 | −21.13 | 1 | Thr332 |
| H3 | −3.21 | −6.74 | −21.42 | 1 | Thr333 |
| 4 | −3.11 | −5.13 | −15.96 | - | - |
| 5 | - | −6.11 | −20.09 | 1 | Thr332 |
| H5 | - | −6.77 | −20.83 | 1 | His228 |
| 6 | - | −5.81 | −17.26 | - | - |
| H6 | - | −6.05 | −18.73 | 1 | Thr333 |
| 7 | - | −6.24 | −21.05 | 1 | Thr332 |
| H7 | −2.57 | −6.72 | −21.41 | 1 | Thr333 |
| 8 | - | −6.03 | −19.26 | - | - |
| 8 | −1.22 | −6.15 | −19.58 | 1 | Thr333 |
| 5 9 | - | −6.10 | −20.27 | 1 | Gln203 |
| H9 | - | −6.79 | −20.97 | 1 | Thr333 |
| 10 | −3.49 | −8.22 | −27.34 | 1 | Thr332 |
| H10 | −5.88 | −10.03 | −29.78 | 2 | Thr332, Thr333 |
| 11 | −5.36 | −9.61 | −29.14 | 2 | Gln203, Thr332 |
| H11 | −5.41 | −13.25 | −35.41 | 4 | Gln224, His226, His228, Thr332 |
| 12 | −3.18 | −5.28 | −16.47 | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kartsev, V.; Geronikaki, A.; Bua, S.; Nocentini, A.; Petrou, A.; Lichitsky, B.; Frasinyuk, M.; Leitans, J.; Kazaks, A.; Tars, K.; et al. Extending the Inhibition Profiles of Coumarin-Based Compounds Against Human Carbonic Anhydrases: Synthesis, Biological, and In Silico Evaluation. Molecules 2019, 24, 3580. https://doi.org/10.3390/molecules24193580
Kartsev V, Geronikaki A, Bua S, Nocentini A, Petrou A, Lichitsky B, Frasinyuk M, Leitans J, Kazaks A, Tars K, et al. Extending the Inhibition Profiles of Coumarin-Based Compounds Against Human Carbonic Anhydrases: Synthesis, Biological, and In Silico Evaluation. Molecules. 2019; 24(19):3580. https://doi.org/10.3390/molecules24193580
Chicago/Turabian StyleKartsev, Victor, Athina Geronikaki, Silvia Bua, Alessio Nocentini, Anthi Petrou, Boris Lichitsky, Mykhaylo Frasinyuk, Janis Leitans, Andris Kazaks, Kaspars Tars, and et al. 2019. "Extending the Inhibition Profiles of Coumarin-Based Compounds Against Human Carbonic Anhydrases: Synthesis, Biological, and In Silico Evaluation" Molecules 24, no. 19: 3580. https://doi.org/10.3390/molecules24193580






